A Miniaturized Impedimetric Immunosensor for the Competitive Detection of Adrenocorticotropic Hormone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
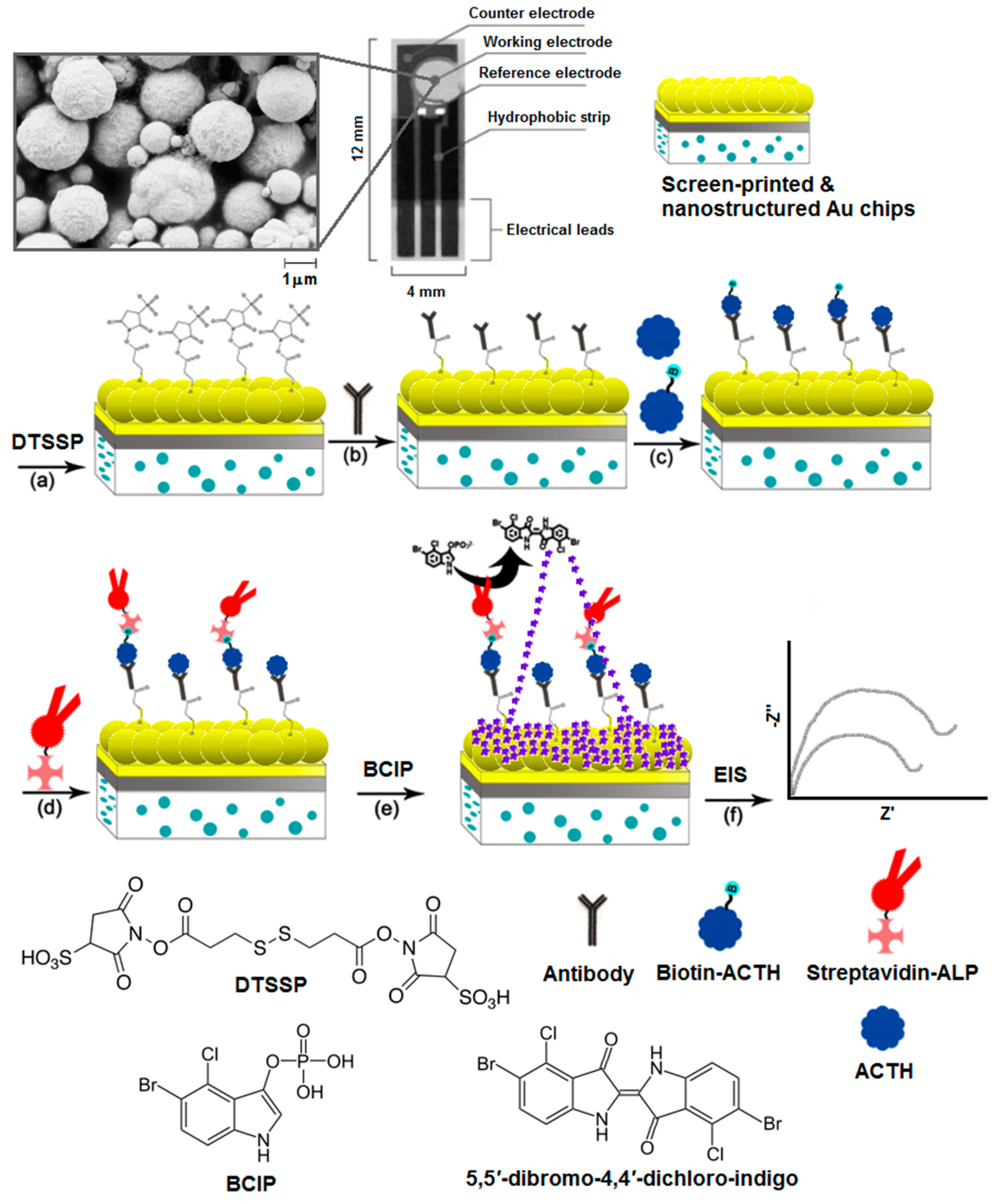
2.2. Surface Modification and Electrochemical Impedance Spectroscopy
2.3. Scanning Electron Microscopy (SEM)
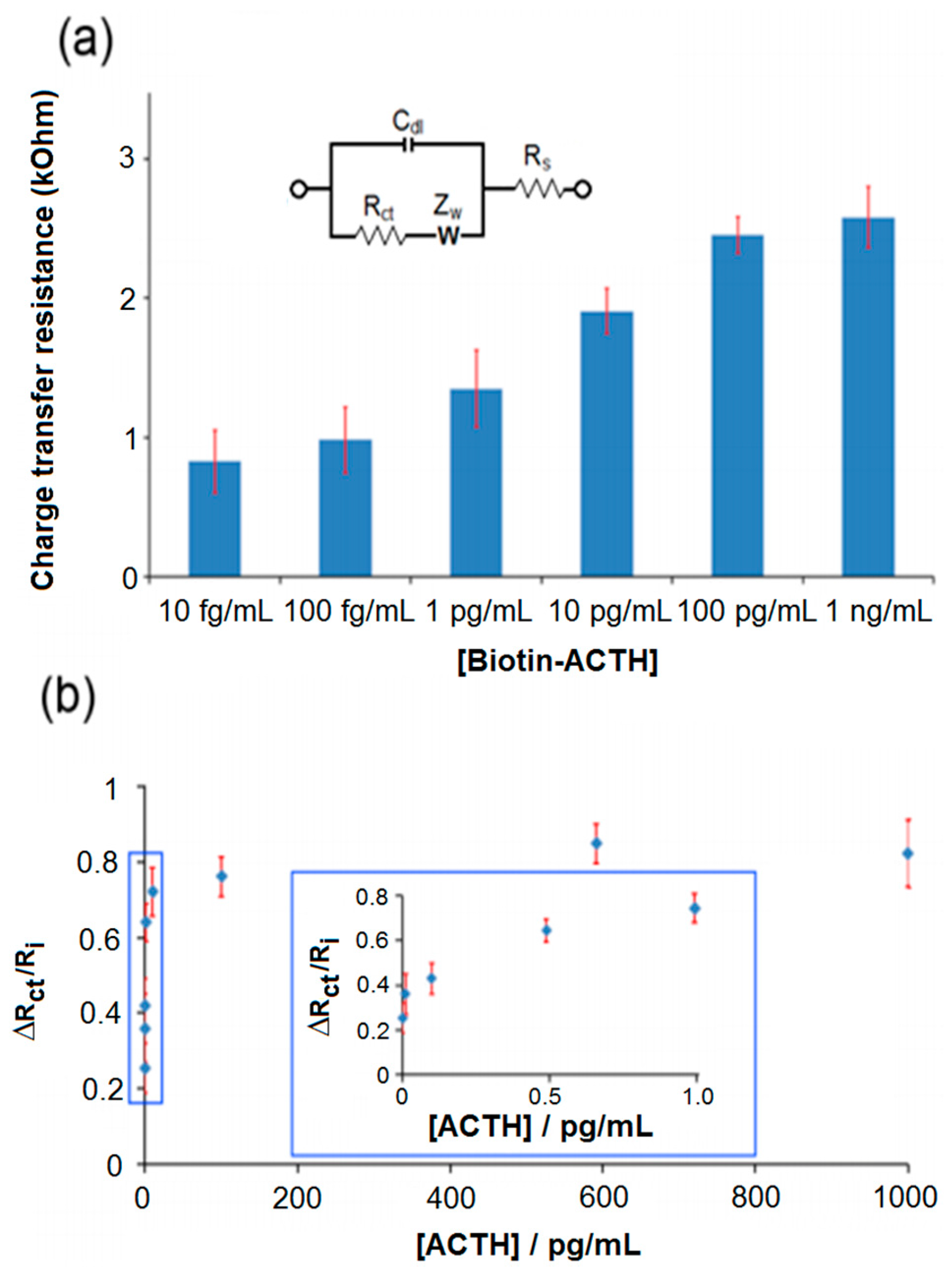
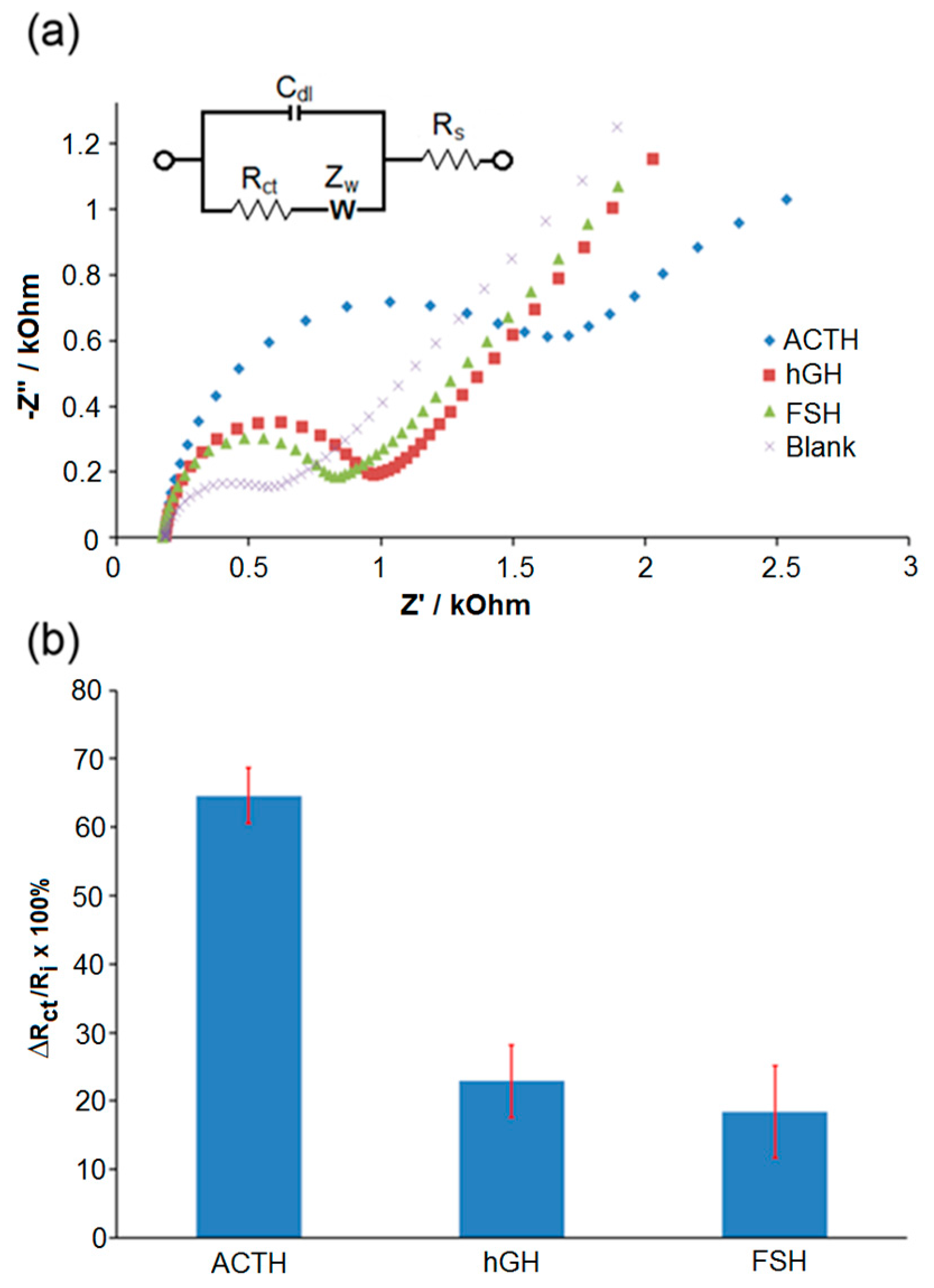
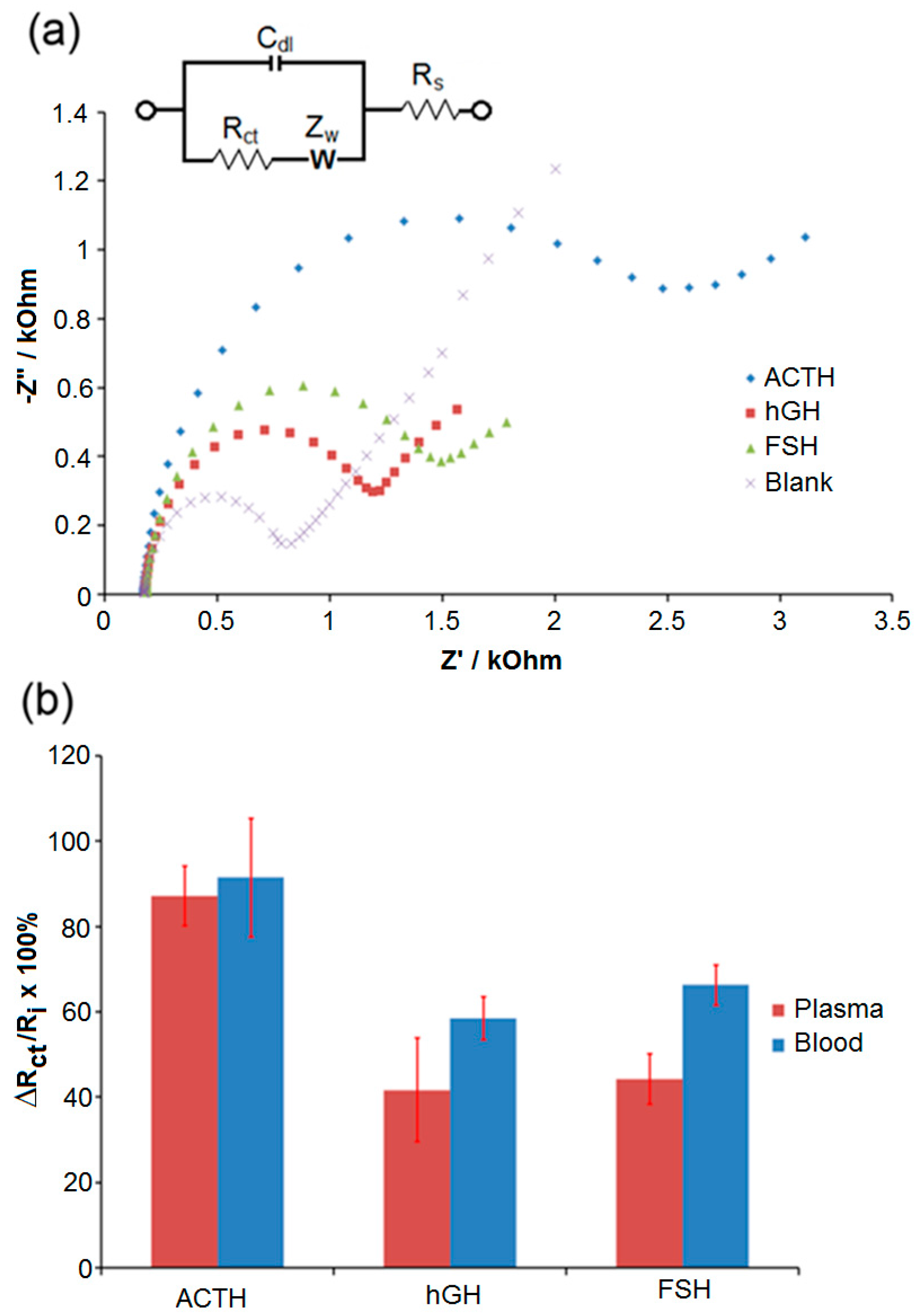
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- White, A.A. ACTH: Cellular peptide hormone synthesis and secretory pathways. Results Probl. Cell Differ. 2010, 50, 63–84. [Google Scholar]
- Duda, T.; Pertzev, A.; Rameshwar, K.S. Ca(2+) modulation of ANF-RGC: New signalling paradigm interlocked with blood pressure regulation. Biochemistry 2012, 51, 9394–9405. [Google Scholar] [PubMed]
- Varadhan, L.; Aror, A.; Walker, A.B.; Varughese, G.I. Cushing’s disease: Establishing the diagnosis and management approach. J. Assoc. Physicians India 2013, 61, 278–280. [Google Scholar] [PubMed]
- Hale, A.C.; Besser, G.M.; Rees, L.H. Characterization of pro-opiomelanocortin-derived peptides in pituitary and ectopic adrenocorticotrophin-secreting tumours. J. Endocrinol. 1986, 108, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, V.K.; Shalet, S.M. Aetiology, diagnosis, and management of hypopituitarism in adult life. Postgrad. Med. J. 2006, 82, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Newell-Prince, J.; Bertagna, X.; Grossman, A.B.; Nieman, L.K. Cushing’s syndrome. Lancet 2006, 367, 1605–1617. [Google Scholar] [CrossRef]
- Gibson, S.; Crosby, S.R.; Stewart, M.F.; Jennings, A.M.; McCall, E.; White, A. Cushing’s syndrome. J. Clin. Endocrinol. Metab. 1993, 78, 835–841. [Google Scholar]
- Rees, L.H.; Bloomfield, G.A.; Gilkes, J.J.; Jeffcoate, W.J.; Besser, G.M. ACTH as a tumor marker. Ann. N. Y. Acad. Sci. 1977, 297, 603–620. [Google Scholar] [CrossRef] [PubMed]
- Maragliano, R.; Vanoli, A.; Albarello, L.; Milione, M.; Basturk, O.; Klimstra, D.S.; Wachtel, A.; Uccella, S.; Vicari, E.; Milesi, M.; et al. ACTH-secreting pancreatic neoplasms associated with Cushing syndrome: Clinicooathologic study of 11 cases and review of the literature. Am. J. Surg. Pathol. 2015, 39, 374–382. [Google Scholar] [PubMed]
- Rees, L.H.; Cook, D.M.; Kendall, J.W.; Allen, C.F.; Kramer, R.M.; Ratcliffe, J.G.; Knight, R.A. A radioimmunoassay for rat plasma ACTH. Endocrinology 1971, 89, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, G.; Bourcier, B.; Cailla, H.; Jean, F. Immunoradiometric assay of succinylated corticotropin: An improved method for quantification of ACTH. Clin. Chem. 1998, 44, 78–85. [Google Scholar] [PubMed]
- Crosby, S.R.; Stewart, M.F.; Ratcliffe, J.G.; White, A. Direct measurement of the precursors of adrenocorticotropin in human plasma by two-site immunoradiometric assay. J. Clin. Endocrinol. Metab. 1988, 67, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Hakanson, R.; Sundler, F. Fluorometric determination of N-terminal tryptophan-peptides after formaldehyde condensation. Biochem. Pharmacol. 1971, 20, 3223–3225. [Google Scholar] [CrossRef]
- Vogeser, M.; Kuhnel, W.; Lambrecht, H.G.; Sitte, J. Fluorometric determination of N-terminal tryptophan-peptides after formaldehyde condensation. Clin. Lab. 1999, 45, 27–45. [Google Scholar]
- Ojeda, I.; Moreno-Guzmán, M.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. A disposable electrochemical immunosensor for the determination of leptin in serum and breast milk. Analyst 2013, 138, 4284–4291. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Brahmendra, A.; Veloso, A.J.; Prashar, A.; Cheng, X.R.; Hung, V.W.S.; Kerman, K. Disposable immunochips for the detection of Legionella pneumophila using electrochemical impedance spectroscopy. Anal. Chem. 2012, 84, 3485–3488. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chow, A.M.; Ganesh, H.V.S.; Brown, I.R.; Kerman, K. Quantum dot based fluorometric detection of cancer TF-antigen. Anal. Chem. 2013, 85, 9699–9704. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; González, V.M.; Rincón, E.; Domingo, A.; Domínguez, E. Aptasensor based on the selective electrodeposition of protein-linked gold nanoparticles on screen-printed electrodes. Analyst 2011, 136, 1810–1815. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Macia, L.; Morrin, A.; Smyth, M.R.; Killard, A.J. Advanced printing and deposition methodologies for the fabrication of biosensors and biodevices. Analyst 2010, 135, 845–867. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, N.; Le Tissier, P.; Grattan, D.R.; Kerman, K. Miniaturized electrochemical immunosensor for label-free detection of growth hormone. Electroanalysis 2012, 24, 1272–1276. [Google Scholar] [CrossRef]
- Sadik, O.A.; Wanekaya, A.K.; Andreescu, S. Advances in analytical technologies for environmental protection and public safety. J. Environ. Monit. 2004, 6, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Pejcic, B.; Marco, R.E.; Parkinson, G. The role of biosensors in the detection of emerging infectious diseases. Analyst 2006, 131, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Dounin, V.; Veloso, A.J.; Schulze, H.; Bachmann, T.T.; Kerman, K. Disposable electrochemical printed gold chips for the analysis of acetylcholinesterase inhibition. Anal. Chim. Acta 2010, 669, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Willner, I. Probing biomolecular interactions at conductive and semiconductive surfaces by impedance spectroscopy: routes to impedimetric immunosensors, DNA-sensors, and enzyme biosensors. Electroanalysis 2003, 15, 913–947. [Google Scholar] [CrossRef]
- Kaatz, M.; Schulze, H.; Ciani, I.; Lisdat, F.; Mount, A.R.; Bachmann, T.T. Alkaline phosphatase enzymatic signal amplification for fast, sensitive impedimetric DNA detection. Analyst 2012, 137, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Witte, C.; Lisdat, F. Alkaline phosphatase enzymatic signal amplification for fast, sensitive impedimetric DNA detection. Electroanalysis 2011, 23, 339–346. [Google Scholar] [CrossRef]
- Lucarelli, F.; Marrazza, G.; Mascini, M. Enzyme-based impedimetric detection of PCR products using oligonucleotide-modified screen-printed gold electrodes. Biosens. Bioelectron. 2005, 20, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- Ravalli, A.; Lozzi, L.; Marrazza, G. Micro-flow immunosensor based on thin-film interdigitated gold array microelectrodes for cancer biomarker detection. Curr. Drug Deliv. 2016, 13, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Z.; Liu, J.Q.; Davis, T.P.; Gooding, J.J. Electrochemical impedance immunosensor based on gold nanoparticles and aryl diazonium salt functionalized gold electrodes for the detection of antibody. Biosens. Bioelectron. 2011, 26, 3660–3665. [Google Scholar] [CrossRef] [PubMed]
- Tolba, M.; Ahmed, M.U.; Tlili, C.; Eichenseher, F.; Loessner, M.J.; Zourob, M. A bacteriophage endolysin-based electrochemical impedance biosensor for the rapid detection of Listeria cells. Analyst 2012, 137, 5749–5756. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Davis, J.J. Electrical biosensors and the label free detection of protein disease biomarkers. Chem. Soc. Rev. 2013, 42, 5944–5962. [Google Scholar] [CrossRef] [PubMed]
- Voccia, D.; Bettazzi, F.; Baydemir, G.; Palchetti, I. Alkaline-phosphatase-based nanostructure assemblies for electrochemical detection of microRNAs. J. Nanosci. Nanotechnol. 2015, 15, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Voccia, D.; Bettazzi, F.; Fratini, E.; Berti, D.; Palchetti, I. Improving impedimetric nucleic acid detection by using enzyme0decorated liposomes and nanostructured screen-printed electrodes. Anal. Bioanal. Chem. 2016, 408, 7271–7281. [Google Scholar] [CrossRef] [PubMed]
- Selvolini, G.; Marrazza, G. MIP-based sensors: Promising new tools for cancer biomarker determination. Sensors 2017, 17, 718. [Google Scholar] [CrossRef] [PubMed]
- Miodek, A.; Regan, E.M.; Bhalla, N.; Hopkins, N.A.; Goodchild, S.A.; Estrela, P. Optimisation and characterisation of anti-fouling ternary SAM layers for impedance-based aptasensors. Sensors 2015, 15, 25015–25032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, J.; O’Kelley, S. Tuning the bacterial detection sensitivity of nanostructured microelectrodes. Anal. Chem. 2013, 85, 7333–7338. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Guzmá, M.; Ojeda, I.; Villalonga, R.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Ultrasensitive detection of adrenocorticotropin hormone (ACTH) using disposable phenylboronic-modified electrochemical immunosensors. Biosens. Bioelectron. 2012, 35, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Talbot, J.A.; Kane, J.W.; White, A. Analytical and clinical aspects of adrenocorticotrophin determination. Ann. Clin. Biochem. 2003, 40, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Gooding, J.J.; Mearns, F.; Yang, W.; Liu, J. Self-assembled monolayers into the 21st century: Recent advances and applications. Electroanalysis 2003, 15, 81–96. [Google Scholar] [CrossRef]
- Steel, A.B.; Levicky, R.I.; Herne, T.M.; Tarlov, M.J. Immobilization of nucleic acids at solid surfaces: Effect of oligonucleotide length on layer assembly. Biophys. J. 2000, 79, 975–981. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Larin, E.M.; Kerman, K. A Miniaturized Impedimetric Immunosensor for the Competitive Detection of Adrenocorticotropic Hormone. Sensors 2017, 17, 2836. https://doi.org/10.3390/s17122836
Li N, Larin EM, Kerman K. A Miniaturized Impedimetric Immunosensor for the Competitive Detection of Adrenocorticotropic Hormone. Sensors. 2017; 17(12):2836. https://doi.org/10.3390/s17122836
Chicago/Turabian StyleLi, Nan, Egor M. Larin, and Kagan Kerman. 2017. "A Miniaturized Impedimetric Immunosensor for the Competitive Detection of Adrenocorticotropic Hormone" Sensors 17, no. 12: 2836. https://doi.org/10.3390/s17122836




