Recent Advances in Fluorescence Lifetime Analytical Microsystems: Contact Optics and CMOS Time-Resolved Electronics
Abstract
:1. Introduction
2. FLM Techniques
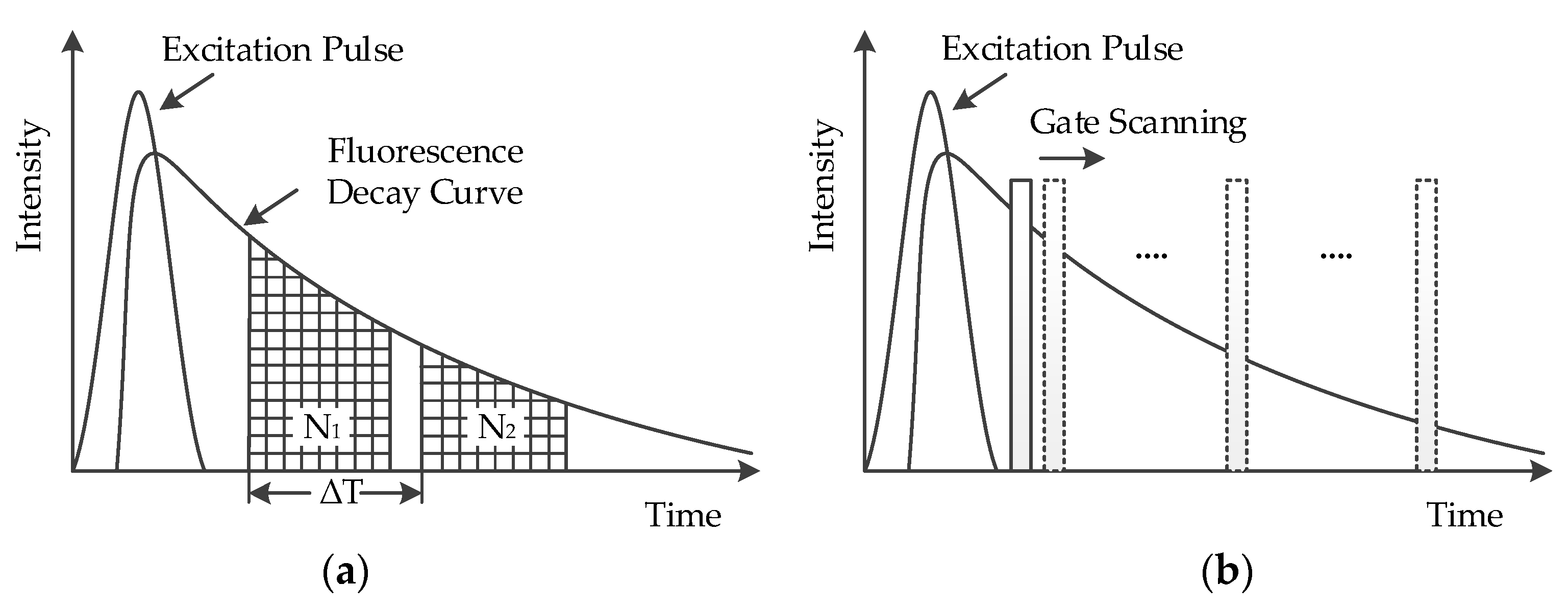
2.1. Time-Gating
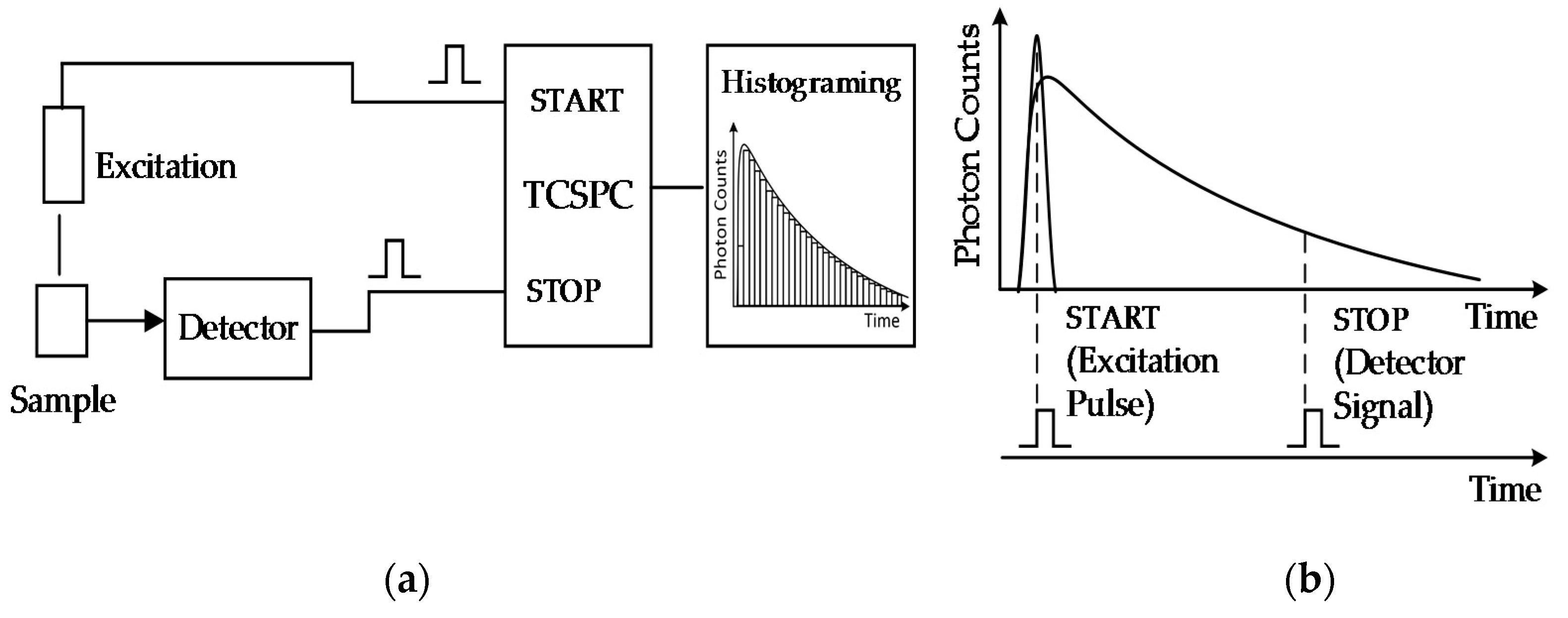
2.2. TCSPC
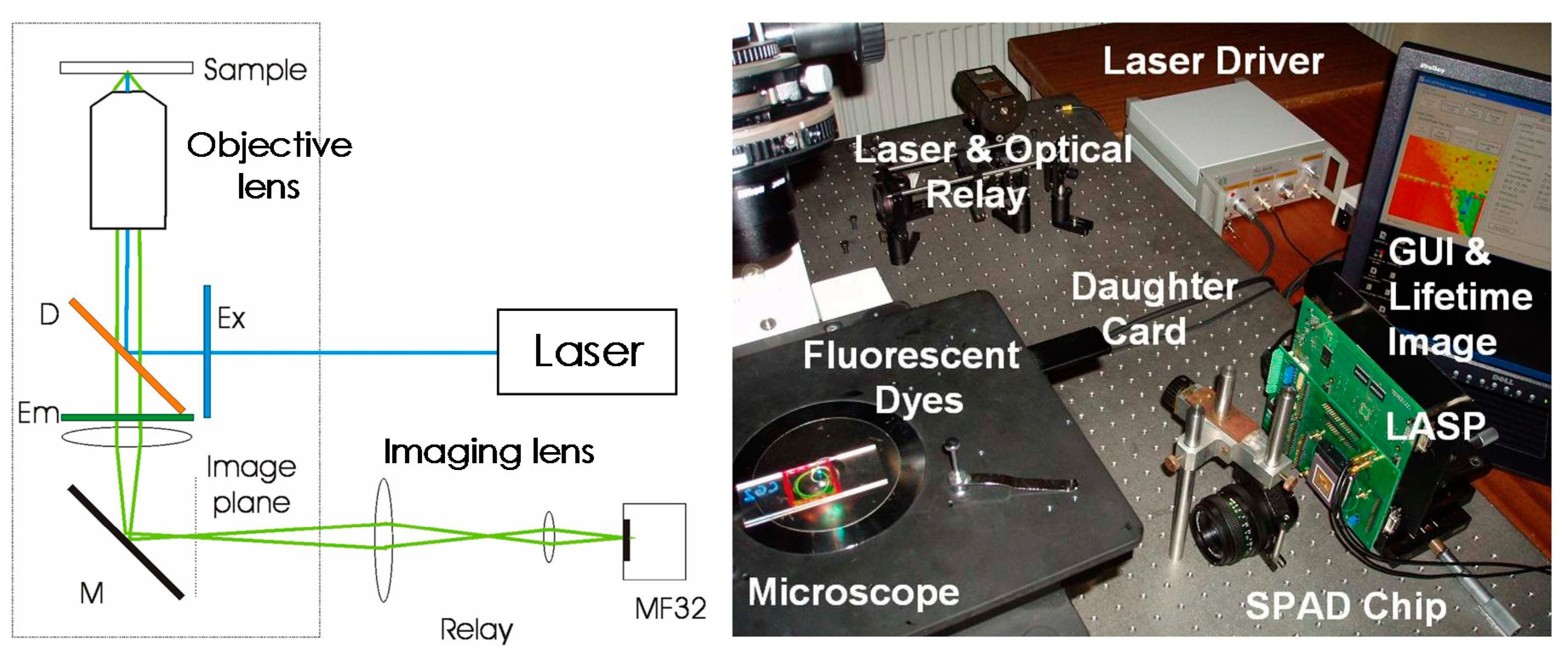
3. CMOS Implementation of FLM
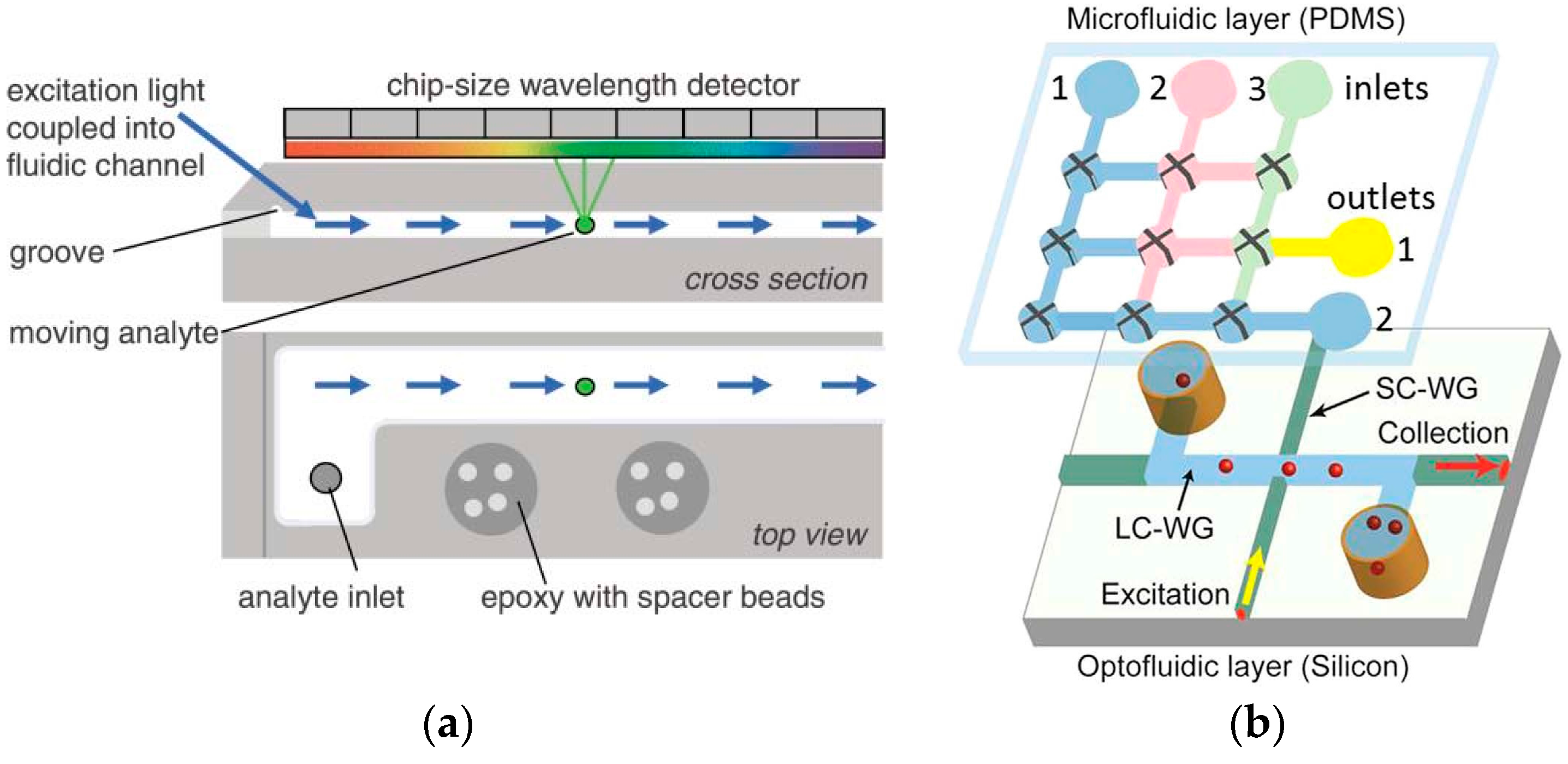
4. Recent Advances in Contact Sensing
4.1. Steady-State Contact Sensing
4.2. Time-Gated Contact Sensing
5. Perspective
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Villa, F.; Lussana, R.; Tamborini, D.; Tosi, A.; Zappa, F. High-Fill-Factor 60 × 1 SPAD Array with 60 Subnanosecond Integrated TDCs. IEEE Photonics Technol. Lett. 2015, 27, 1261–1264. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Isikman, S.O.; Mudanyali, O.; Greenbaum, A.; Ozcan, A. Optical imaging techniques for point-of-care diagnostics. Lab Chip 2013, 13, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Myers, F.B.; Lee, L.P. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip 2008, 8, 2015–2031. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.H.; Cui, Y.Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S.M. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Rowley, M.I.; Coolen, A.C.C.; Vojnovic, B.; Barber, P.R. Robust Bayesian Fluorescence Lifetime Estimation, Decay Model Selection and Instrument Response Determination for Low-Intensity FLIM Imaging. PLoS ONE 2016, 11, e0158404. [Google Scholar] [CrossRef]
- Rocca, F.M.D.; Nedbal, J.; Tyndall, D.; Krstajić, N.; Li, D.D.-U.; Ameer-Beg, S.M.; Henderson, R.K. Real-time fluorescence lifetime actuation for cell sorting using a CMOS SPAD silicon photomultiplier. Opt. Lett. 2016, 41, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Feng, L.; Jiang, Y.; Wong, Y.-T.; He, Y.; Zheng, G.; He, J.; Tan, Y.; Sun, H.; Ho, D. A reaction-based near-infrared fluorescent sensor for Cu2+ detection in aqueous buffer and its application in living cells and tissues imaging. Biosens. Bioelectron. 2017, 94, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Ormo, M.; Cubitt, A.B.; Kallio, K.; Gross, L.A.; Tsien, R.Y.; Remington, S.J. Crystal structure of the Aequorea victoria green fluorescent protein. Sci. Wash. 1996, 273, 1392. [Google Scholar] [CrossRef]
- Crivat, G.; Taraska, J.W. Imaging proteins inside cells with fluorescent tags. Trends Biotechnol. 2012, 30, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer Science & Business Media: New York, NY, USA, 2007; ISBN 0-387-46312-7. [Google Scholar]
- Becker, W. Advanced Time-Correlated Single Photon Counting Techniques; Springer: Berlin, Germany, 2005; Chapter 2; pp. 14–15. [Google Scholar]
- Sytsma, J.; Vroom, J.M.; De Grauw, C.J.; Gerritsen, H.C. Time-gated fluorescence lifetime imaging and microvolume spectroscopy using two-photon excitation. J. Microsc.-Oxf. 1998, 191, 39–51. [Google Scholar] [CrossRef]
- Cominelli, A.; Acconcia, G.; Peronio, P.; Rech, I.; Ghioni, M. Readout Architectures for High Efficiency in Time-Correlated Single Photon Counting Experiments-Analysis and Review. IEEE Photonics J. 2017, 9, 1–15. [Google Scholar] [CrossRef]
- Pancheri, L.; Massari, N.; Stoppa, D. SPAD Image Sensor with Analog Counting Pixel for Time-Resolved Fluorescence Detection. IEEE Trans. Electron Devices 2013, 60, 3442–3449. [Google Scholar] [CrossRef]
- Tyndall, D.; Rae, B.R.; Li, D.D.-U.; Arlt, J.; Johnston, A.; Richardson, J.A.; Henderson, R.K. A High-Throughput Time-Resolved Mini-Silicon Photomultiplier with Embedded Fluorescence Lifetime Estimation in 0.13 µm CMOS. IEEE Trans. Biomed. Circuits Syst. 2012, 6, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Becker, W. The bh TCSPC Handbook, 5th ed.; Becker & Hickl GmbH: Berlin, Germany, 2012. [Google Scholar]
- Horiba DeltaPro Lifetime System. Available online: http://www.horiba.com/fileadmin/uploads/Scientific/Documents/Fluorescence/DeltaPro_Spec_Sheet.pdf (accessed on 1 January 2015).
- PicoQuant FluoTime 300. Available online: https://www.picoquant.com/products/category/fluorescence-spectrometers/fluotime-300-high-performance-fluorescence-lifetime-spectrometer (accessed on 1 January 2015).
- Sunaga, Y.; Haruta, M.; Yamaguchi, T.; Motoyama, M.; Ohta, Y.; Takehara, H.; Noda, T.; Sasagawa, K.; Tokuda, T.; Ohta, J. An implantable green fluorescence imaging device using absorption filters with high excitation light rejection ratio. In Proceedings of the 2014 IEEE Biomedical Circuits and Systems Conference (BioCAS), Lausanne, Switzerland, 22–24 October 2014; pp. 248–251. [Google Scholar]
- Patounakis, G.; Shepard, K.L.; Levicky, R. Active CMOS array sensor for time-resolved fluorescence detection. IEEE J. Solid-State Circuits 2006, 41, 2521–2530. [Google Scholar] [CrossRef]
- Moon, S.; Keles, H.O.; Ozcan, A.; Khademhosseini, A.; Haeggstrom, E.; Kuritzkes, D.; Demirci, U. Integrating microfluidics and lensless imaging for point-of-care testing. Biosens. Bioelectron. 2009, 24, 3208–3214. [Google Scholar] [CrossRef] [PubMed]
- Gurkan, U.A.; Moon, S.; Geckil, H.; Xu, F.; Wang, S.; Lu, T.J.; Demirci, U. Miniaturized lensless imaging systems for cell and microorganism visualization in point-of-care testing. Biotechnol. J. 2011, 6, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Coskun, A.F.; Su, T.-W.; Ozcan, A. Wide field-of-view lens-free fluorescent imaging on a chip. Lab Chip 2010, 10, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Scully, A.D.; Ostler, R.B.; Phillips, D.; O’Neill, P.; Townsend, K.M.S.; Parker, A.W.; MacRobert, A.J. Application of fluorescence lifetime imaging microscopy to the investigation of intracellular PDT mechanisms. Bioimaging 1997, 5, 9–18. [Google Scholar] [CrossRef]
- Ballew, R.M.; Demas, J.N. An error analysis of the rapid lifetime determination method for the evaluation of single exponential decays. Anal. Chem. 1989, 61, 30–33. [Google Scholar] [CrossRef]
- Gerritsen, H.; Asselbergs, M.; Agronskaia, A.; Van Sark, W. Fluorescence lifetime imaging in scanning microscopes: Acquisition speed, photon economy and lifetime resolution. J. Microsc. 2002, 206, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Tamborini, D.; Portaluppi, D.; Villa, F.; Zappa, F. Eight-Channel 21 ps Precision ps Precision 10us Range Time-to-Digital Converter Module. IEEE Trans. Instrum. Meas. 2016, 65, 423–430. [Google Scholar] [CrossRef]
- Liping, W.; Derek, H. Design of CMOS Microsystems for Time-Correlated Single-Photon Counting Fluorescence Lifetime Analysis. In Advances in Imaging and Sensing; Taylor & Francis Group: Abingdon, UK, 2016; pp. 169–189. [Google Scholar]
- Cantoral-Ceballos, J.A.; Ozanyan, K.B. Parallel-data reconstruction for limited views tomography sensors by sinusoidal hough transform. IEEE Sens. J. 2013, 13, 581–588. [Google Scholar] [CrossRef]
- Kima, K.; Lee, Y.-W. Creation of the BMA ensemble for SST using a parallel processing technique. In Proceedings of the High-Performance Computing in Remote Sensing III, Dresden, Germany, 25–26 October 2013; Volume 8895. [Google Scholar] [CrossRef]
- Kursun, V.; Friedman, E.G. Sleep switch dual threshold voltage domino logic with reduced standby leakage current. IEEE Trans. Very Large Scale Integr. Syst. 2004, 12, 485–496. [Google Scholar] [CrossRef]
- Xiao, J.; Peterchev, A.; Zhang, J.; Sanders, S. An ultra-low-power digitally-controlled buck converter IC for cellular phone applications. In Proceedings of the Nineteenth Annual IEEE Applied Power Electronics Conference and Exposition, Anaheim, CA, USA, 22–26 February 2004; Volume 1, pp. 383–391. [Google Scholar]
- Rae, B.R.; Yang, J.; McKendry, J.; Gong, Z.; Renshaw, D.; Girkin, J.M.; Gu, E.; Dawson, M.D.; Henderson, R.K. A Vertically Integrated CMOS Microsystem for Time-Resolved Fluorescence Analysis. IEEE Trans. Biomed. Circuits Syst. 2010, 4, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Rae, B.R.; Muir, K.R.; Gong, Z.; McKendry, J.; Girkin, J.M.; Gu, E.; Renshaw, D.; Dawson, M.D.; Henderson, R.K. A CMOS Time-Resolved Fluorescence Lifetime Analysis Micro-System. Sensors 2009, 9, 9255–9274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, J.; Walker, R.; Grant, L.; Stoppa, D.; Borghetti, F.; Charbon, E.; Gersbach, M.; Henderson, R.K. A 32 × 32 50ps Resolution 10 bit Time to Digital Converter Array in 130 nm CMOS for Time Correlated Imaging. In Proceedings of the IEEE 2009 Custom Integrated Circuits Conference, Rome, Italy, 13–16 September 2009; ISBN 978-1-4244-4072-6. [Google Scholar]
- Li, D.D.U.; Arlt, J.; Tyndall, D.; Walker, R.; Richardson, J.; Stoppa, D.; Charbon, E.; Henderson, R.K. Video-rate fluorescence lifetime imaging camera with CMOS single-photon avalanche diode arrays and high-speed imaging algorithm. J. Biomed. Opt. 2011, 16. [Google Scholar] [CrossRef] [PubMed]
- Mandai, S.; Charbon, E. A 128-Channel, 8.9-ps LSB, Column-Parallel Two-Stage TDC Based on Time Difference Amplification for Time-Resolved Imaging. IEEE Trans. Nucl. Sci. 2012, 59, 2463–2470. [Google Scholar] [CrossRef]
- Markovic, B.; Tisa, S.; Villa, F.A.; Tosi, A.; Zappa, F. A High-Linearity, 17 ps Precision Time-to-Digital Converter Based on a Single-Stage Vernier Delay Loop Fine Interpolation. IEEE Trans. Circuits Syst. I Regul. Pap. 2013, 60, 557–569. [Google Scholar] [CrossRef]
- Burri, S.; Maruyama, Y.; Michalet, X.; Regazzoni, F.; Bruschini, C.; Charbon, E. Architecture and applications of a high resolution gated SPAD image sensor. Opt. Express 2014, 22, 17573–17589. [Google Scholar] [CrossRef] [PubMed]
- Bronzi, D.; Villa, F.; Bellisai, S.; Markovic, B.; Tisa, S.; Tosi, A.; Zappa, F.; Weyers, S.; Durini, D.; Brockherde, W.; Paschen, U. Low-noise and large-area CMOS SPADs with timing response free from slow tails. In Proceedings of the European Solid-State Device Research Conference (ESSDERC), Bordeaux, France, 17–21 September 2012; pp. 230–233. [Google Scholar]
- Bronzi, D.; Villa, F.; Tisa, S.; Tosi, A.; Zappa, F.; Durini, D.; Weyers, S.; Brockherde, W. 100,000 Frames/s 64 × 32 Single-Photon Detector Array for 2-D Imaging and 3-D Ranging. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 354–363. [Google Scholar] [CrossRef]
- Villa, F.; Lussana, R.; Bronzi, D.; Tisa, S.; Tosi, A.; Zappa, F.; Mora, A.D.; Contini, D.; Durini, D.; Weyers, S.; et al. CMOS Imager with 1024 SPADs and TDCs for Single-Photon Timing and 3-D Time-of-Flight. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 364–373. [Google Scholar] [CrossRef]
- Field, R.M.; Realov, S.; Shepard, K.L. A 100 fps, Time-Correlated Single-Photon-Counting-Based Fluorescence-Lifetime Imager in 130 nm CMOS. IEEE J. Solid-State Circuits 2014, 49, 867–880. [Google Scholar] [CrossRef]
- Schwartz, D.E.; Charbon, E.; Shepard, K.L. A Single-Photon Avalanche Diode Array for Fluorescence Lifetime Imaging Microscopy. IEEE J. Solid-State Circuits 2008, 43, 2546–2557. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, Y.; Blacksberg, J.; Charbon, E. A 1024 × 8, 700-ps Time-Gated SPAD Line Sensor for Planetary Surface Exploration with Laser Raman Spectroscopy and LIBS. IEEE J. Solid-State Circuits 2014, 40, 179–189. [Google Scholar] [CrossRef]
- Krstajić, N.; Levitt, J.; Poland, S.; Ameer-Beg, S.; Henderson, R. 256 × 2 SPAD line sensor for time resolved fluorescence spectroscopy. Opt. Express 2015, 23, 5653–5669. [Google Scholar] [CrossRef] [PubMed]
- Pavia, J.M.; Scandini, M.; Lindner, S.; Wolf, M.; Charbon, E. A 1 × 400 Backside-Illuminated SPAD Sensor with 49.7 ps Resolution, 30 pJ/Sample TDCs Fabricated in 3D CMOS Technology for Near-Infrared Optical Tomography. IEEE J. Solid-State Circuits 2015, 50, 2406–2418. [Google Scholar] [CrossRef]
- Yoon, H.J.; Itoh, S.; Kawahito, S. A CMOS Image Sensor with In-Pixel Two-Stage Charge Transfer for Fluorescence Lifetime Imaging. IEEE Trans. Electron Devices 2009, 56, 214–221. [Google Scholar] [CrossRef]
- Li, Z.; Kawahito, S.; Yasutomi, K.; Kagawa, K.; Ukon, J.; Hashimoto, M.; Niioka, H. A Time-Resolved CMOS Image Sensor with Draining-Only Modulation Pixels for Fluorescence Lifetime Imaging. IEEE Trans. Electron Devices 2012, 59, 2715–2722. [Google Scholar] [CrossRef]
- Seo, M.W.; Kagawa, K.; Yasutomi, K.; Kawata, Y.; Teranishi, N.; Li, Z.; Halin, I.A.; Kawahito, S. A 10 ps Time-Resolution CMOS Image Sensor with Two-Tap True-CDS Lock-In Pixels for Fluorescence Lifetime Imaging. IEEE J. Solid-State Circuits 2016, 51, 141–154. [Google Scholar] [CrossRef]
- Palubiak, D.P.; Deen, M.J. CMOS SPADs: Design Issues and Research Challenges for Detectors, Circuits, and Arrays. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 409–426. [Google Scholar] [CrossRef]
- Tosi, A.; Zappa, F. MiSPIA: Microelectronic Single-Photon 3D Imaging Arrays for low-light high-speed Safety and Security Applications. Proc. SPIE 2013, 87270L. [Google Scholar] [CrossRef]
- Gersbach, M.; Maruyama, Y.; Trimananda, R.; Fishburn, M.W.; Stoppa, D.; Richardson, J.A.; Walker, R.; Henderson, R.; Charbon, E. A Time-Resolved, Low-Noise Single-Photon Image Sensor Fabricated in Deep-Submicron CMOS Technology. IEEE J. Solid-State Circuits 2012, 47, 1394–1407. [Google Scholar] [CrossRef]
- Giraud, G.; Schulze, H.; Li, D.-U.; Bachmann, T.T.; Crain, J.; Tyndall, D.; Richardson, J.; Walker, R.; Stoppa, D.; Charbon, E. Fluorescence lifetime biosensing with DNA microarrays and a CMOS-SPAD imager. Biomed. Opt. Express 2010, 1, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Perenzoni, M.; Massari, N.; Perenzoni, D.; Gasparini, L.; Stoppa, D. A 160 × 120 Pixel Analog-Counting Single-Photon Imager with Time-Gating and Self-Referenced Column-Parallel A/D Conversion for Fluorescence Lifetime Imaging. IEEE J. Solid-State Circuits 2016, 51, 155–167. [Google Scholar] [CrossRef]
- Li, D.-U.; Arlt, J.; Richardson, J.; Walker, R.; Buts, A.; Stoppa, D.; Charbon, E.; Henderson, R. Real-time fluorescence lifetime imaging system with a 32 × 32 0.13 μm CMOS low dark-count single-photon avalanche diode array. Opt. Express 2010, 18, 10257–10269. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Sander, D.; Haas, A.; Abshire, P.A. Contact Imaging: Simulation and Experiment. IEEE Trans. Circuits Syst. I Regul. Pap. 2007, 54, 1698–1710. [Google Scholar] [CrossRef]
- Adams, M.L.; Enzelberger, M.; Quake, S.; Scherer, A. Microfluidic integration on detector arrays for absorption and fluorescence micro-spectrometers. Sens. Actuators A Phys. 2003, 104, 25–31. [Google Scholar] [CrossRef]
- Li, W.; Knoll, T.; Sossalla, A.; Bueth, H.; Thielecke, H. On-chip integrated lensless fluorescence microscopy/spectroscopy module for cell-based sensors. Proc. SPIE 2011, 7894. [Google Scholar] [CrossRef]
- Nelson, N.; Sander, D.; Dandin, M.; Prakash, S.B.; Sarje, A.; Abshire, P. Handheld Fluorometers for Lab-on-a-Chip Applications. IEEE Trans. Biomed. Circuits Syst. 2009, 3, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Campopiano, S.; Zeni, L. Silicon micromachined hollow optical waveguides for sensing applications. IEEE J. Sel. Top. Quantum Electron. 2002, 8, 106–110. [Google Scholar] [CrossRef]
- Schmidt, O.; Bassler, M.; Kiesel, P.; Knollenberg, C.; Johnson, N. Fluorescence spectrometer-on-a-fluidic-chip. Lab Chip 2007, 7, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Parks, J.W.; Wall, T.A.; Stott, M.A.; Stambaugh, A.; Alfson, K.; Griffiths, A.; Mathies, R.A.; Carrion, R.; Patterson, J.L.; et al. Optofluidic analysis system for amplification-free, direct detection of Ebola infection. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.R.; Ho, D.; Nilchi, A.; Gulak, G.; Yau, P.; Genov, R. A CMOS/Thin-Film Fluorescence Contact Imaging Microsystem for DNA Analysis. IEEE Trans. Circuits Syst. I Regul. Pap. 2010, 57, 1029–1038. [Google Scholar] [CrossRef]
- Maruyama, Y.; Sawada, K.; Takao, H.; Ishida, M. A novel filterless fluorescence detection sensor for DNA analysis. IEEE Trans. Electron Devices 2006, 53, 553–558. [Google Scholar] [CrossRef]
- Ho, D.; Noor, M.O.; Krull, U.J.; Gulak, G.; Genov, R. CMOS spectrally-multiplexed Fret-on-a-Chip for DNA analysis. IEEE Trans. Biomed. Circuits Syst. 2013, 7, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Crowley, B.; Gaudet, V. LED die-on-chip integration for fluorescence-detection applications. Electron. Lett. 2013, 49, 1553–1555. [Google Scholar] [CrossRef]
- Stoppa, D.; Mosconi, D.; Pancheri, L.; Gonzo, L. Single-photon avalanche diode CMOS sensor for time-resolved fluorescence measurements. IEEE Sens. J. 2009, 9, 1084–1090. [Google Scholar] [CrossRef]
- Roulet, J.-C.; Volkel, R.; Herzig, H.-P.; Verpoorte, E.; de Rooij, N.F.; Dandliker, R. Fabrication of multilayer systems combining microfluidic and microoptical elements for fluorescence detection. J. Microelectromech. Syst. 2001, 10, 482–491. [Google Scholar] [CrossRef]
- Maruyama, Y.; Charbon, E. An all-digital, time-gated 128 × 128 spad array for on-chip, filter-less fluorescence detection. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference (TRANSDUCERS), Beijing, China, 5–9 June 2011; pp. 1180–1183. [Google Scholar]
- Lee, C.; Johnson, B.; Molnar, A. An On-chip 72 × 60 Angle-Sensitive Single Photon Image Sensor Array for Lens-less Time-resolved 3-D Fluorescence Lifetime Imaging. In Proceedings of the 2014 Symposium on VLSI Circuits Digest of Technical Papers, Honolulu, HI, USA, 10–13 June 2014; pp. 1–2. [Google Scholar]
- Sivaramakrishnan, S.; Wang, A.; Gill, P.; Molnar, A. Design and Characterization of Enhanced Angle Sensitive Pixels. IEEE Trans. Electron Devices 2016, 63, 113–119. [Google Scholar] [CrossRef]
- Norian, H.; Field, R.M.; Kymissis, I.; Shepard, K.L. An integrated CMOS quantitative-polymerase-chain-reaction lab-on-chip for point-of-care diagnostics. Lab Chip 2014, 14, 4076–4084. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Sorgenfrei, S.; Gong, P.; Levicky, R.; Shepard, K.L. A 0.18-µm CMOS Array Sensor for Integrated Time-Resolved Fluorescence Detection. IEEE J. Solid-State Circuits 2009, 44, 1644–1654. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.C.; Paul, S.; Gong, P.; Levicky, R.; Kymissis, J.; Amundson, S.A.; Shepard, K.L. Gene expression analysis with an integrated CMOS microarray by time-resolved fluorescence detection. Biosens. Bioelectron. 2011, 26, 2660–2665. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.E.; Gong, P.; Shepard, K.L. Time-resolved Forster-resonance-energy-transfer DNA assay on an active CMOS microarray. Biosens. Bioelectron. 2008, 24, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.-H.; Yoo, J.-C. Miniaturized Fluorometer Based on Total Internal Reflector and Condensing Mirror. J. Opt. Soc. Korea 2013, 17, 81–85. [Google Scholar] [CrossRef]
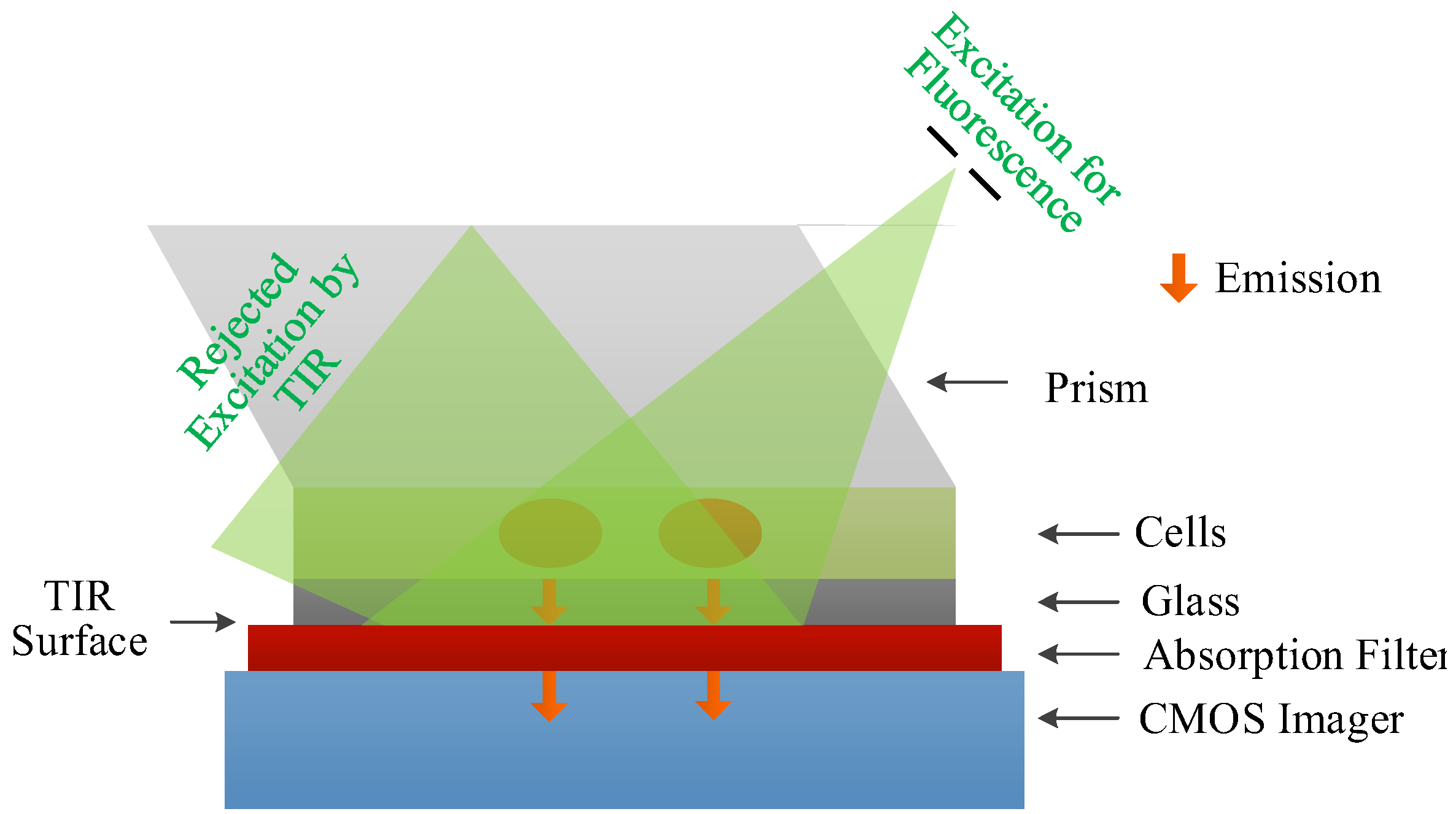
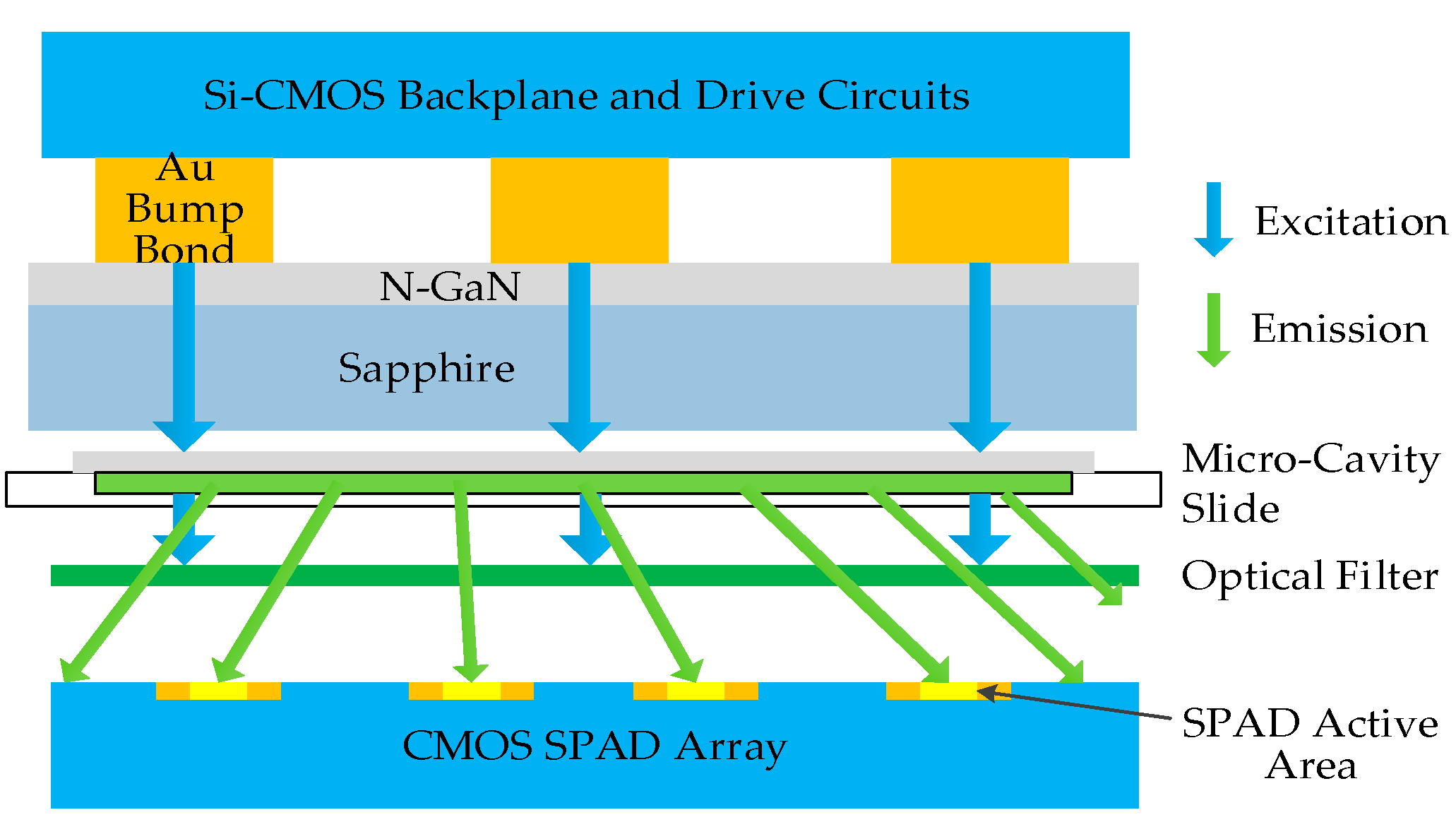
| Detector Type | Pixel Area 1 | Array Size | Total Area 2 | Time Resolution 4 | Power Consumption | Integration Level | Technique | Refs |
|---|---|---|---|---|---|---|---|---|
| SPAD | 200 × 200 | 16 × 4 | 3.18 × 3.1 | 80 ps 4a | Nil | Pixel-level | TG | [34,35] |
| SPAD | 50 × 50 | 32 × 32 | Nil | 50 ps | Nil | Pixel-level | TCSPC | [36] |
| SPAD | Nil | 32 × 32 | Nil | 54 ps | Nil | Pixel-level | TCSPC | [37] |
| SPAD | Φ 6 μm 3 | 128 × 1 | 3.12 × 1.1 | 21.4–8.9 ps | Nil | Pixel-level | TCSPC | [38] |
| SiPM | Φ 8 μm | 32 × 32 | 1. 3 × 1.7 | 50 ps | 9.5 mW | Chip-level | TCSPC | [16] |
| SPAD | 250 × 250 | 1 × 1 | 0.3 | 10 ps | <80 mW | Pixel-level | TCSPC | [39] |
| SPAD | 25 × 25 | 32 × 32 | Nil | ~ns 4b | Nil | Pixel-level | TG | [15] |
| SPAD | 24 × 24 | 512 × 128 | 13.5 × 3.5 | 4 ns 4b | 1.65 W | Pixel-level | TG | [40] |
| SPAD | Φ 30 μm | 64 × 32 | 9.6 × 4.8 | 120 ps 4b | 50 mW | Pixel-level | TG | [41,42] |
| SPAD | Φ 30 μm | 32 × 32 | 9 × 9 | 312 ps | 430 mW | Pixel-level | TCSPC | [43] |
| SPAD | 48 × 48 | 64 × 64 | Nil | 62.5 ps | 8.79 W | Pixel-level | TCSPC | [44] |
| SPAD | 40 × 40 | 64 × 64 | 4 × 4 | 350ps | 1.4 W | Chip-level | TCSPC and TG | [45] |
| SPAD | Nil | 1024 × 8 | 24.7 × 0. 8 | 250 ps | Nil | - | TG | [46] |
| SPAD | 23.8 × 100 | 256 × 2 | 6.61 × 0.958 | 320 ps | Nil | Pixel-level | TCSPC | [47] |
| SPAD | 64 × 47 | 1 × 400 | 0.77 × 5 | 49.7 ps | 7 mW | Column-level | TCSPC | [48] |
| SPAD | Φ 100 μm | 60 × 1 | 9.3 × 2 | 250 ps | Nil | Pixel-level | TCSPC | [1] |
| Lock-in pixel | 7.5 × 7.5 | 256 × 256 | Nil | 250 ps 4b | Nil | Pixel-level | TG | [49] |
| Lock-in pixel (DOM) | 7.5 × 7.5 | 256 × 256 | Nil | ~ns | Nil | Pixel-level | TG | [50] |
| Lock-in pixel (LEFM) | 11.2 × 5.6 | 256 × 512 | 7 × 9.3 | 10 ps 4c | 540 mW | Pixel-level | TG | [51] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, L.; Yan, W.; Ho, D. Recent Advances in Fluorescence Lifetime Analytical Microsystems: Contact Optics and CMOS Time-Resolved Electronics. Sensors 2017, 17, 2800. https://doi.org/10.3390/s17122800
Wei L, Yan W, Ho D. Recent Advances in Fluorescence Lifetime Analytical Microsystems: Contact Optics and CMOS Time-Resolved Electronics. Sensors. 2017; 17(12):2800. https://doi.org/10.3390/s17122800
Chicago/Turabian StyleWei, Liping, Wenrong Yan, and Derek Ho. 2017. "Recent Advances in Fluorescence Lifetime Analytical Microsystems: Contact Optics and CMOS Time-Resolved Electronics" Sensors 17, no. 12: 2800. https://doi.org/10.3390/s17122800





