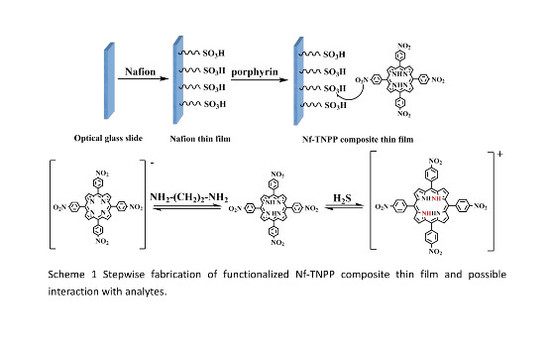
A Functionalized Tetrakis(4-Nitrophenyl)Porphyrin Film Optical Waveguide Sensor for Detection of H2S and Ethanediamine Gases
Abstract
:1. Introduction
2. Methods
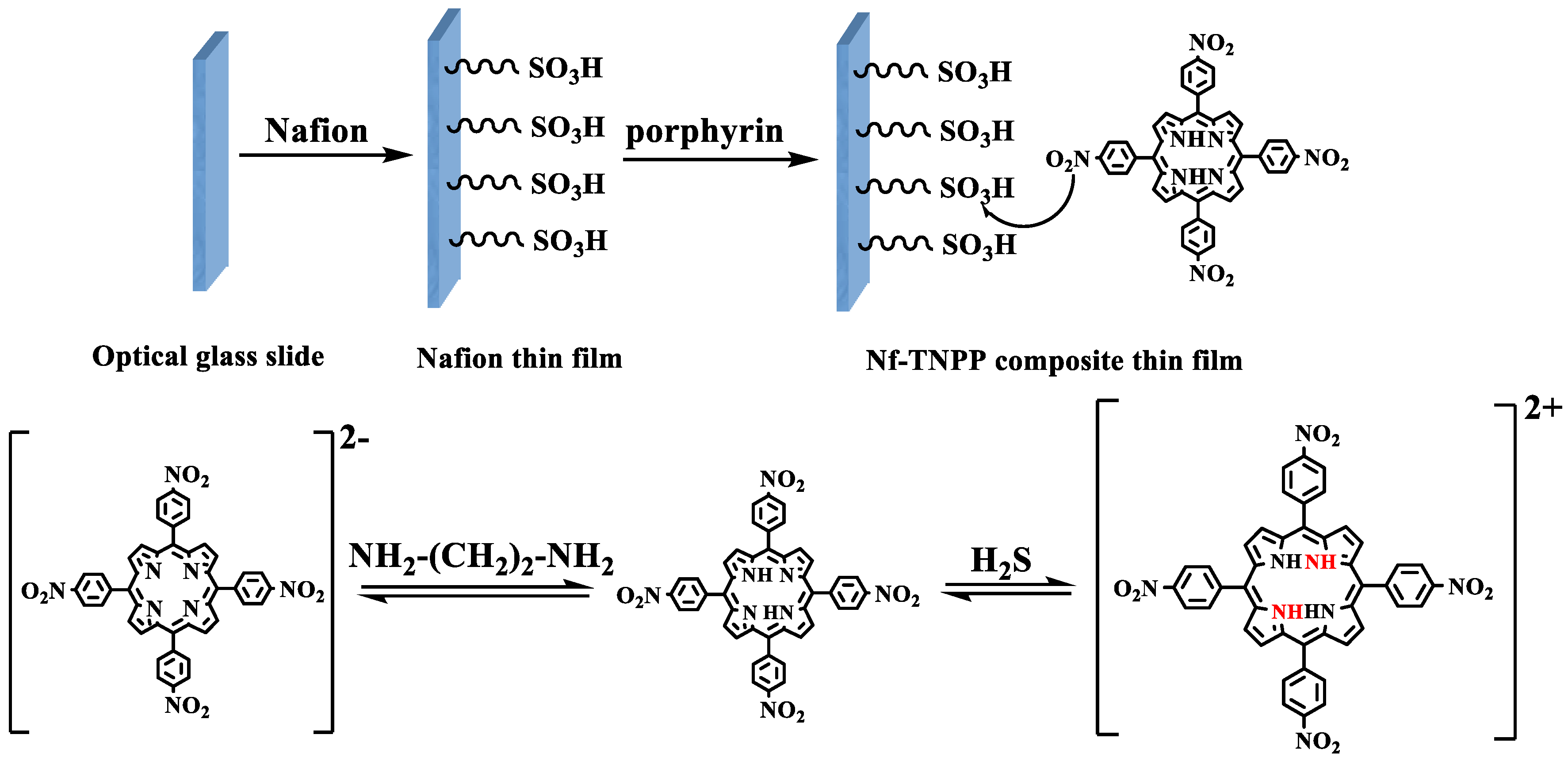
2.1. Synthesis and Characterization of Meso-tetrakis(4-Nitrophenyl) Porphyrin (TNPP)
2.2. Preparation of Nafion Solution
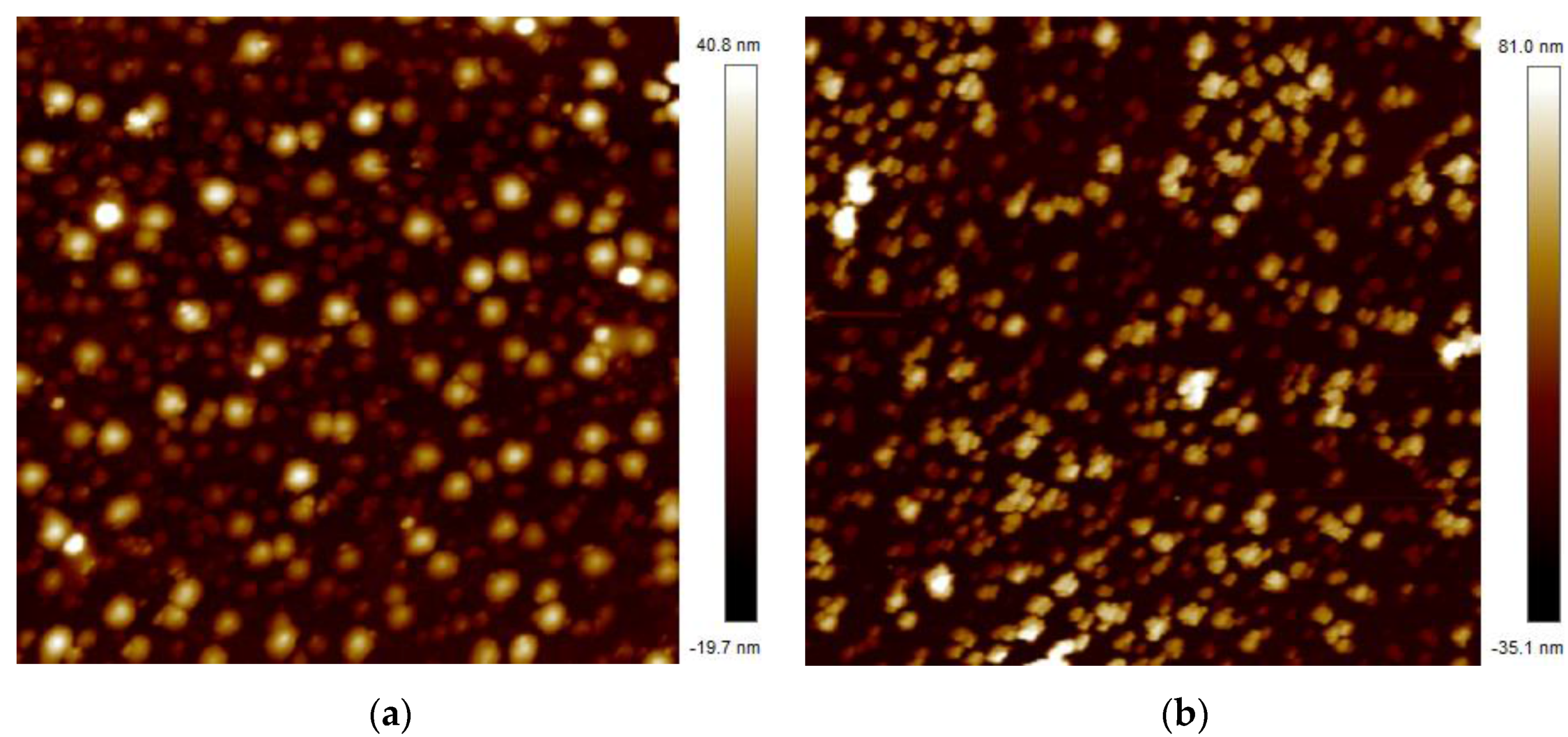
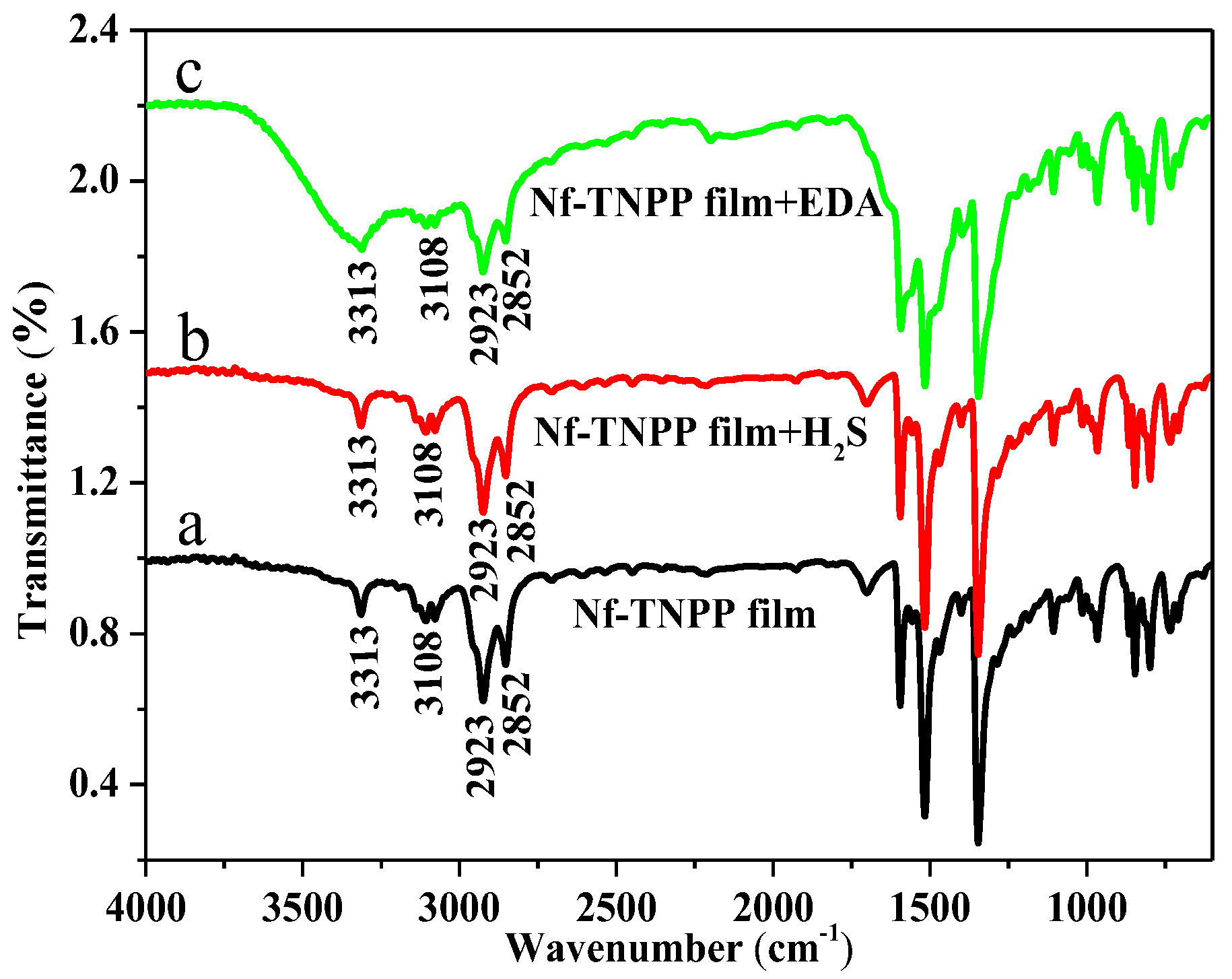
2.3. Preparation and Analysis of Sensing Element
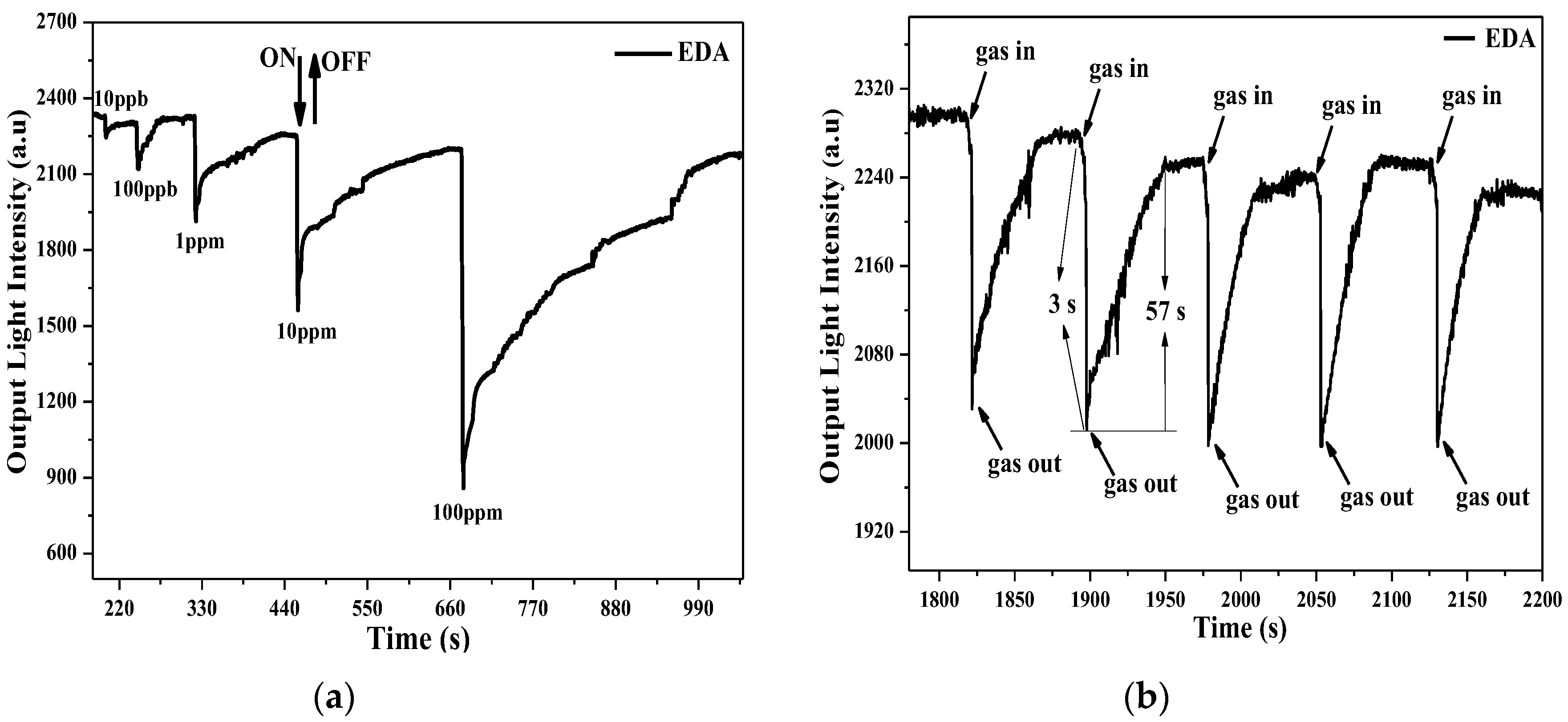
3. Sensing Apparatus and Procedure
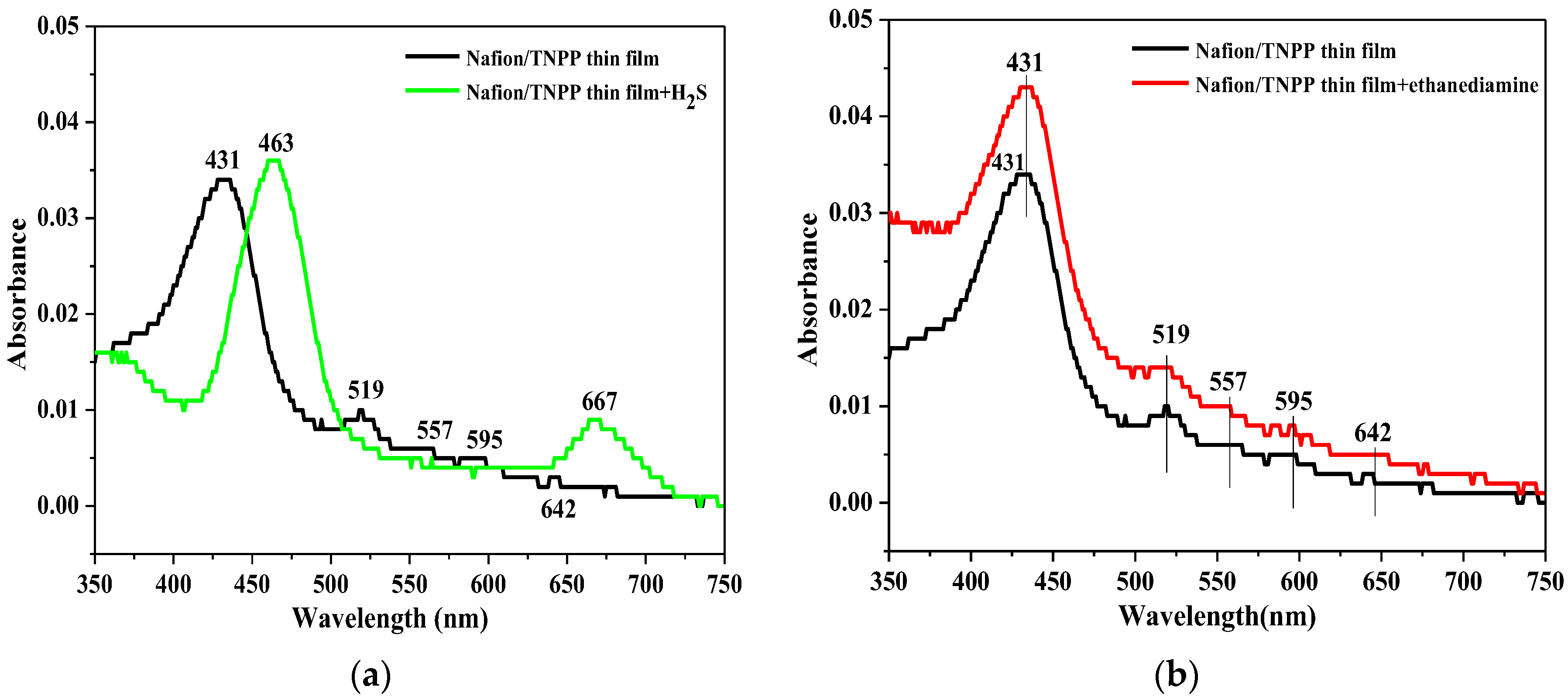
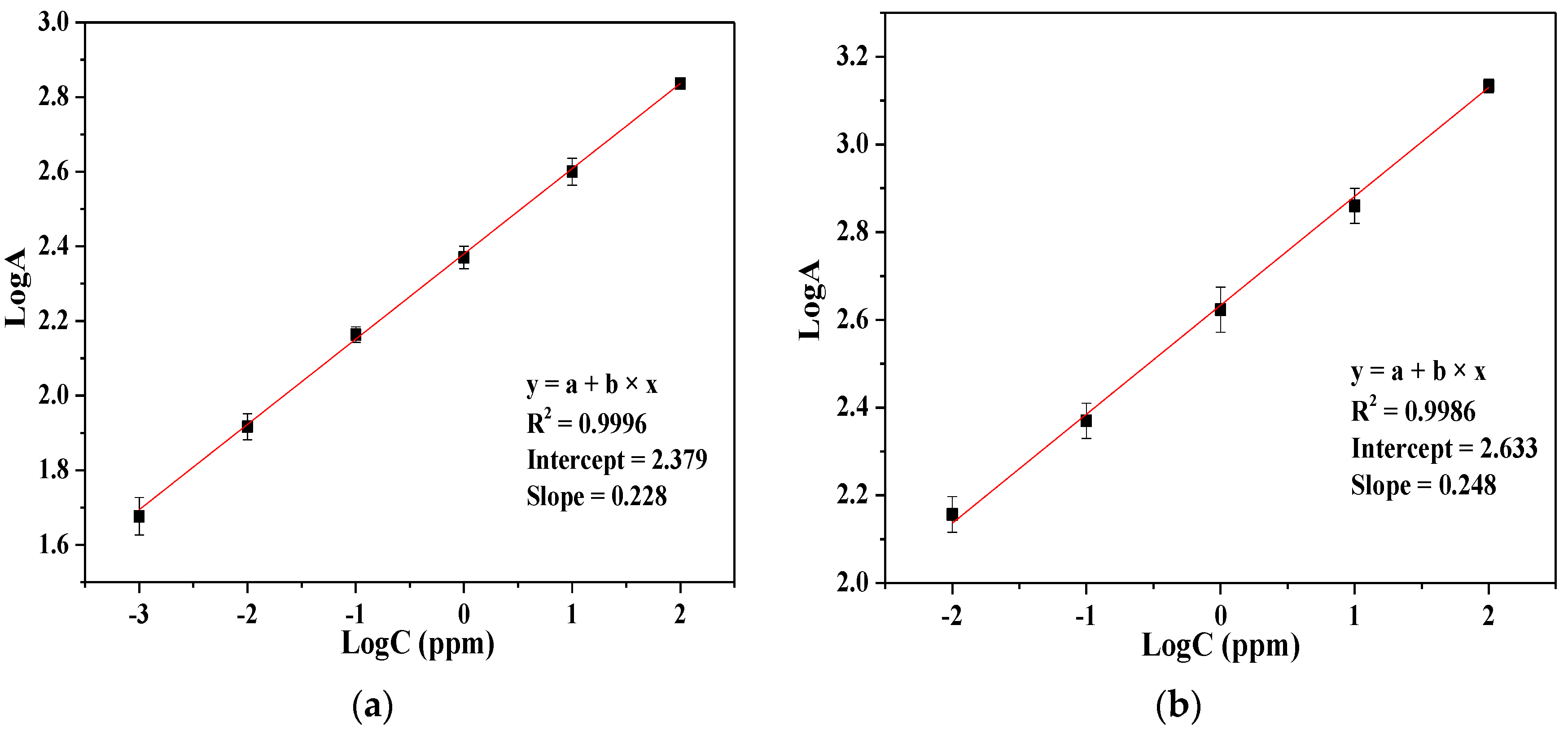
4. Result and Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kong, L.; Zhang, Y.; Mao, H.; Pan, X.; Tian, Y.; Tian, Z.; Zeng, X.; Shi, J.; Tong, B.; Dong, Y. Dimalononitrile-containing probe based on aggregation-enhanced emission features for the multi-mode fluorescence detection of volatile amines. Faraday Discuss. 2017, 196, 101–111. [Google Scholar] [CrossRef] [PubMed]
- The National Institute for Occupational Safety and Health (NIOSH). NIOSH Pocket Guide to Chemical Hazards. 11 April 2016. Available online: https://www.cdc.gov/niosh/npg/npgd0026.html (accessed on 24 November 2017).
- Quan, K.; Li, G.; Tao, L.; Xie, Q.; Yuan, Q.; Wang, X. Diaminopropionic acid reinforced graphene sponge and its use for hemostasis. ACS Appl. Mater. Interfaces 2016, 8, 7666–7673. [Google Scholar] [CrossRef] [PubMed]
- Demessence, A.; D’Alessandro, D.M.; Foo, M.L.; Long, J.R. Strong CO2 binding in a water-stable, triazolate-bridged metal-organic frame work functionalized with ethylenediamine. J. Am. Chem. Soc. 2009, 131, 8784–8786. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Li, X.; Zhu, J.Q.; Li, W.C.; Xu, H.; Guan, Q.M.; Zhang, M.T.; Yuan, Y.J. Optimization of ethylenediamine pretreatment and enzymatic hydrolysis to produce fermentable sugars from corn stover. Ind. Crops. Prod. 2017, 102, 51–57. [Google Scholar] [CrossRef]
- Hu, P.; Ben-David, Y.; Milstein, D. Rechargeable hydrogen storage system based on the dehydrogenative coupling of ethylenediamine with ethanol. Angew. Chem. 2015, 128, 1073–1076. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Wang, Q.; Fu, J.; Zhao, L.; Liu, Z.; Jin, D. Highly responsive ethylenediamine vapor sensor based on a perylenediimide-camphorsulfonic acid complex via ionic self-assembly. J. Mater. Chem. C. 2017, 5, 7644–7651. [Google Scholar] [CrossRef]
- Zhang, X.; Li, K.; Li, H.; Lu, J.; Fu, Q.; Jia, Y.; Li, W. Electrochemical sensing of ethylenediamine based on cuprous oxide/graphene hybrid structures. J. Mater. Sci. 2015, 50, 4288–4299. [Google Scholar] [CrossRef]
- Li, P.; Yang, D.; Li, H. Luminescence ethylenediamine sensor based on terbium complexes entrapment. Dyes Pigm. 2016, 132, 306–309. [Google Scholar] [CrossRef]
- Castillero, P.; Roales, J.; Lopescosta, T.; Sánchezvalencia, J.R.; Barranco, A.; Pedrosa, J.M. Optical gas sensing of ammonia and amines based on protonated porphyrin/TiO2 composite thin films. Sensors 2017, 17, 24. [Google Scholar] [CrossRef]
- Zhou, X.; Lee, S.; Xu, Z.; Yoon, J. Recent progress on the development of chemosensors for gases. Chem. Rev. 2015, 115, 7944–8000. [Google Scholar] [CrossRef]
- Guidotti, T.L. Hydrogen sulfide: Advances in understanding human toxicity. Int. J. Toxicol. 2010, 29, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Petruci, J.F.S.; Wilk, A.; Cardoso, A.A.; Mizaikoff, B. Online Analysis of H2S and SO2 via advanced mid-infrared gas sensors. Anal. Chem. 2015, 87, 9605–9611. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Wang, N.N.; Lin, Z.J.; Wang, J.Q.; Liu, W.; Sun, K.; Fu, Y.Q.; Wang, Z.G. Room-temperature high-performance H2S sensor based on porous CuO nanosheets prepared by hydrothermal method. ACS Appl. Mater. Interfaces 2016, 8, 20962–20968. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Du, B.S.; Sun, Y.; Yu, M.; Yin, Y.Q.; Tang, W.; Chen, C.; Sun, L.; Yang, B.; Cao, W.W.; et al. Sensitive room temperature photoluminescence-based sensing of H2S with novel CuO-ZnO nanorods. ACS Appl. Mater. Interfaces 2016, 8, 16379–16385. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, J.; Pullithadathil, B. New insights towards electron transport mechanism of highly efficient p-Type CuO(111) nanocuboids-based H2S gas sensor. J. Phys. Chem. C 2016, 120, 4087–4096. [Google Scholar] [CrossRef]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Natale, C.D. Porphyrinoids for chemical sensor applications. Chem. Rev. 2016, 12, 2517–2583. [Google Scholar] [CrossRef]
- Woller, J.G.; Hannestad, J.K.; Albinsson, B. Self-assembled nanoscale DNA-porphyrin complex for artificial light harvesting. J. Am. Chem. Soc. 2013, 135, 2759–2768. [Google Scholar] [CrossRef]
- Wang, T.; Yasukochi, W.; Korposh, S.; James, S.W.; Tatam, R.P.; Lee, S.W. A long period grating optical fiber sensor with nano-assembled porphyrin layers for detecting ammonia gas. Sens. Actuator B-Chem. 2016, 288, 573–580. [Google Scholar] [CrossRef] [Green Version]
- Khade, R.L.; Zhang, Y. Catalytic and biocatalytic iron porphyrin carbene formation: Effects of binding mode, carbene substituent, porphyrin substituent, and protein axial ligand. J. Am. Chem. Soc. 2015, 137, 7560–7563. [Google Scholar] [CrossRef]
- Askim, J.R.; Mahmoudiab, M.; Suslick, K.S. Optical sensor arrays for chemical sensing: The optoelectronic nose. Chem. Soc. Rev. 2013, 22, 1–34. [Google Scholar] [CrossRef]
- Boscher, N.D.; Duday, D.; Heier, P.; Heinze, K.; Hilt, F.; Choquet, P. Atmospheric pressure plasma polymerisation of metalloporphyrins containing mesoporous membranes for gas sensing applications. Surf. Coat. Tech. 2013, 234, 48–52. [Google Scholar] [CrossRef]
- Kladsomboon, S.; Kerdcharoen, T. A method for the detection of alcohol vapours based on optical sensing of magnesium 5,10,15,20-tetraphenyl porphyrin thin film by an optical spectrometer and principal component analysis. Anal. Chim. Acta 2012, 757, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Roales, J.; Pedrosa, J.M.; Guillen, M.G.; Lopes-Costa, T.; Castillero, P.; Barranco, A.; Gonzalez-Elipe, A. Free-base carboxyphenyl porphyrin films using a TiO2 columnar matrix: Characterization and application as NO2 sensors. Sensors 2015, 15, 11118–11132. [Google Scholar] [CrossRef] [PubMed]
- Fagadar-Cosma, E.; Tarabukina, E.; Zakharova, N.; Birdeanu, M.; Taranu, B.; Palade, A.; Creanga, I.; Lascu, A.; Fagadar-Cosma, G. Hybrids formed between polyvinylpyrrolidone and an A3B porphyrin dye: Behaviour in aqueous solutions and chemical response to CO2 presence. Polym. Int. 2015, 31, 95–96. [Google Scholar]
- Bae, S.K.; Heo, C.H.; Choi, D.J.; Sen, D.; Joe, E.; Cho, B.R.; Kim, H.M. A ratiometric two-photon fluorescent probe reveals reduction in mitochondrial H2S production in parkinson’s disease gene knockout astrocytes. J. Am. Chem. Soc. 2013, 135, 9915–9923. [Google Scholar] [CrossRef] [PubMed]
- Zilberman, Y.; Chen, Y.; Sonkusale, S.R. Dissolved ammonia sensing in complex mixtures using metalloporphyrin-based optoelectronic sensor and spectroscopic detection. Sens. Actuator B-Chem. 2014, 202, 976–983. [Google Scholar] [CrossRef]
- Giancane, G.; Borovkov, V.; Inoue, Y.; Valli, L. Conformational switching in bis(zinc porphyrin) Langmuir-Schaefer film as an effective tool for selectively sensing aromatic amines. J. Colloid Interf. Sci. 2012, 385, 282–284. [Google Scholar] [CrossRef]
- Muthukumar, P.; John, S.A. Optochemical ammonia gas sensing properties of meso-substituted porphyrin derivatives immobilized Nafion film on glass slide. Sens. Actuator B-Chem. 2012, 174, 74–80. [Google Scholar] [CrossRef]
- Rubatat, L.G.; Gebel, A.; Diat, O. Fibrillar Structure of Nafion: Matching Fourier and Real Space Studies of Corresponding Films and Solutions. Macromolecules 2017, 37, 7772–7783. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, J.; Kaneko, M. Electron transfer in the redox reaction of cobalt tetraphenylporphyrin incorporated in a Nafion film. J. Porphyr. Phthalocyanines 2015, 4, 158–167. [Google Scholar] [CrossRef]
- Roales, J.; Pedrosa, J.M.; Guillén, M.G.; Lopes-Costa, T.; Pinto, S.M.A.; Calvete, M.J.F.; Pereira, M.M. Optical detection of amine vapors using ZnTriad porphyrin thin films. Sens. Actuator B-Chem. 2015, 210, 28–35. [Google Scholar] [CrossRef]
- Evyapan, M.; Dunbar, A.D.F. Controlling surface adsorption to enhance the selectivity of porphyrin based gas sensors. Appl. Surf. Sci. 2016, 362, 191–201. [Google Scholar] [CrossRef]
- Xu, P.; Tao, D.Q.; Liu, W.W. Rapid detection of H2S in heart blood by chemical method and GC/MS. Appl. Mech. Mater. 2014, 496–500, 528–531. [Google Scholar] [CrossRef]
- Heusler, H.; Richter, E.; Epping, J.; Schmidt, M. Quantitative analysis of ethylenediamine in plasma by capillary column gas chromatography. J. Sep. Sci. 2015, 9, 548–554. [Google Scholar] [CrossRef]
- Smock, P.L.; Orofino, T.A.; Wooten, G.W.; Spencer, W.S. Vapor phase determination of blood ammonia by an optical waveguide technique. Anal. Chem. 1979, 51, 505–509. [Google Scholar] [CrossRef]
- Zinoviev, K.; Dominguez, C.; Plaza, J.A.; Busto, V.J.C.; Lechuga, L.M. A novel optical waveguide microcantilever sensor for the detection of nanomechanical forces. J. Lightw. Technol. 2006, 24, 2132–2138. [Google Scholar] [CrossRef] [Green Version]
- Butt, M.A.; Kozlova, E.S.; Khonina, S.N.; Skidanov, R.V. Optical planar waveguide sensor based on (Yb,Nb):RTP/RTP(001) system for the estimation of metal coated cells. CEUR Workshop Proc. 2016, 1638, 16–23. [Google Scholar]
- Kehl, F.; Etlinger, G.; Gartmann, T.E.; Tscharner, N.S.R.U.; Heub, S.; Follonier, S. Introduction of an angle interrogated, mems-based, optical waveguide grating system for label-free biosensing. Sens. Actuator B-Chem. 2016, 226, 135–143. [Google Scholar] [CrossRef]
- Fan, Y.; Ding, Y.; Ma, H.; Teramae, N.; Sun, S.Q.; He, Y.H. Optical waveguide sensor based on silica nanotube arrays for label-free biosensing. Biosens. Bioelectron. 2015, 67, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Kooriyaden, F.R.; Sujatha, S.; Arunkumar, C. Synthesis, spectral, structural and antimicrobial studies of fluorinated porphyrins. Polyhedron 2015, 97, 66–74. [Google Scholar] [CrossRef]
- Kangwanwong, T.; Pluempanupat, W.; Parasuk, W.; Keenan, H.E.; Songsasen, A. Using 5,10,15,20-tetrakis(4-nitrophenyl)porphyrin as a fluorescent chemosensor to determine Ru3+. Sci. Asia 2012, 38, 278–282. [Google Scholar] [CrossRef]
- Meng, G.G.; James, B.R.; Skov, K.A. Porphyrin chemistry pertaining to the design of anti-cancer drugs; part 1, the synthesis of porphyrins containing meso-pyridyl and meso-substituted phenyl functional groups [J]. Cana. J. Chem. 1994, 72, 2447–2457. [Google Scholar] [CrossRef]
- Turdi, G.; Yimit, A.; Tursun, A.; Turgun, A. Zinc tetraphenylporphyrin film optical waveguide sensor for detection of volatile organic compound gases. Chin. J. Appl. Chem. 2015, 32, 232–238. [Google Scholar]
- Ablat, H.; Yimit, A.; Mahmut, M.; Itoh, K. Nafion film/K+-exchanged glass optical waveguide sensor for BTX detection. Anal. Chem. 2008, 80, 7678–7683. [Google Scholar] [CrossRef]
- Nizamidin, P.; Yimit, A.; Itoh, K. Synthesis and optical-electrochemical gas sensing applications of Ni-doped LiFePO4 nano-particles. New J. Chem. 2016, 40, 295–301. [Google Scholar]
- Nizamidin, P.; Yimit, A.; Wang, J.D.; Itoh, K. Optical properties and sensing applications of lithium iron phosphate thin films. Thin Solid Films 2012, 520, 6250–6255. [Google Scholar] [CrossRef]
- He, Q.G.; Mugadza, T.; Kang, X.W.; Zhu, X.B.; Chen, S.W.; Kerr, J.; Nyokong, T. Molecular catalysis of the oxygen reduction reaction by iron porphyrin catalysts tethered into Nafion layers: An electrochemical study in solution and a membrane-electrode-assembly study in fuel cells. J. Power Sources 2012, 216, 67–75. [Google Scholar] [CrossRef]
- Yeager, H.L.; Steck, A. Cation and water diffusion in Nafion ion exchange membranes: Influence of polymer structure. J. Electrochem. Soc. 1981, 128, 1880–1884. [Google Scholar] [CrossRef]
- Lou, Z.; Li, F.; Deng, J.A.; Wang, L.L.; Zhang, T. Branch-like hierarchical heterostructure (α-Fe2O3/TiO2): A novel sensing material for trimethylamine gas sensor. ACS Appl. Mater. Interfaces 2013, 5, 12310–12316. [Google Scholar] [CrossRef]
- Geng, J.; Jung, H. Porphyrin functionalized graphene sheets in aqueous suspensions: From the preparation of graphene sheets to highly conductive graphene films. J Phys. Chem. C. 2010, 114, 8227–8234. [Google Scholar] [CrossRef]
- Rogero, C.; Pickup, D.F.; Colchero, J.; Azaceta, E.; Tena-Zaera, R.; Palacios-Lidon, E. Nanophotoactivity of porphyrin functionalized polycrystalline ZnO films. ACS Appl. Mater. Interfaces 2016, 8, 16783–16790. [Google Scholar] [CrossRef] [PubMed]
- Yivlialin, R.; Bussetti, G.; Penconi, M.; Bossi, A.; Ciccacci, F.; Finazzi, M.; Duò, L. Vacuum-deposited porphyrin protective films on graphite: Electrochemical atomic force microscopy investigation during anion intercalation. ACS Appl. Mater. Interfaces 2017, 1, 4100–4105. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, H.; Itoh, K.; Murabayashi, M.; Qi, Z.M. A composite optical waveguide-based polarimetric interferometer for chemical and biological sensing applications. J. Lightw. Technol. 2002, 18, 1106–1110. [Google Scholar]
- Qi, Z.M.; Yimit, A.; Itoh, K.; Murabayashi, M.; Matsuda, N.; Takatsu, A.; Kato, K. Composite optical waveguide composed of a tapered film of bromothymol blue evaporated onto a potassium ion-exchanged waveguide and its application as a guided wave absorption-based ammonia-gas sensor. Opt. Lett. 2001, 26, 629–633. [Google Scholar] [CrossRef]
- Wang, B.; Zuo, X.; Wu, Y.Q.; Che, Z.M. Preparation, characterization and gas sensing properties of lead tetra-(tert-butyl)-5,10,15,20-tetraazaporphyrin spin-coating films. Sens. Actuator B-Chem. 2007, 125, 268–273. [Google Scholar] [CrossRef]
- Ma, J.Q.; Zhang, W.L.; Li, Z.; Lin, Q.; Xu, J.; Han, Y.S. Competition of major forces dominating the structures of porphyrin assembly. Cryst. Growth Des. 2016, 16, 1942–1947. [Google Scholar] [CrossRef]
- Alsaif, M.M.; Field, M.R.; Murdoch, B.J.; Daeneke, T.; Latham, K.; Chrimes, A.F.; Zoolfakar, A.S.; Russo, S.P.; Ou, J.Z.; Kalantar-zadeh, K. Substoichiometric two-dimensional molybdenum oxide flakes: A plasmonic gas sensing platform. Nanoscale 2014, 6, 12780–12791. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Hirami, H.; Izawa, T.; Terashima, Y. Density, viscosity, and glass transition of an ethylenediamine–ethylene glycol binary system. J. Solution Chem. 2017, 1, 1–17. [Google Scholar] [CrossRef]
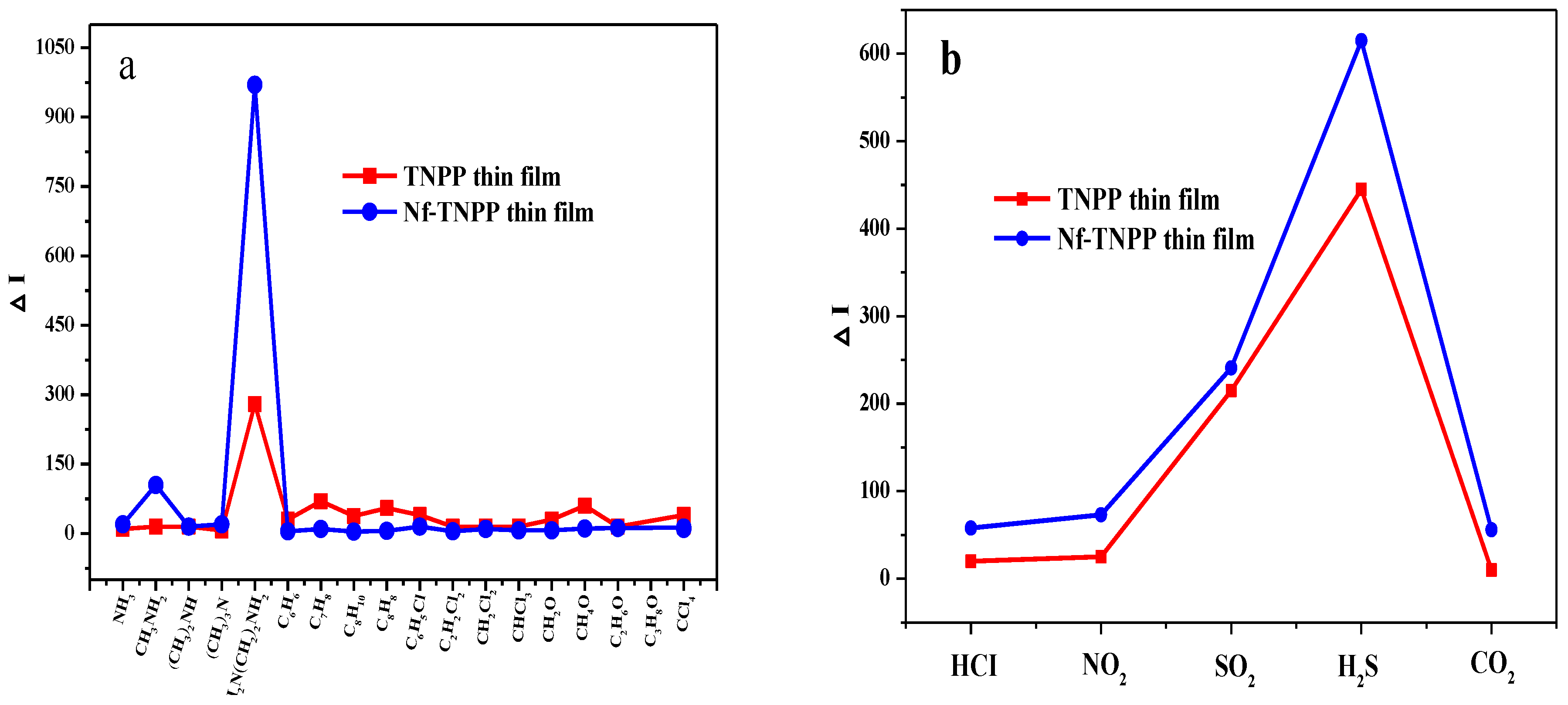



| Gas Sample | ΔAbs (532 nm) | ΔAbs (650 nm) | ΔI (532 nm) | ΔI (650 nm) |
|---|---|---|---|---|
| H2S | 0.001 | 0.005 | 100 | 530 |
| EDA | 0.005 | 0.002 | 970 | 212 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuerdi, G.; Kari, N.; Yan, Y.; Nizamidin, P.; Yimit, A. A Functionalized Tetrakis(4-Nitrophenyl)Porphyrin Film Optical Waveguide Sensor for Detection of H2S and Ethanediamine Gases. Sensors 2017, 17, 2717. https://doi.org/10.3390/s17122717
Tuerdi G, Kari N, Yan Y, Nizamidin P, Yimit A. A Functionalized Tetrakis(4-Nitrophenyl)Porphyrin Film Optical Waveguide Sensor for Detection of H2S and Ethanediamine Gases. Sensors. 2017; 17(12):2717. https://doi.org/10.3390/s17122717
Chicago/Turabian StyleTuerdi, Gulimire, Nuerguli Kari, Yin Yan, Patima Nizamidin, and Abliz Yimit. 2017. "A Functionalized Tetrakis(4-Nitrophenyl)Porphyrin Film Optical Waveguide Sensor for Detection of H2S and Ethanediamine Gases" Sensors 17, no. 12: 2717. https://doi.org/10.3390/s17122717