A Novel Detection Method of Human Serum Albumin Based on the Poly(Thymine)-Templated Copper Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Apparatus
2.3. Detection of HSA
3. Results and Discussion
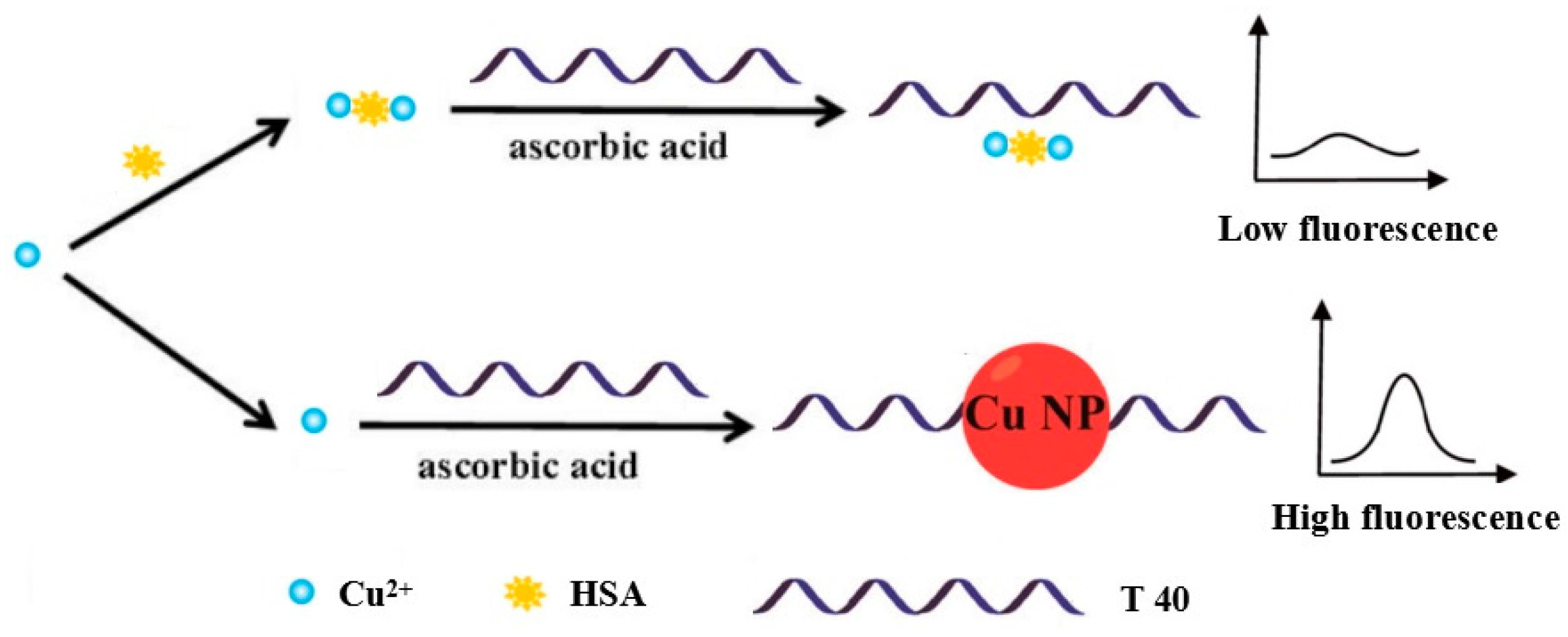
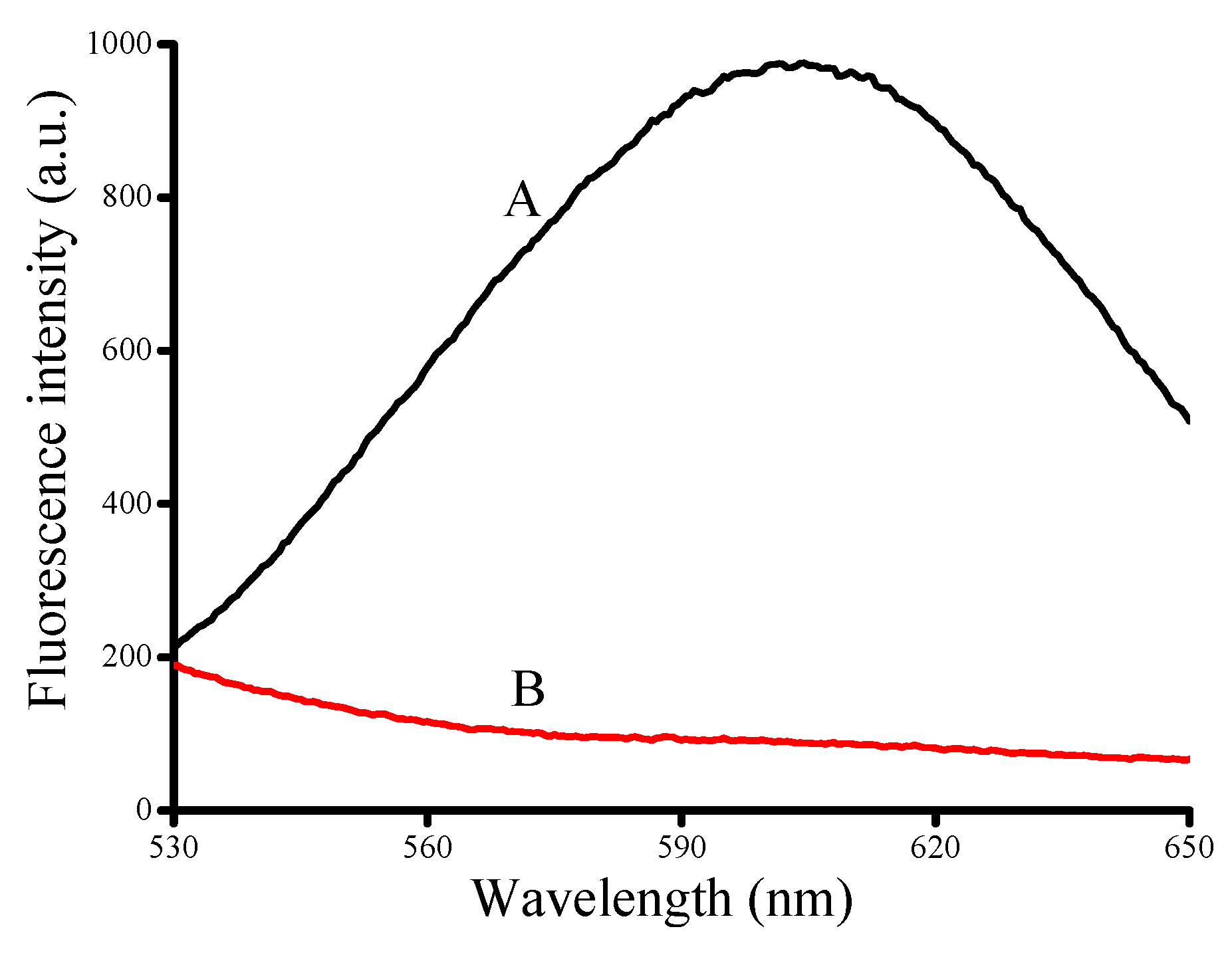
3.1. Verification of the Feasibility of HSA Detection Using Poly T-Templated CuNPs
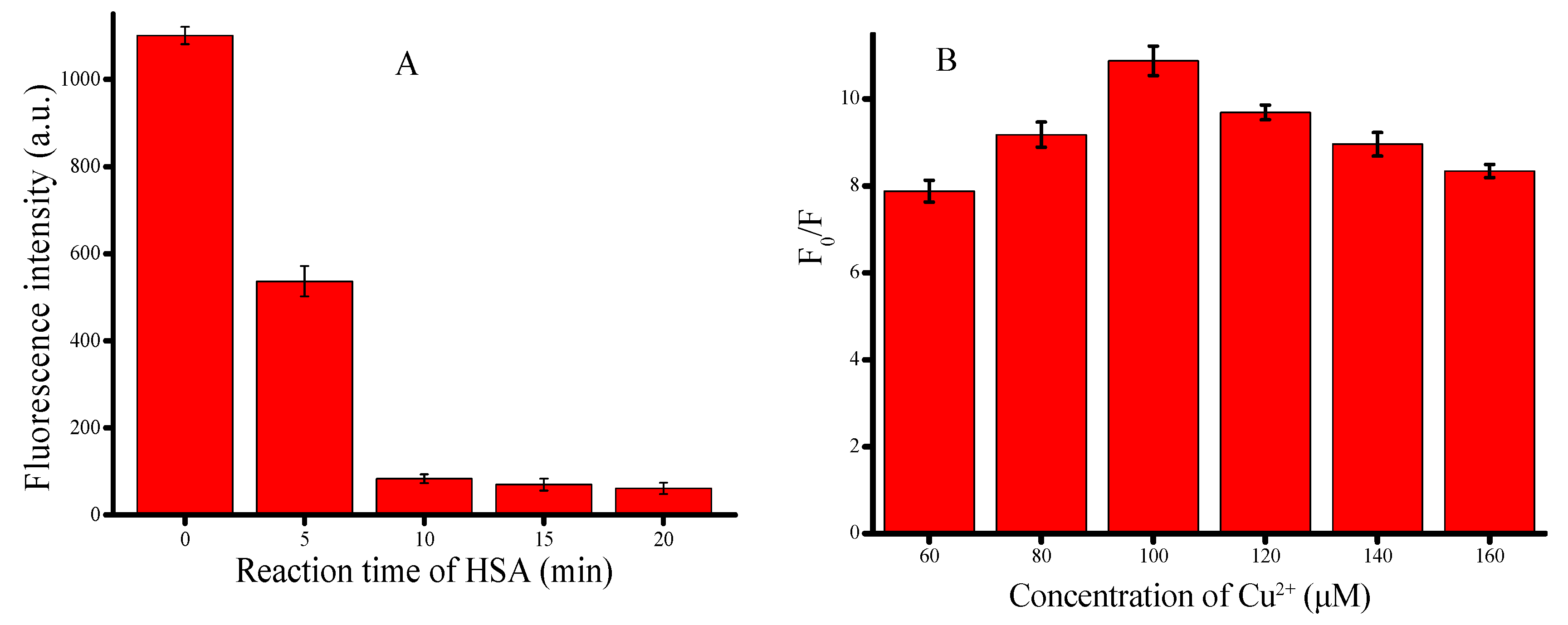
3.2. Optimization of Experimental Conditions
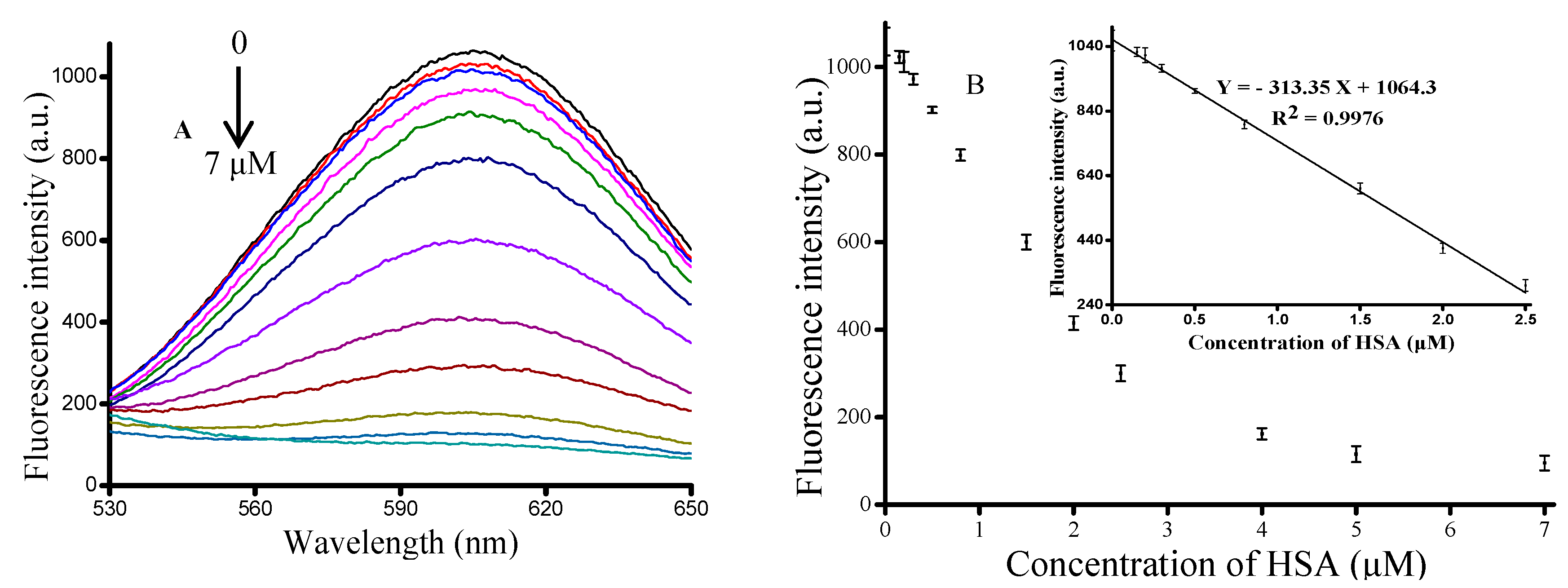
3.3. Quantitative Measurement of HSA
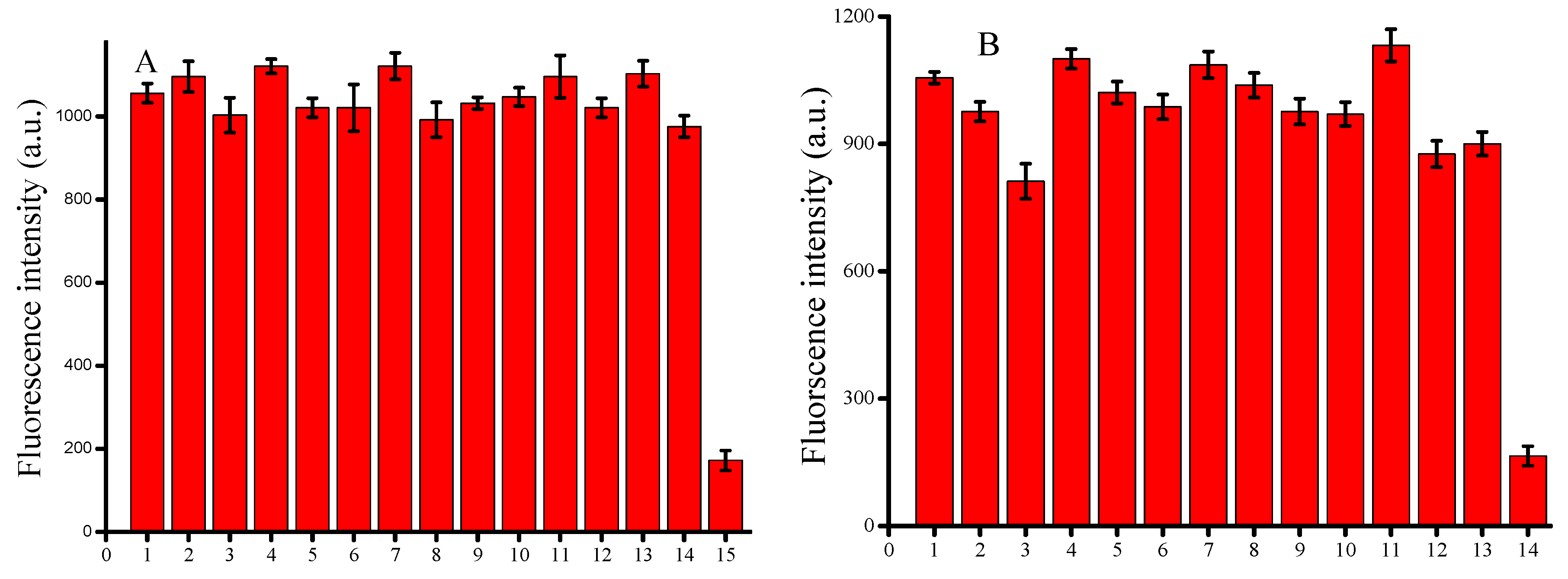
3.4. Study of Interferences
3.5. Application of the Proposed Assay in Biological Systems
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Y.; Guo, Y.M.; Xian, Y.L.; Chen, W.W.; Zhao, Y.Y.; Jiang, X.Y. Nanomaterials for Ultrasensitive Protein Detection. Adv. Mater. 2013, 25, 3802–3819. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Li, F.F.; Liao, C.Y.; Zheng, B.Z.; Du, J.; Xiao, D. A selective and sensitive fluorescent probe for the determination of HSA and trypsin. Talanta 2017, 170, 562–568. [Google Scholar] [CrossRef] [PubMed]
- McCallum, M.M.; Pawlak, A.J.; Shadrick, W.R.; Simeonov, A.; Jadhav, A.; Yasgar, A.; Maloney, D.J.; Arnold, L.A. A fluorescence-based high throughput assay for the determination of small molecule-human serum albumin protein binding. Anal. Bioanal. Chem. 2014, 406, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Gui, W.Y.; Chen, X.Q.; Ma, Q. A novel detection method of human serum albumin based on CuInZnS quantum dots—Co2+ sensing system. Anal. Bioanal. Chem. 2017, 409, 3871–3876. [Google Scholar] [CrossRef] [PubMed]
- Fanali, G.; Masi, A.D.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human Serum Albumin: From Bench to Bedside. Mol. Aspects Med. 2012, 33, 209–290. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.Z.; Wang, H.N.; Yang, W.S. Gold Nanoparticle-Based Facile Detection of Human Serum Albumin and Its Application as an INHIBIT Logic Gate. ACS Appl. Mater. Interfaces 2015, 7, 8990–8999. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.X.; Xiang, J.F.; Zhang, X.F.; Chen, H.B.; Yang, Q.F.; Li, Q.; Guan, A.J.; Shang, Q.; Tang, Y.L.; Xu, G.Z. A Colorimetric and Fluorometric Dual-Modal Supramolecular Chemosensor and Its Application for HSA Detection. Analyst 2014, 139, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.D.; Ma, M.S.; Zhu, B.C.; Zhu, L. A near infrared fluorescence probe for sensitive determination of human serum albumin. Anal. Sci. 2016, 32, 1291–1294. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, Z.; Erdossy, J.; Keltai, K.; Scheller, F.W.; Gyurcsanyi, R.E. Electrosynthesized moleculary imprinted polyscopoletin nanofilms for human serum albumin detection. Anal. Chim. Acta 2017, 977, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, M.; Cieplak, M.; Sharma, P.S.; Borowicz, P.; Noworyta, K.; Lisowski, W.; Souza, F.D.; Kuhn, A. Hierarchical templating in deposition of semi-covalently imprinted inverse opal polythiophene film for femtomolar determination of human serum albumin. Biosens. Bioelectron. 2017, 94, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Caballero, D.; Martinez, E.; Bausells, J.; Errachid, A.; Samitier, J. Impedimetric Immunosensor for Human Serum Albumin Detection on a Direct Aldehyde-Functionalized Silicon Nitride Surface. Anal. Chim. Acta 2012, 720, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, V.B.; Pavicevic, I.D.; Takic, M.M.; Penezic-Romanjuk, A.Z.; Acimovic, J.M.; Mandic, L.M. The influence of fatty acids on determination of human serum albumin thiol group. Anal. Biochem. 2014, 448, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.C.; Chang, Y.Z.; Kang, Y.T.; Chang, H.Y.; Chang, P.; Yew, T.R. A quantum dot-based optical immunosensor for human serum albumin detection. Biosens. Bioelectron. 2012, 34, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Contois, J.H.; Hartigan, C.; Rao, L.V.; Snyder, L.M.; Thompson, M.J. Analytical Validation of an HPLC Assay for Urinary Albumin. Clin. Chim. Acta 2006, 367, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Ermolenko, Y.; Anshakova, A.; Osipova, N.; Kamentsev, M.; Maksimenko, O.; Balabanyan, V.; Gelperina, S. Simultaneous determination of rifabutin and human serum albumin in pharmaceutical formulations by capillary electrophoresis. J. Pharmacol. Toxicol. Methods 2017, 85, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Crow, F.W.; Babic, N.; Lutz, W.H.; Lieske, J.C.; Larson, T.S.; Kumar, R. A Liquid Chromatography−Mass Spectrometry Method for the Quantification of Urinary Albumin Using a Novel 15N-Isotopically Labeled Albumin Internal Standard. Clin. Chem. 2007, 53, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.; Zhu, L.; Yang, S.; Cao, Z.; He, X.; Wang, K.; Yang, R. In situ formation of fluorescent copper nanoparticles for ultrafast zero-background Cu2+, detection and its toxicides screening. Biosens. Bioelectron. 2016, 78, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, C.; Wang, E.; Dong, S. A label-free and enzyme-free system for operating various logic devices using poly(thymine)-templated CuNPs and SYBR Green I as signal transducers. Nanoscale 2016, 8, 14243–14249. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.M.; Cao, E.P.; Lei, X.L.; Mang, L.H.; Cheng, S.J.; Song, J.T. Fluorescent copper nanoparticles: Recent advances in synthesis and applications for sensing metal ions. Nanoscale 2016, 8, 4852–4863. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ji, X.; Tinnefeld, P.; He, Z. Multifunctional Dumbbell-Shaped DNA-Templated Selective Formation of Fluorescent Silver Nanoclusters or Copper Nanoparticles for Sensitive Detection of Biomolecules. ACS Appl. Mater. Interfaces 2016, 8, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.S.; Zhang, L.; Cai, Q.Y.; Dong, Z.Z.; Geng, X.; Ge, J.; Li, Z.H. A facile label-free aptasensor for detecting ATP based on fluorescence enhancement of poly(thymine)-templated copper nanoparticles. Anal. Bioanal. Chem. 2016, 408, 6711–6717. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Si, L.; Bao, J.; Wang, Z.; Dai, Z. Fluorescence Regulation of Poly(thymine)-templated Copper Nanoparticles via an Enzyme-triggered Reaction towards Sensitive and Selective Detection of Alkaline Phosphatase. Anal. Chem. 2017, 89, 3681–3686. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, T.; Xu, X.L.; Wang, L.; Yang, X. A fluorescent ELISA based on the enzyme-triggered synthesis of poly(thymine)-templated copper nanoparticles. Nanoscale 2016, 8, 16846–16850. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Yang, X.; Wang, K.; Wang, Q.; Huang, J.; Liu, J.; Liu, W.; Liu, P. Label-free and non-enzymatic detection of DNA based on hybridization chain reaction amplification and dsDNA-templated copper nanoparticles. Anal. Chim. Acta 2014, 827, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Ning, Y.; Kong, J.; Zhang, X. Formation of copper nanoparticles on poly(thymine) through surface-initiated enzymatic polymerization and its application for DNA detection. Analyst 2015, 140, 5678–5684. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Y.; Li, B.; Jin, Y. High-throughput identification of telomere-binding ligands based on the fluorescence regulation of DNA-copper nanoparticles. Biosens. Bioelectron. 2017, 87, 915–917. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Hu, S.; Cao, Y.; Zhang, B.; Li, G. Electrochemical detection of protein based on hybridization chain reaction-assisted formation of copper nanoparticles. Biosens. Bioelectron. 2015, 66, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Zhang, H.D.; Chen, Y.; Liu, Y.M. A fluorescent biosensor for protein detection based on poly(thymine)-templated copper nanoparticles and terminal protection of small molecule-linked DNA. Biosens. Bioelectron. 2015, 74, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Qing, T.B.; He, X.X.; He, D.G.; He, X.; Shangguan, J.; Liu, J.; Yuan, B.; Wang, K. Dumbbell DNA-templated CuNPs as a nano-fluorescent probe for detection of enzymes involved in ligase-mediated DNA repair. Biosens. Bioelectron. 2017, 94, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Z.; Dong, J.J.; Zhou, F.L.; Li, B.X. One facile fluorescence strategy for Sensitive detection of endonuclease activity using DNA-templated copper nanoclusters as signal indicators. Sens. Actuators B Chem. 2017, 238, 828–833. [Google Scholar] [CrossRef]
- Cai, Q.Y.; Ge, J.; Xu, H.H.; Zhang, L.; Hu, Y.L.; Huang, Z.M.; Li, Z.H. A label-free aptasensor for highly sensitive ATP detection by using exonuclease I and oligonucleotide-templated fluorescent copper nanoparticles. Anal. Methods 2017, 9, 2710–2714. [Google Scholar] [CrossRef]
- Chi, B.Z.; Liang, R.P.; Qiu, W.B.; Yuan, Y.H.; Qiu, J.D. Direct fluorescence detection of microRNA based on enzymatically engineered primer extension poly-thymine (EPEPT) reaction using copper nanoparticles as nano-dye. Biosens. Bioelectron. 2016, 87, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Park, K.W.; Batule, B.S.; Kang, K.S.; Park, K.S.; Park, H.G. Rapid and ultrasensitive detection of microRNA by target-assisted isothermal exponential amplification coupled with poly (thymine)-templated fluorescent copper nanoparticles. Nanotechnology 2016, 27, 425–502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cai, Q.; Li, J.; Ge, J.; Wang, J.; Dong, Z.; Li, Z.H. A label-free method for detecting biothiols based on poly(thymine)-templated copper nanoparticles. Biosens. Bioelectron. 2015, 69, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, H.; Cao, Z.; Lau, C.; Lu, J. Additive and enhanced fluorescence effects of hairpin DNA template-based copper nanoparticles and their application for the detection of NAD. Talanta 2016, 154, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.Q.; Liu, M.D.; Gu, C.C.; Nie, H.G.; Xu, X.J.; Li, Z.; Yang, Z.; Huang, S.M. A novel label-free fluorescence strategy for methyltransferase activity assay based on dsDNAtemplated copper nanoparticles coupled with an endonuclease-assisted signal transduction system. Analyst 2016, 141, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.; He, X.; He, D.; Wang, K.; Xu, F.; Qing, T.; Yang, X. Poly(thymine)-Templated Selective Formation of Fluorescent Copper Nanoparticles. Angew. Chem. Int. Ed. 2013, 52, 9719–9722. [Google Scholar] [CrossRef] [PubMed]
- Eertmans, F.; Bogaert, V.; Puype, B. Development and validation of a high-performance liquid chromatography (HPLC) method for the determination of human serum albumin (HSA) in medical devices. Anal. Methods 2011, 3, 1296–1302. [Google Scholar] [CrossRef]
- Ramezani, A.M.; Manzoori, J.; Amjadi, M.; Jouyban, A. Spectrofluorimetric Determination of Human Serum Albumin Using Terbium-Danofloxacin Probe. Sci. World. J. 2012, 1, 940541–940550. [Google Scholar] [CrossRef] [PubMed]
- Boiero, C.; Allemandi, D.; Longhi, M.; Llabot, J.M. RP-HPLC method development for simultaneous determination of timolol maleate and human serum albumin in albumin nanoparticles. J. Pharm. Biomed. Anal. 2015, 111, 186–189. [Google Scholar] [CrossRef] [PubMed]

| Sample | HSA in Plasma (μM) | Added (μM) | Detected (μM) | Recovery (%) |
|---|---|---|---|---|
| 1 | 0.36 | 0.5 | 0.834 ± 0.036 | 97.0 |
| 2 | 0.36 | 1 | 1.374 ± 0.064 | 101.0 |
| 3 | 0.36 | 1.5 | 1.854 ± 0.094 | 99.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Xiang, X.; Wu, K.; He, H.; Chen, H.; Ma, C. A Novel Detection Method of Human Serum Albumin Based on the Poly(Thymine)-Templated Copper Nanoparticles. Sensors 2017, 17, 2684. https://doi.org/10.3390/s17112684
Chen M, Xiang X, Wu K, He H, Chen H, Ma C. A Novel Detection Method of Human Serum Albumin Based on the Poly(Thymine)-Templated Copper Nanoparticles. Sensors. 2017; 17(11):2684. https://doi.org/10.3390/s17112684
Chicago/Turabian StyleChen, Mingjian, Xinying Xiang, Kefeng Wu, Hailun He, Hanchun Chen, and Changbei Ma. 2017. "A Novel Detection Method of Human Serum Albumin Based on the Poly(Thymine)-Templated Copper Nanoparticles" Sensors 17, no. 11: 2684. https://doi.org/10.3390/s17112684




