Choice of Magnetometers and Gradiometers after Signal Space Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Theoretical Reasoning
2.2. Experimental Source Reconstructions from Magnetometers and Gradiometers
MEG Acquisition and Source Estimation
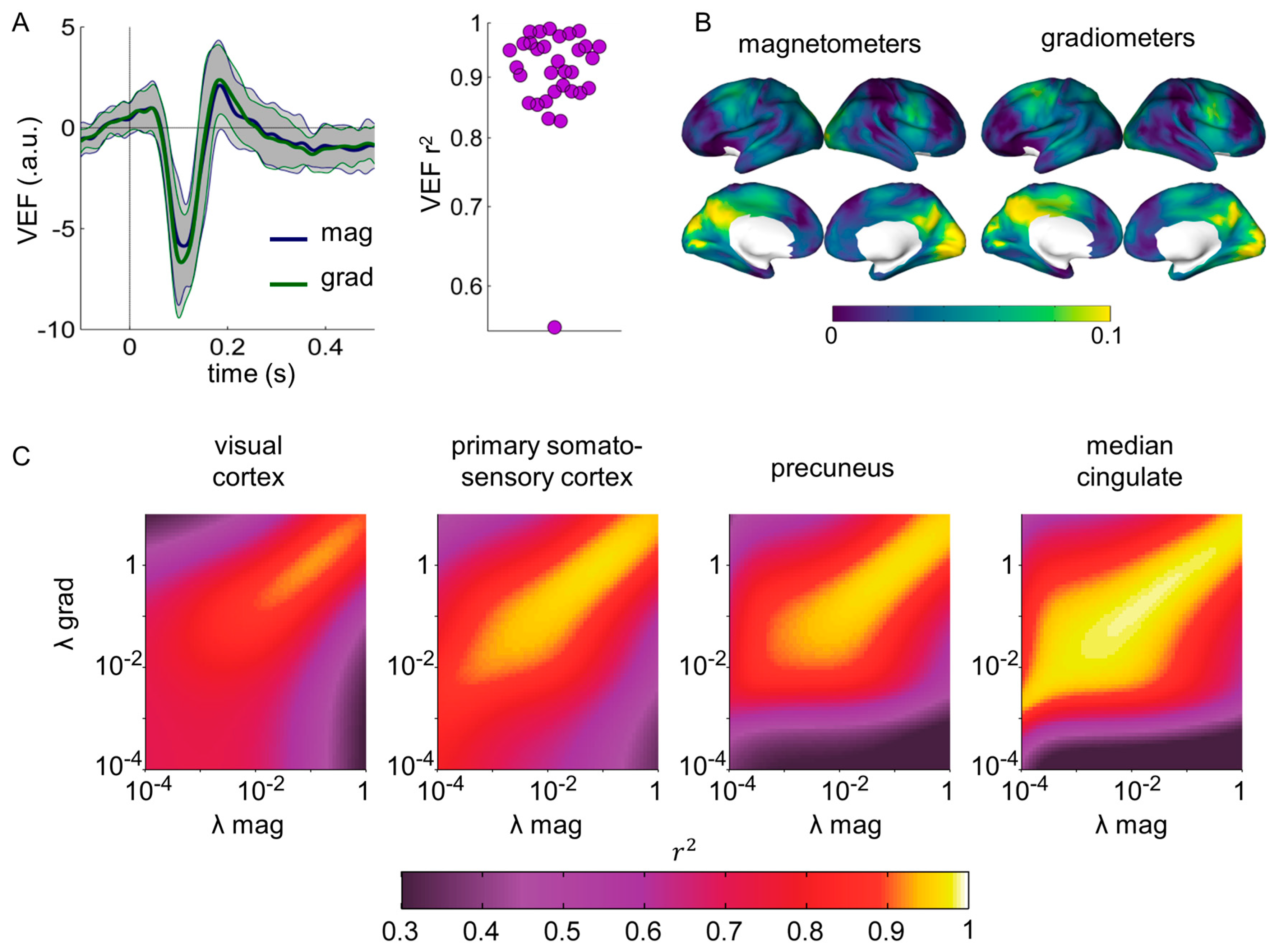
3. Results
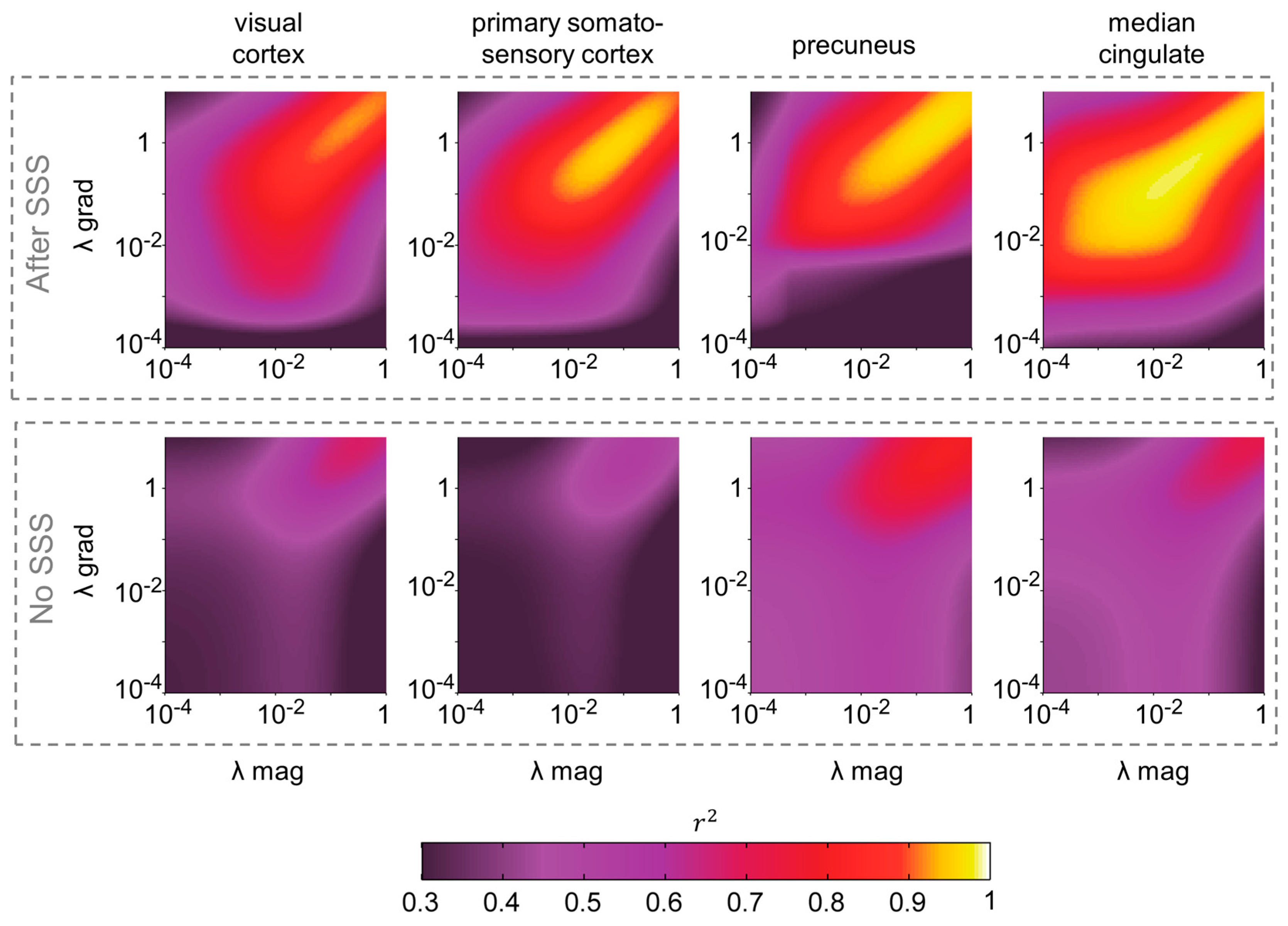
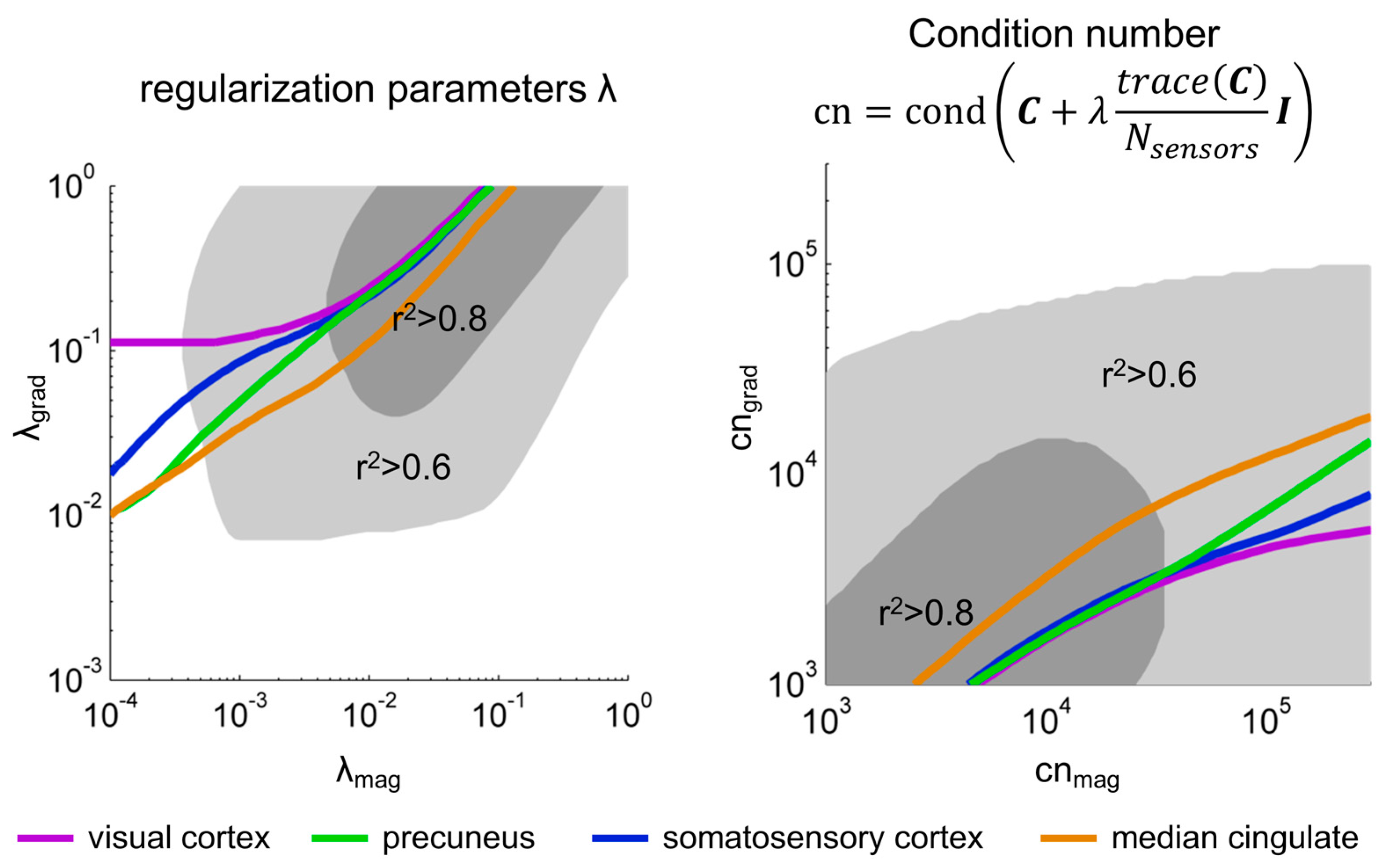
3.1. Correlation between Magnetometer and Gradiometer Source Reconstructions after SSS, as a Function of the Regularization Factor λ
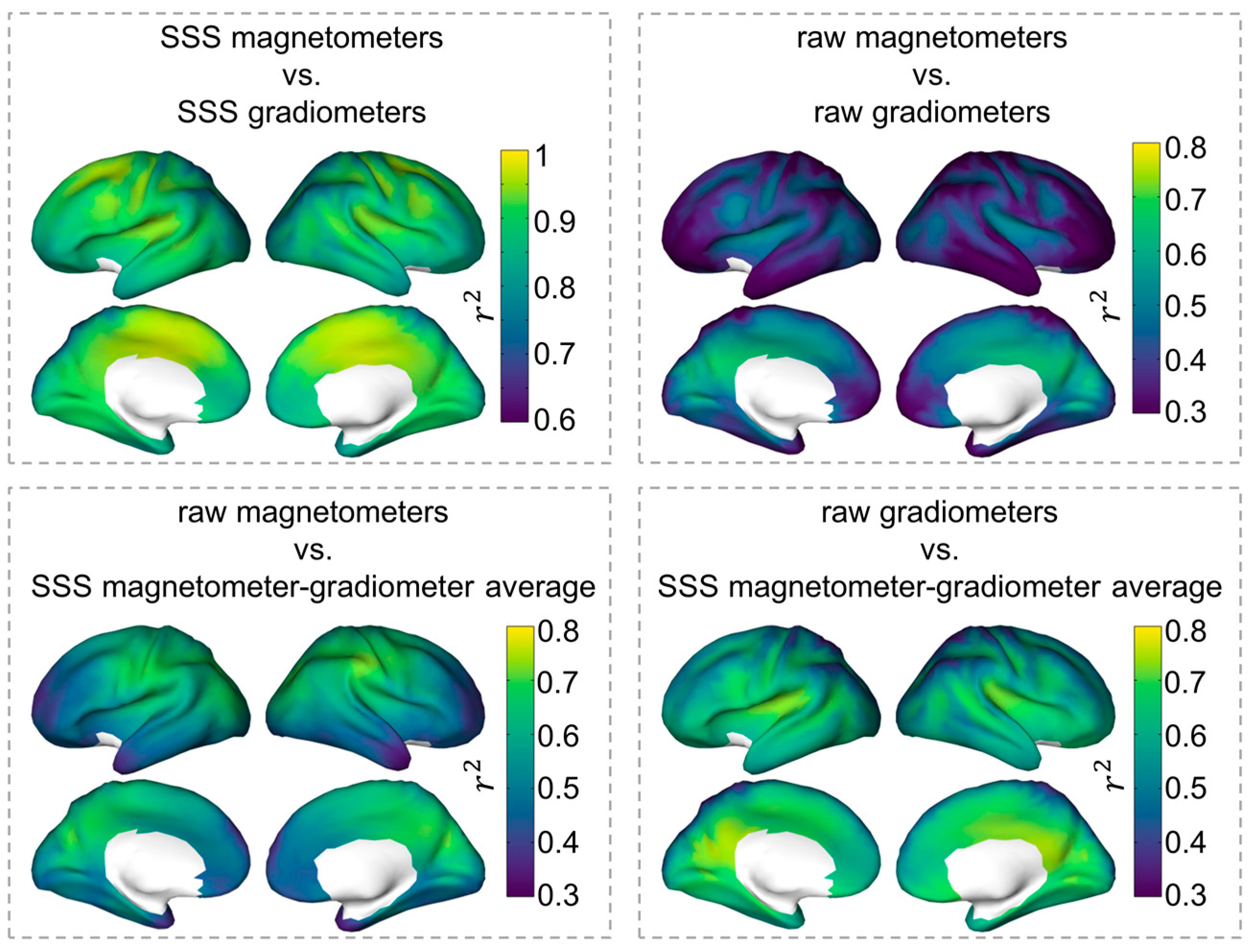
3.2. Spatial Dependence of the Correlation between Magnetometer and Gradiometer Source Reconstructions
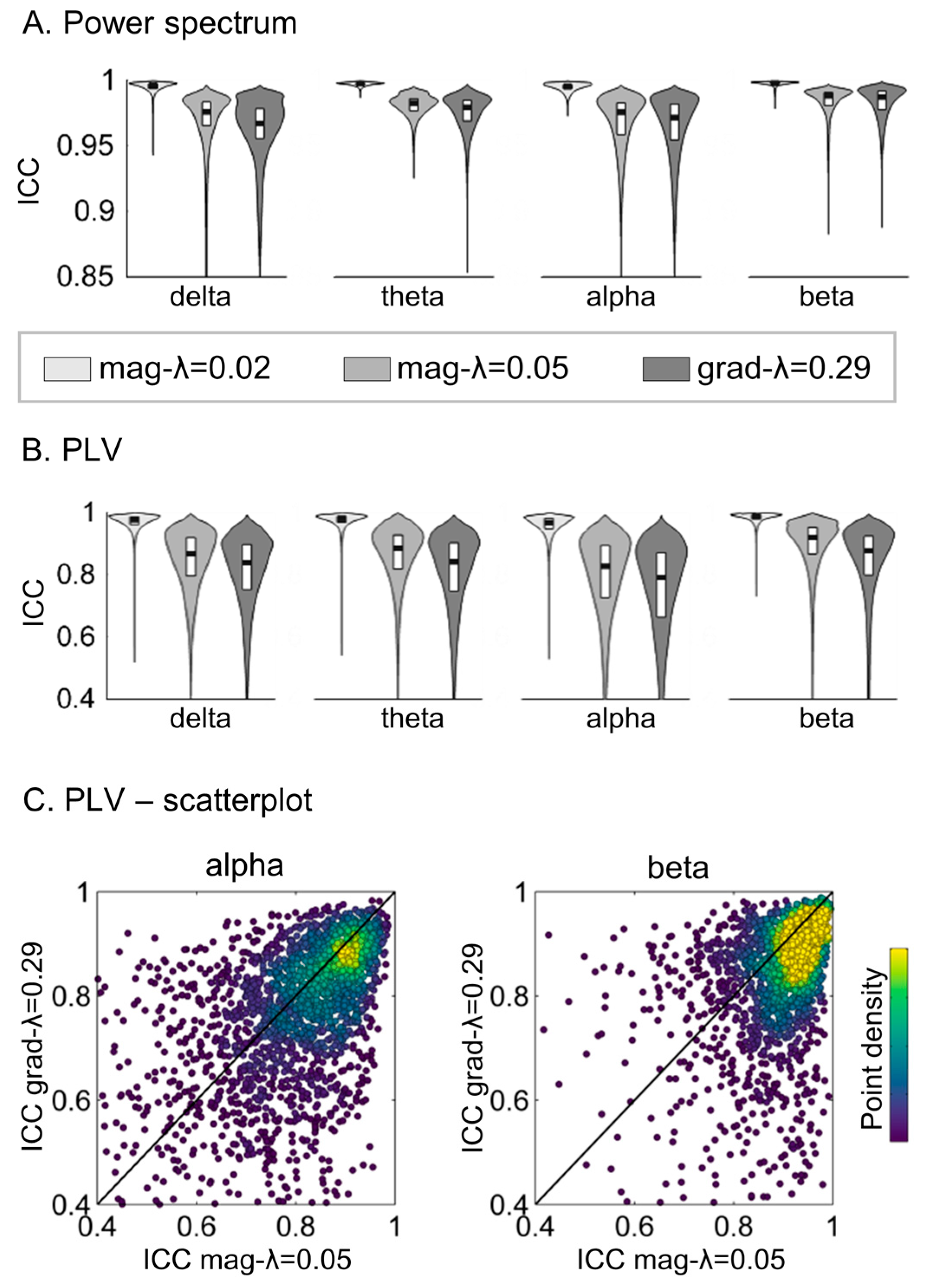
3.3. Inter-Pipeline Reliability of Power and Functional Connectivity Values: Impact on the Choice of Magnetometers or Gradiometers
3.4. Generalizability of the Previous Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Taulu, S.; Kajola, M. Presentation of electromagnetic multichannel data: The signal space separation method. J. Appl. Phys. 2005, 97, 124905. [Google Scholar] [CrossRef]
- Taulu, S.; Simola, J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol. 2006, 51, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Taulu, S.; Simola, J.; Kajola, M. Applications of the signal space separation method. IEEE Trans. Signal Process. 2005, 53, 3359–3372. [Google Scholar] [CrossRef]
- Hillebrand, A.; Fazio, P.; de Munck, J.C.; van Dijk, B.W. Feasibility of clinical Magnetoencephalography (MEG) functional mapping in the presence of dental artefacts. Clin. Neurophysiol. 2013, 124, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Goj, R.; Jas, M.; Brooks, T.; Parkkonen, L.; et al. MEG and EEG data analysis with MNE-Python. Front. Neurosci. 2013, 7, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elekta-Neuroscience MaxFilter User’s Guide; Software Version 2.2 2010; Elektra Neuroscience: Helsinki, Finland, 2010.
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Parkkonen, L.; Hämäläinen, M.S. MNE software for processing MEG and EEG data. Neuroimage 2014, 86, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Elekta-Neuromag. Elekta Neuromag® System Hardware User Manual; Elekta: Helsinki, Finland, 2005. [Google Scholar]
- Malmivuo, J.; Plonsey, R. Magnetoencephalography. In Bioelectromagnetism: Principles and Applications of Bioelectric and Biomagnetic Fields; Oxford University Press: Oxford, NY, USA, 1995. [Google Scholar]
- Vrba, J.; Robinson, S.E. SQUID sensor array configurations for magnetoencephalography applications. Supercond. Sci. Technol. 2002, 15, R51–R89. [Google Scholar] [CrossRef]
- Engemann, D.A.; Gramfort, A. Automated model selection in covariance estimation and spatial whitening of MEG and EEG signals. Neuroimage 2015, 108, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Gauss, C.F. Allgemeine Theorie des Erdmagnetismus. In Werke; Springer: Berlin/Heidelberg, Germany, 1877; pp. 119–193. [Google Scholar]
- Ahonen, A.I.; Hämruäinen, M.S.; Ilmoniemi, R.J.; Kajola, M.J.; Knuutila, J.E.T.; Simola, J.T.; Vilkman, V.A. Sampling Theory for Neuromagnetic Detector Arrays. IEEE Trans. Biomed. Eng. 1993, 40, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Garcés, P.; Martín-Buro, M.C.; Maestú, F. Quantifying the Test-Retest Reliability of Magnetoencephalography Resting-State Functional Connectivity. Brain Connect. 2016, 6, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.-M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 2011, 156869. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; van der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole Brain Segmentation. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef]
- Ségonne, F.; Pacheco, J.; Fischl, B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans. Med. Imaging 2007, 26, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Van Veen, B.D.; van Drongelen, W.; Yuchtman, M.; Suzuki, A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng. 1997, 44, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Litvak, V.; Eusebio, A.; Jha, A.; Oostenveld, R.; Barnes, G.R.; Penny, W.D.; Zrinzo, L.; Hariz, M.I.; Limousin, P.; Friston, K.J.; et al. Optimized beamforming for simultaneous MEG and intracranial local field potential recordings in deep brain stimulation patients. Neuroimage 2010, 50, 1578–1588. [Google Scholar] [CrossRef] [PubMed]
- El Ghaoui, L. Inversion error, condition number, and approximate inverses of uncertain matrices. Linear Algebra Appl. 2002, 343, 171–193. [Google Scholar] [CrossRef]
- Shrout, P.; Fleiss, J. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Hunt, L.T.; Woolrich, M.W.; Rushworth, M.F.S.; Behrens, T.E.J. Trial-Type Dependent Frames of Reference for Value Comparison. PLoS Comput. Biol. 2013, 9, e1003225. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Martín-Buro, M.C.; Garcés, P.; Maestú, F. Test-retest reliability of resting-state magnetoencephalography power in sensor and source space. Hum. Brain Mapp. 2016, 37, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Taylor, J.R.; Williams, N.; Cusack, R.; Auer, T.; Shafto, M.A.; Dixon, M.; Tyler, L.K.; Cam-CAN; Henson, R.N. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) data repository: Structural and functional MRI, MEG, and cognitive data from a cross-sectional adult lifespan sample. Neuroimage 2017, 144, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Shafto, M.A.; Tyler, L.K.; Dixon, M.; Taylor, J.R.; Rowe, J.B.; Cusack, R.; Calder, A.J.; Marslen-Wilson, W.D.; Duncan, J.; Dalgleish, T.; et al. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) study protocol: A cross-sectional, lifespan, multidisciplinary examination of healthy cognitive ageing. BMC Neurol. 2014, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Price, D.; Tyler, L.K.; Neto Henriques, R.; Campbell, K.L.; Williams, N.; Treder, M.S.; Taylor, J.R.; Brayne, C.; Bullmore, E.T.; Calder, A.C.; et al. Age-related delay in visual and auditory evoked responses is mediated by white- and grey-matter differences. Nat. Commun. 2017, 8, 15671. [Google Scholar] [CrossRef] [PubMed]
- García-Pacios, J.; Garcés, P.; Del Río, D.; Maestú, F. Early detection and late cognitive control of emotional distraction by the prefrontal cortex. Sci. Rep. 2015, 5, 10046. [Google Scholar] [CrossRef] [PubMed]
- Wens, V.; Bourguignon, M.; Goldman, S.; Marty, B.; Op de Beeck, M.; Clumeck, C.; Mary, A.; Peigneux, P.; Van Bogaert, P.; Brookes, M.J. Inter- and intra-subject variability of neuromagnetic resting state networks. Brain Topogr. 2014, 27, 620–634. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, A.; Tewarie, P.; van Dellen, E.; Yu, M.; Carbo, E.W.S.; Douw, L.; Gouw, A.A.; van Straaten, E.C.W.; Stam, C.J. Direction of information flow in large-scale resting-state networks is frequency-dependent. Proc. Natl. Acad. Sci. USA 2016, 113, 3867–3872. [Google Scholar] [CrossRef] [PubMed]
- Henson, R.N.; Mouchlianitis, E.; Friston, K.J. MEG and EEG data fusion: Simultaneous localisation of face-evoked responses. Neuroimage 2009, 47, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Haberkorn, W.; Steinhoff, U.; Burghoff, M.; Kosch, O.; Morguet, A.; Koch, H. Pseudo current density maps of electrophysiological heart, nerve or brain function and their physical basis. Biomagn. Res. Technol. 2006, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Sekihara, K.; Nagarajan, S.S. Adaptive Spatial Filters for Electromagnetic Brain Imaging; Springer: Berlin, Germany, 2008; ISBN 3540793704. [Google Scholar]
- Dalal, S.S.; Zumer, J.M.; Guggisberg, A.G.; Trumpis, M.; Wong, D.D.E.; Sekihara, K.; Nagarajan, S.S. MEG/EEG source reconstruction, statistical evaluation, and visualization with NUTMEG. Comput. Intell. Neurosci. 2011, 2011, 758973. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, A.; Barnes, G.R. Beamformer Analysis of MEG Data. Int. Rev. Neurobiol. 2005, 68, 149–171. [Google Scholar] [CrossRef] [PubMed]
- Brookes, M.J.; Vrba, J.; Robinson, S.E.; Stevenson, C.M.; Peters, A.M.; Barnes, G.R.; Hillebrand, A.; Morris, P.G. Optimising experimental design for MEG beamformer imaging. Neuroimage 2008, 39, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Engemann, D.; Strohmeier, D.; Larson, E.; Gramfort, A. Mind the Noise Covariance When Localizing Brain Sources with M/EEG. In Proceedings of the 2015 International Workshop on Pattern Recognition in NeuroImaging (PRNI 2015), Stanford, CA, USA, 10–12 June 2015; pp. 9–12. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcés, P.; López-Sanz, D.; Maestú, F.; Pereda, E. Choice of Magnetometers and Gradiometers after Signal Space Separation. Sensors 2017, 17, 2926. https://doi.org/10.3390/s17122926
Garcés P, López-Sanz D, Maestú F, Pereda E. Choice of Magnetometers and Gradiometers after Signal Space Separation. Sensors. 2017; 17(12):2926. https://doi.org/10.3390/s17122926
Chicago/Turabian StyleGarcés, Pilar, David López-Sanz, Fernando Maestú, and Ernesto Pereda. 2017. "Choice of Magnetometers and Gradiometers after Signal Space Separation" Sensors 17, no. 12: 2926. https://doi.org/10.3390/s17122926





