A Mediated BOD Biosensor Based on Immobilized B. Subtilis on Three-Dimensional Porous Graphene-Polypyrrole Composite
Abstract
:1. Introduction
2. Experiment Section
2.1. Materials and Reagents
2.2. Bacterial Cultivation
2.3. Preparation of 3D Porous rGO-PPy Composite
2.4. Microorganisms Immobilization and Bioreactor Fabrication
2.5. Microscopic Observations
2.6. Electrochemical Experiments
2.7. BOD Measurement Procedures
2.8. Measurement of Real Water
3. Results and Discussion
3.1. The Detection Mechanism of the Mediated BOD Biosensor

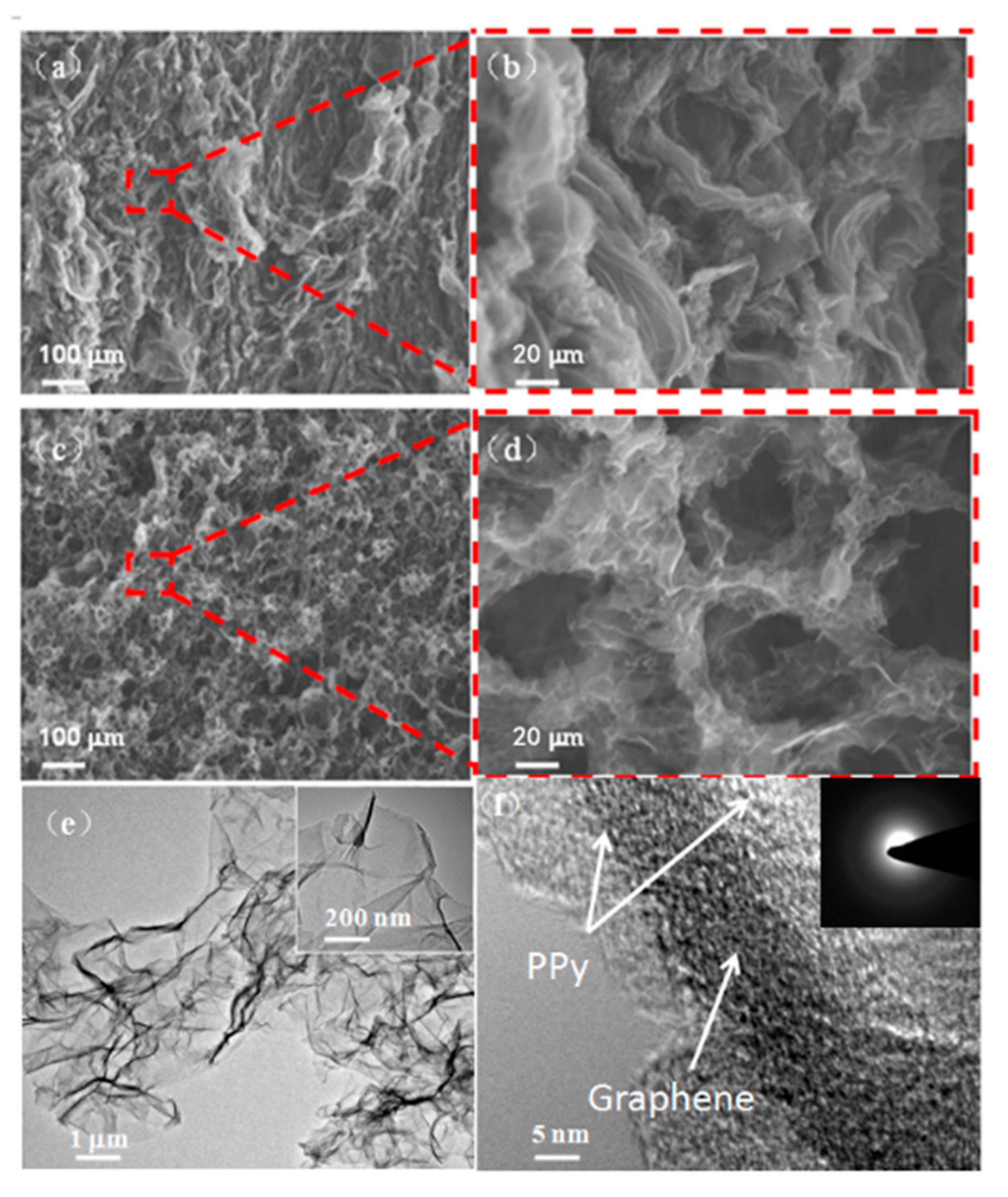
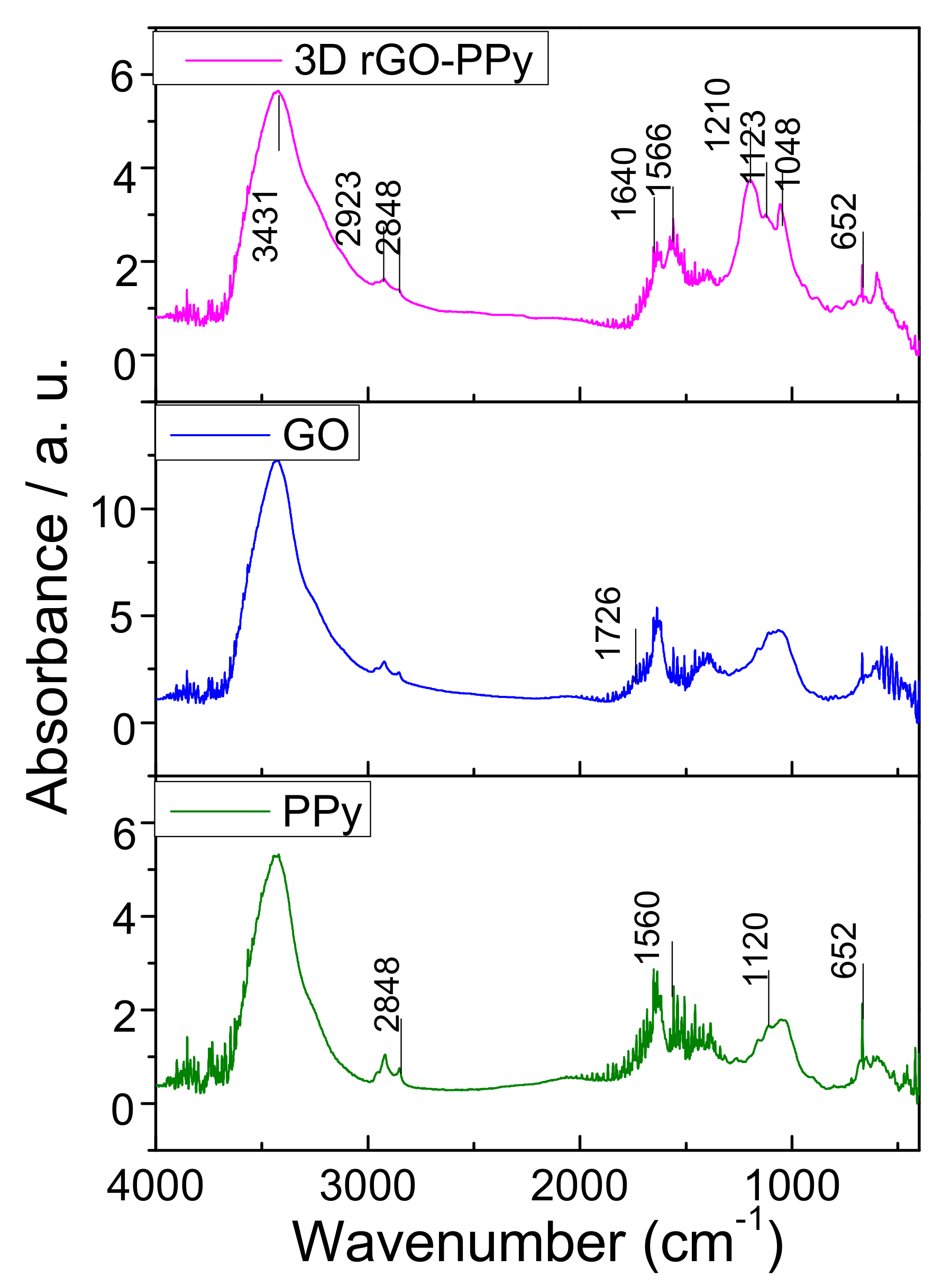
3.2. Characterization of 3D rGO-PPy Composite
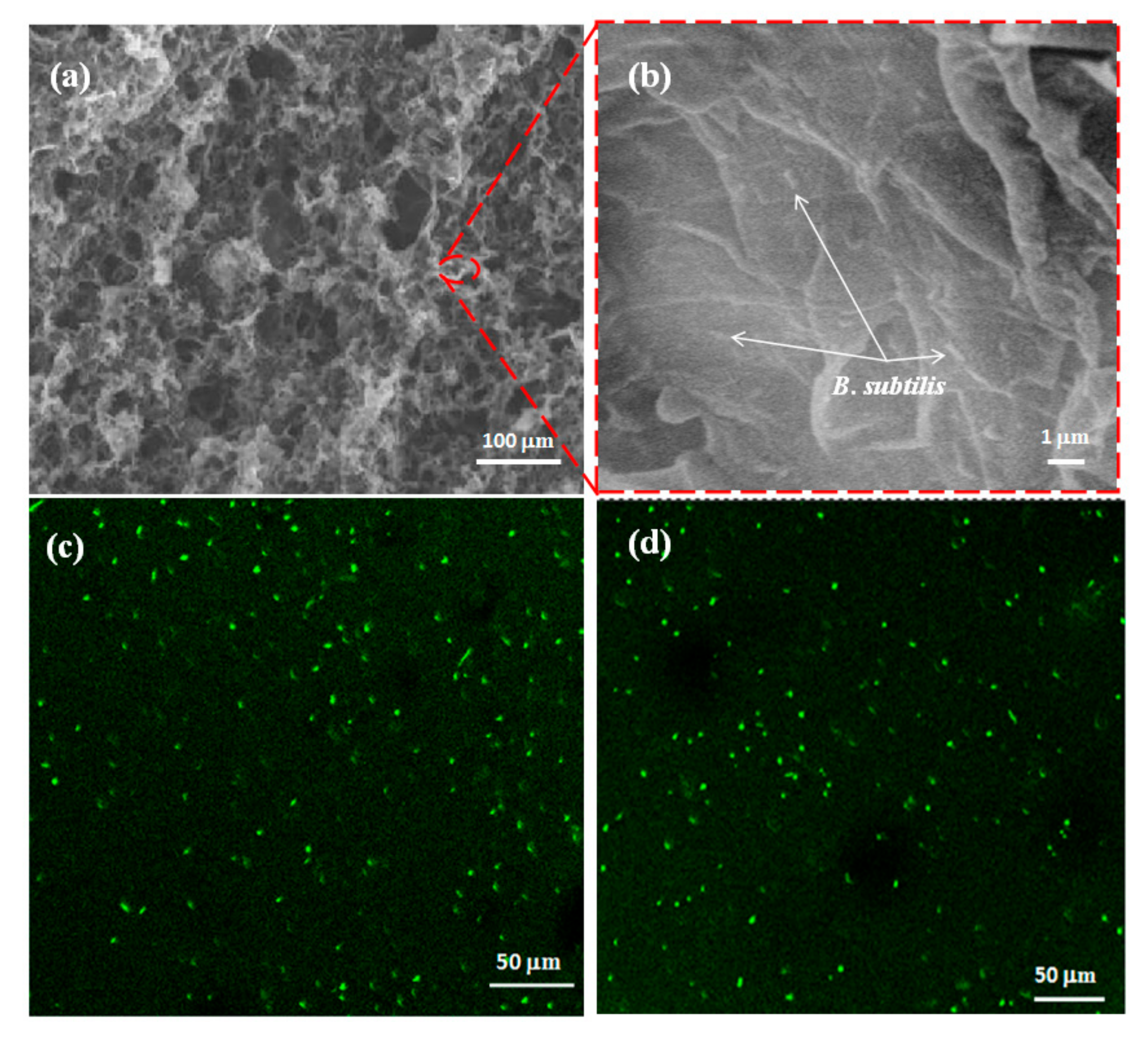
3.3. Characterization of rGO-PPy-B Microbial Biofilm
3.4. Optimization of Experimental Conditions
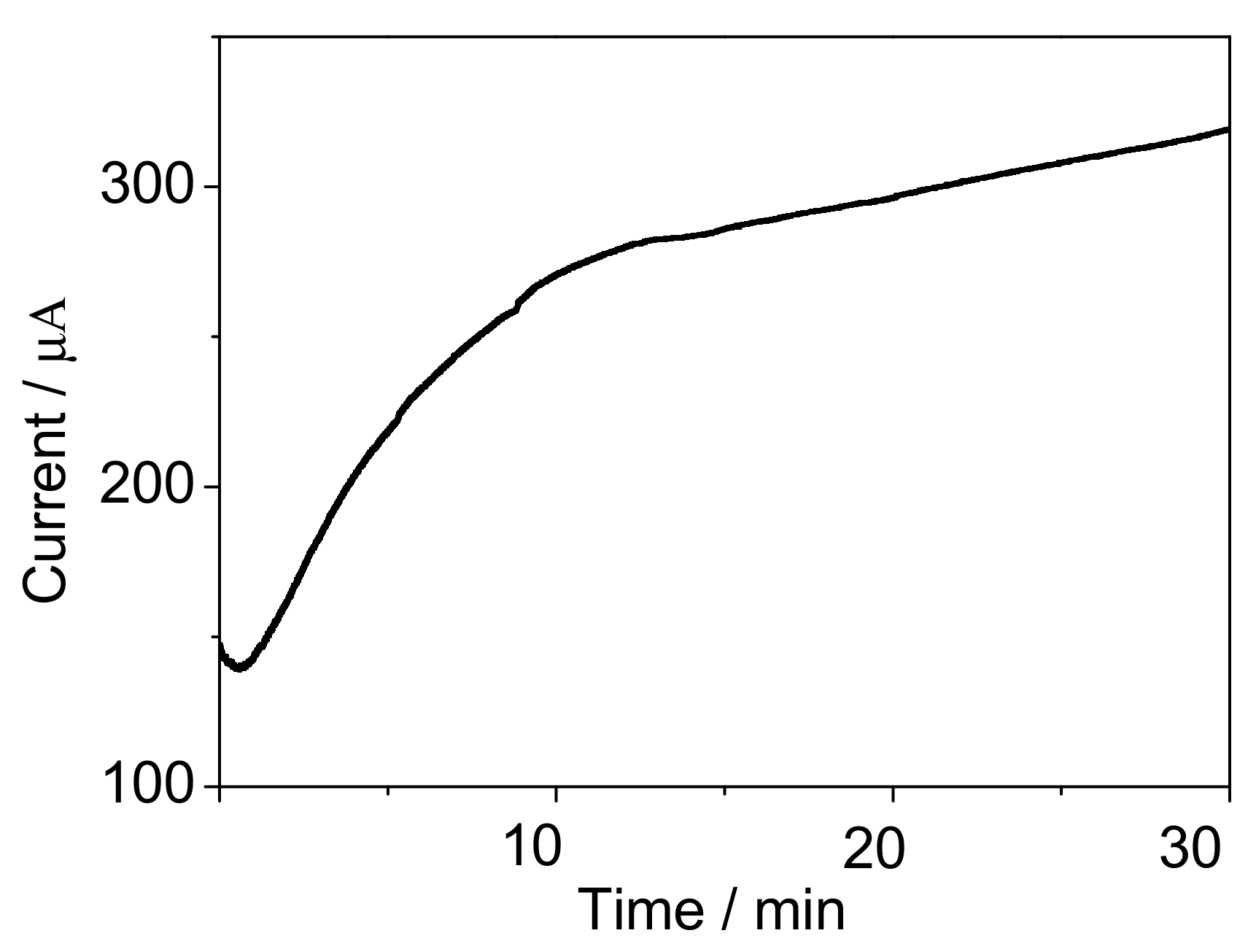
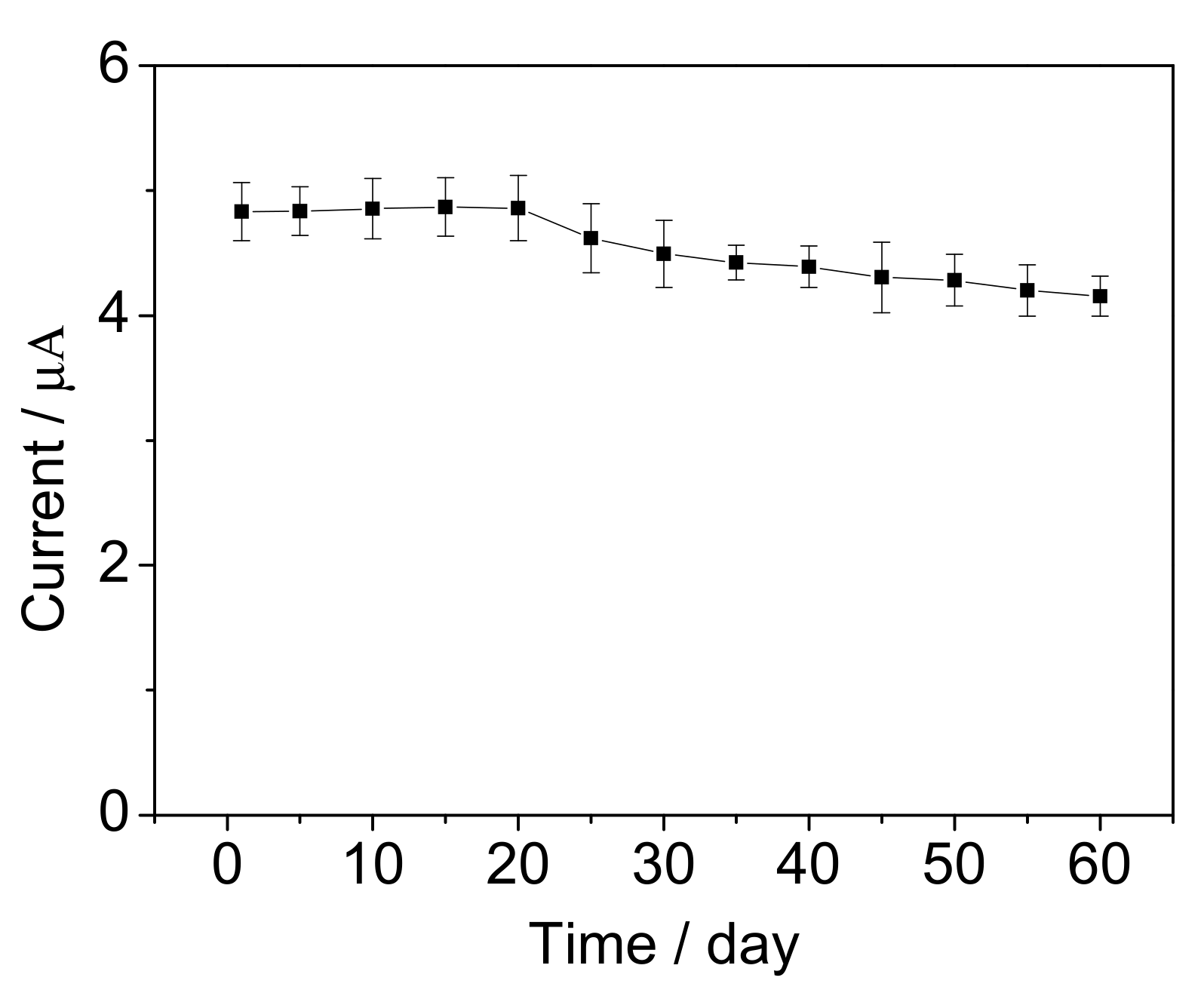
3.5. Performance of the Mediated BOD Biosensor
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oturan, M.A. Electrochemical advanced oxidation technologies for removal of organic pollutants from water. Environ. Sci. Pollut. Res. 2014, 21, 8333–8335. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Z.; Jiang, D.; Jia, J.; Zhang, Y.; Chai, Y.; Cheng, X.; Dong, S. Demonstration study of biofilm reactor based rapid biochemical oxygen demand determination of surface water. Sens. Bio-Sens. Res. 2016, 8, 8–13. [Google Scholar] [CrossRef]
- European Union. Environmental Standard of the European Union, Data Sheets for Surface Water Quality Standards; Directive 2008/105/EC; European Union: Brussels, Belgium, 2008. [Google Scholar]
- Ministry of Environmental Protection of the People’s Republic of China. National Standard of the People’s Republic of China, Environmental Quality Standards for Surface Water; GB 3838-2002; MEP: Beijing, China, 2002.
- Ministry of Environmental Protection of the People’s Republic of China. National Standard of the People’s Republic of China, Environmental Quality Standards for Domestic Wastewater Discharge Standard; GB 18918-2002; MEP: Beijing, China, 2002.
- Zaitseva, A.S.; Arlyapov, V.A.; Yudina, N.Y.; Alferov, S.V.; Reshetilov, A.N. Use of one- and two-mediator systems for developing a BOD biosensor based on the yeast Debaryomyces hansenii. Enzyme Microb. Technol. 2017, 98, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Welsh, D.T.; John, R.; Catterall, K.; Teasdale, P.R. A sensitive ferricyanide-mediated biochemical oxygen demand assay for analysis of wastewater treatment plant influents and treated effluents. Water Res. 2013, 47, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Dhall, P.; Kumar, R.; Kumar, A. BOD beads—A ready to use seeding material for estimation of organic load of wastewater. Anal. Methods 2012, 4, 4101–4106. [Google Scholar]
- Sergio, A.M.D.; Bustos, T.Y. Biodegradation of wastewater pollutants by activated sludge encapsulated inside calcium-alginate beads in a tubular packed bed reactor. Biodegradation 2009, 20, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Elkahlout, K.; Alipour, S.; Eroglu, I.; Gunduz, U.; Yucel, M. Long-term biological hydrogen production by agar immobilized Rhodobacter capsulatus, in a sequential batch photobioreactor. Bioprocess Biosyst. Eng. 2017, 40, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Fijałkowski, K.; Peitler, D.; Rakoczy, R.; Ẑywicka, A. Survival of probiotic lactic acid bacteria immobilized in different forms of bacterial cellulose in simulated gastric juices and bile salt solution. LWT Food Sci. Technol. 2015, 68, 322–328. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Wang, Y.; Xu, R.; Sun, Z.; Lie, Z. An innovative reactor-type biosensor for BOD rapid measurement. Biosens. Bioelectron. 2010, 25, 1705–1709. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yan, S.; Shi, Y. Direct electrochemical analysis of glucose oxidase on a graphene aerogel/gold nanoparticle hybrid for glucose biosensing. J. Solid State Electrochem. 2015, 19, 307–314. [Google Scholar] [CrossRef]
- Qiu, H.; Guan, Y.; Luo, P.; Wang, Y. Recent advance in fabricating monolithic 3D porous graphene and their applications in biosensing and biofuel cells. Biosens. Bioelectron. 2017, 89, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Zanon, E.; Mancini, A.; Pavanello, I.G.; Bertolino, M.; Grosso, M. A Review on Thermal Exfoliation of Graphene Oxide. J. Mater. Res. 2013, 95, 5343–5350. [Google Scholar]
- Liu, J.; Wang, X.; Wang, T.; Li, D.; Xi, F.; Wang, J.; Wang, E. Functionalization of monolithic and porous three-dimensional graphene by one-step chitosan electrodeposition for enzymatic biosensor. ACS Appl. Mater. Interfaces 2014, 6, 19997–20002. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, Q.; Gao, S.; Song, Q.; Huang, R.; Liu, L.; Dai, J.; Tang, M.; Cheng, G. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci. Rep. UK 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Ormategui, N.; Veloso, A.; Leal, G.P.; Rodriguez-Couto, S.; Tomovska, R. Design of Stable and Powerful Nanobiocatalysts, Based on Enzyme Laccase Immobilized on Self-Assembled 3D Graphene/Polymer Composite Hydrogels. ACS Appl. Mater. Interfaces 2015, 7, 14104–14112. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, R.; Chen, K.; Song, Z. Mediated microbial biosensor using Bacillus subtilis for wastewater BOD measurement. Biochem. Eng. J. 2013, 8, 219–225. [Google Scholar]
- Sonnenfeld, E.M.; Beveridge, T.J.; Koch, A.L.; Doyle, R.J. Asymmetric distribution of charge on the cell wall of Bacillus subtilis. J. Bacteriol. 1985, 163, 1167–1170. [Google Scholar] [PubMed]
- Fein, J.B.; Daughney, C.J.; Yee, N.; Davis, T.A. A chemical equilibrium model for metal adsorption onto bacterial surfaces. Geochim. Cosmochim. Acta 1997, 61, 3319–3328. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, H.; Yang, F.; Yang, X. Biofilm-Engineered Nanostructures. Adv. Mater. 2009, 21, 2815–2818. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Zhao, Y.; Cheng, H.; Hu, C.; Jiang, L.; Qu, L. Three-dimensional graphene-polypyrrole hybrid electrochemical actuator. Nanoscale 2012, 4, 7563–7568. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lin, X. Overoxidized polypyrrole film directed DNA immobilization for construction of electrochemical micro-biosensors and simultaneous determination of serotonin and dopamine. Anal. Chim. Acta 2005, 537, 145–151. [Google Scholar] [CrossRef]
- Le, D.Q.; Takai, M.; Suekuni, S. Development of an observation platform for bacterial activity using polypyrrole films doped with bacteria. Anal. Chem. 2015, 87, 4047. [Google Scholar] [CrossRef] [PubMed]
- State Environmental Protection Administration. Water Quality Standard for Determination of Biochemical Oxygen Demand; HJ/T 86-2002; State Environmental Protection Administration: Beijing, China, 2002.
- Li, Y.; Sun, J.; Wang, J.; Bian, C.; Tong, J.; Xia, S. A single-layer structured microbial sensor for fast detection of biochemical oxygen demand. Biochem. Eng. J. 2016, 112, 219–225. [Google Scholar] [CrossRef]
- Hu, J.; Gao, G.; Xia, S. A Mediated BOD Microsensor Based on Poly (Neutral Red) and Bacteria Modified Interdigited Ultramicroelectrode Array. Int. J. Electrochem. Sci. 2016, 11, 6387–6402. [Google Scholar] [CrossRef]
- Morris, K.; Zhao, H.; John, R. Ferricyanide-Mediated Microbial Reactions for Environmental Monitoring. Aust. J. Chem. 2005, 36, 237–245. [Google Scholar] [CrossRef]
- Pasco, N.; Baronian, K.; Jeffries, C.; Hay, J. Biochemical mediator demand—A novel rapid alternative for measuring biochemical oxygen demand. Appl. Microbiol. Biotechnol. 2000, 53, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Chang, H.; Wu, R. Applied novel sensing material graphene/polypyrrole for humidity sensor. Sens. Actuators B Chem. 2013, 181, 326–331. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process. ACS Nano 2010, 4, 4324–4327. [Google Scholar] [CrossRef] [PubMed]
- Samonin, V.V.; Elikova, E.E. A study of the adsorption of bacterial cells on porous materials. Microbiology 2004, 73, 696–701. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Zhou, J.; Bian, L.; Zhu, E.; Hai, J.; Tang, J.; Tang, W. Graphene/polypyrrole intercalating nanocomposites as supercapacitors electrode. Electrochim. Acta 2013, 112, 44–52. [Google Scholar] [CrossRef]
- Sugama, T. Hydrothermally Self-Advancing Hybrid Coatings for Mitigating Corrosion of Carbon Steel; Brook Haven National Laboratory: Upton, NY, USA, 2006. [Google Scholar] [CrossRef]
- Ke, Y.; Dong, H. Handbook of Analytical Chemistry: Spectrochemical Analysis; Chemical Industry Press: Beijing, China, 1999. [Google Scholar]
- Bose, S.; Mishra, A.; Kuila, T.; Kim, N.H.; Park, O.; Lee, J. Tunable electrical conductivity and dielectric properties of triglycine sulfate-polypyrrole composite. Chem. Eng. J. 2012, 187, 334–340. [Google Scholar] [CrossRef]
- Kinoshita, T.; Hatsuoka, Y.; Nguyen, D.Q.; Iwata, R.; Shiigi, H.; Tsutomu, N. Electrochemical Response of Acridine Orange in Bacterial Cell. Electrochem. Soc. Jpn. 2016, 84, 334–337. [Google Scholar] [CrossRef]
- Rathnayake, V.; Megharaj, M.; Krishnamurti, G.S.; Bolan, N.S.; Naidu, R. Heavy metal toxicity to bacteria—Are the existing growth media accurate enough to determine heavy metal toxicity? Chemosphere 2013, 90, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Yano, K.; Morita, T. A mediator-type biosensor as a new approach to biochemical oxygen demand estimation. Analyst 2000, 125, 2280–2284. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ye, T.; Qiu, B.; Chen, G.; Chen, X. A novel approach based on ferricyanide-mediator immobilized in an ion-exchangeable biosensing film for the determination of biochemical oxygen demand. Anal. Chim. Acta 2008, 612, 75. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Deng, L.; Yong, D.; Dong, S. Native biofilm cultured under controllable condition and used in mediated method for BOD measurement. Talanta 2011, 84, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shang, L.; Liu, C.; Zhang, B.; Dong, S. A new mediator method for BOD measurement under non-deaerated condition. Talanta 2010, 81, 1170–1175. [Google Scholar] [CrossRef] [PubMed]



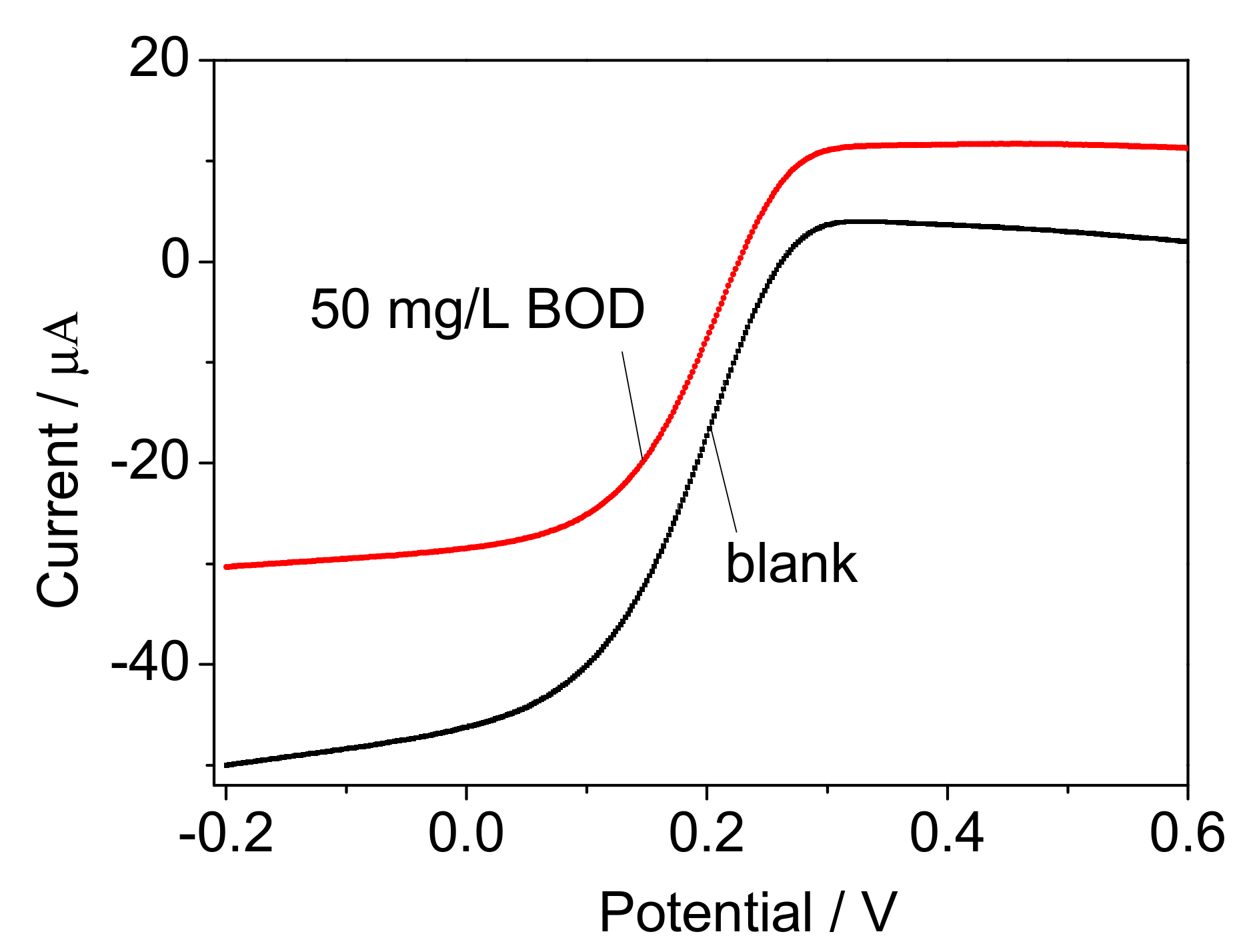

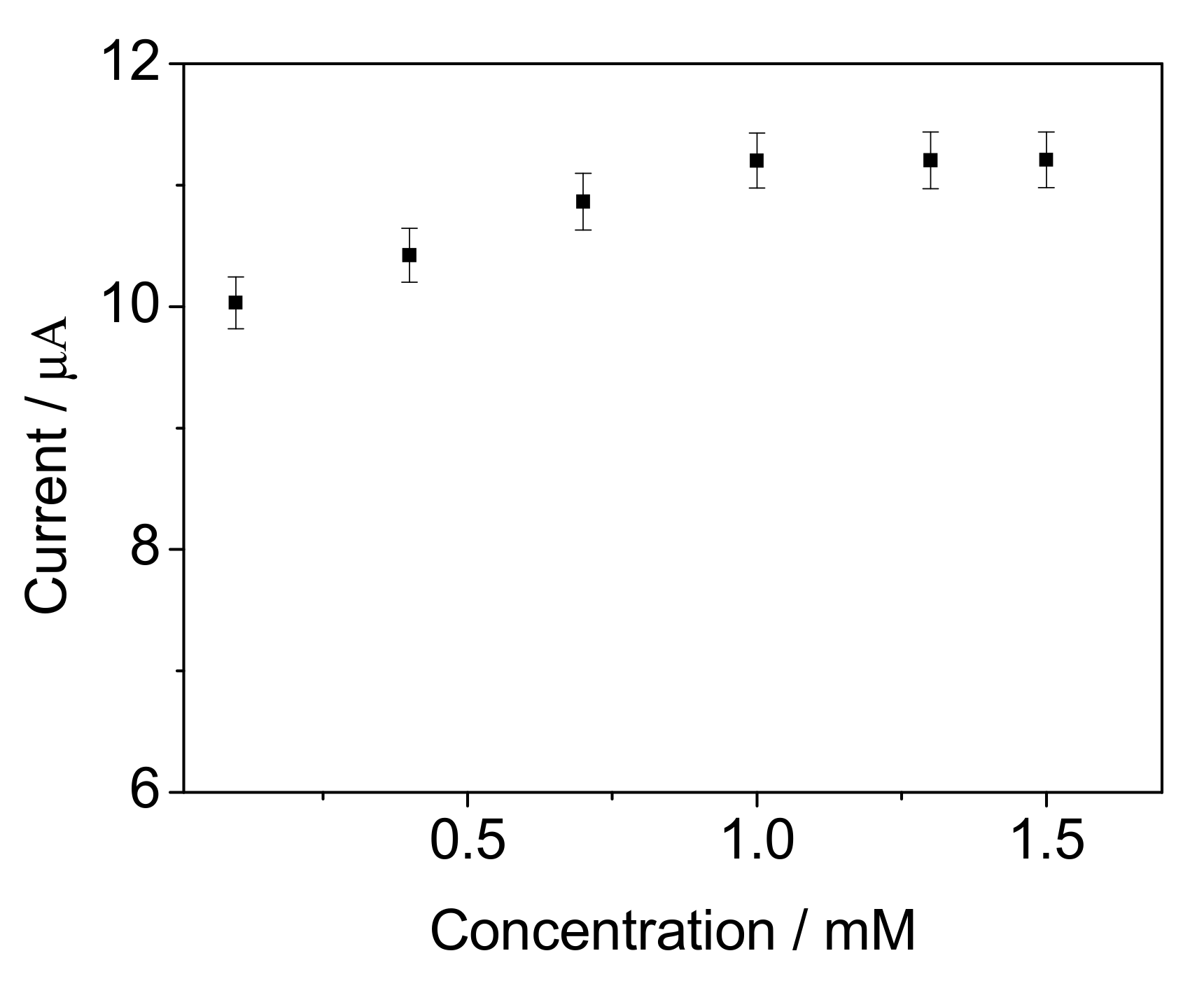

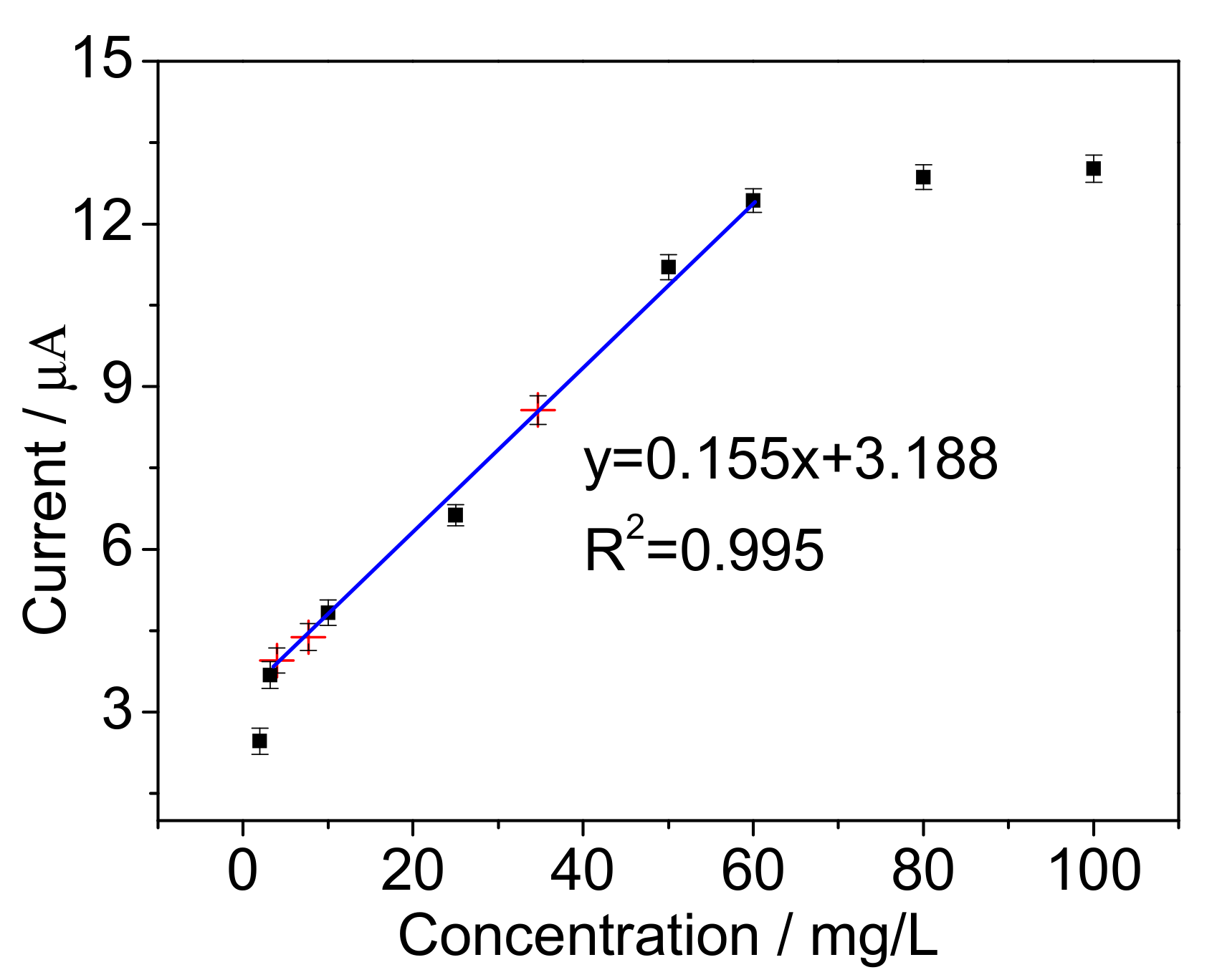
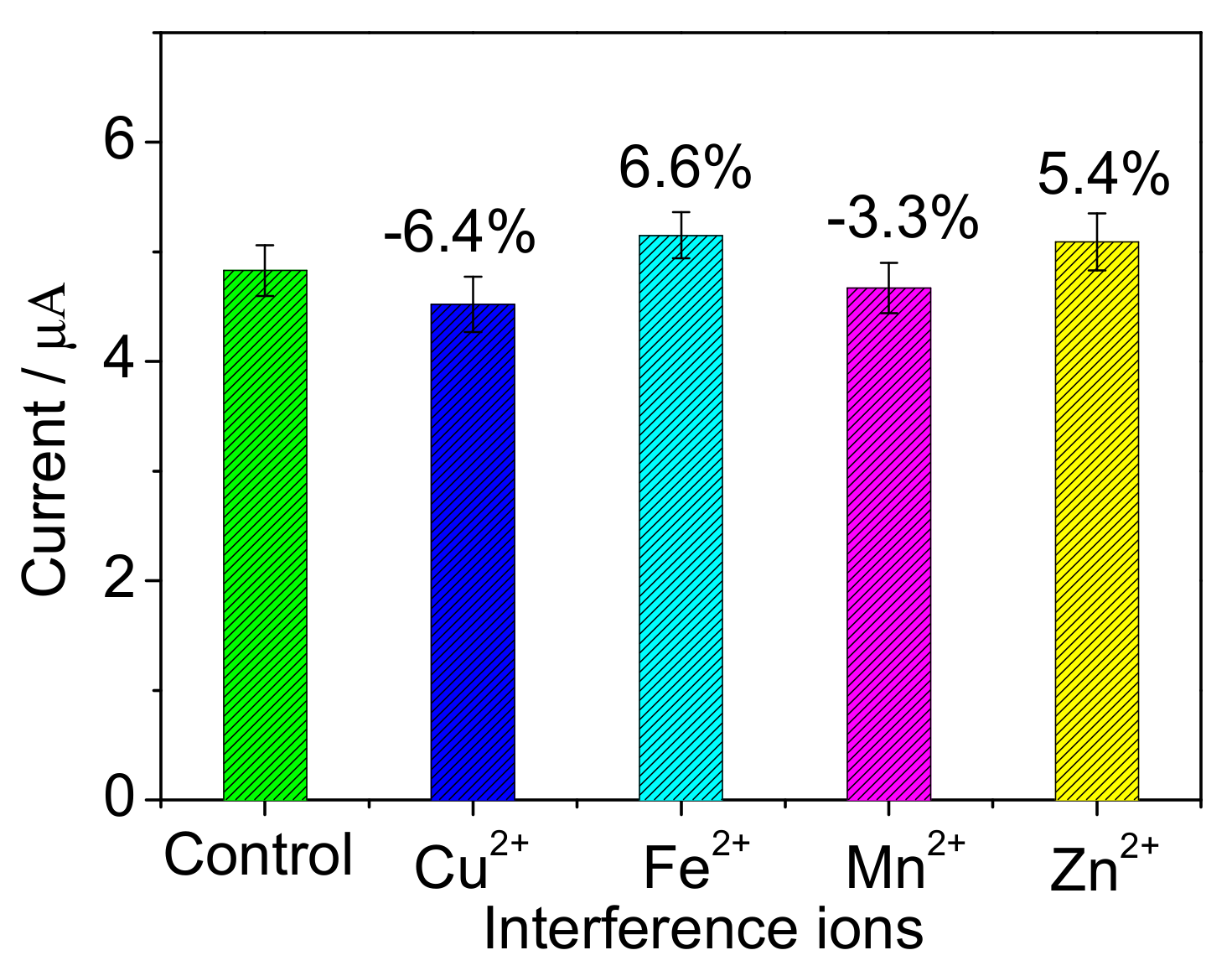
| Support | Before Immobilization (mg) | After Immobilization (mg) | ImmobilizedCells (mg) |
|---|---|---|---|
| rGO | 20 ± 2 | 40 ± 7 | 20 ± 5 |
| rGO-Py | 20 ± 2 | 100 ± 10 | 80 ± 8 |
| rGO-PPy | 20 ± 2 | 140 ± 12 | 120± 10 |
| Cell Concentration (CFU/mL) | Current Value for BOD Measurement (µA) | Regression Equation | Linear Correlation | ||
|---|---|---|---|---|---|
| 5 mg/L BOD | 10 mg/L BOD | 25 mg/L BOD | |||
| 0.2 × 107 | 0.91 ± 0.23 | 1.02 ± 0.21 | 2.1 ± 0.21 | y = 0.083x + 0.37 | 0.938 |
| 1.1 × 107 | 2.01 ± 0.22 | 2.82 ± 0.14 | 3.43 ± 0.21 | y = 0.089x + 1.705 | 0.928 |
| 5.4 × 107 | 2.96 ± 0.19 | 3.63 ± 0.13 | 4.32 ± 0.17 | y = 0.087x + 2.615 | 0.967 |
| 9.8 × 107 | 4.16 ± 0.18 | 4.83 ± 0.23 | 5.99 ± 0.19 | y = 0.122x + 3.553 | 0.998 |
| 19.7 × 107 | 6.03 ± 0.25 | 6.24 ± 0.27 | 6.31 ± 0.24 | y = 0.017x ± 5.995 | 0.794 |
| Support | Microorganism | Mediator | Linear Range (mg/L) | Detection Limit (mg/L) | Response Time (min) | Stability | Reference |
|---|---|---|---|---|---|---|---|
| PVA–SbQ | Bacteria isolated from active sludge | HCF (III) | 15–200 | -- | 15 | 7 | [40] |
| Ormodils-PVA | E. marius, B. horikoshii, H. marina | ferricyanide | 1.2–40 | 0.8 | 30 | 1 h | [41] |
| Carbon fiber felt | Bacteria isolated from waste water | ferricyanide | 12–100 | -- | 60 | 110 d | [42] |
| No support | E. coil | ferricyanide | 5–400 | -- | 60 | -- | [43] |
| PPy | P. aeruginosa | Poly (neutral red) | 5–100 | 3 | 20 | 10 | [29] |
| 3D porous rGO-PPy | B. subtilis | ferricyanide | 4–60 | 1.8 | 15 | 60 | This study |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Li, Y.; Gao, G.; Xia, S. A Mediated BOD Biosensor Based on Immobilized B. Subtilis on Three-Dimensional Porous Graphene-Polypyrrole Composite. Sensors 2017, 17, 2594. https://doi.org/10.3390/s17112594
Hu J, Li Y, Gao G, Xia S. A Mediated BOD Biosensor Based on Immobilized B. Subtilis on Three-Dimensional Porous Graphene-Polypyrrole Composite. Sensors. 2017; 17(11):2594. https://doi.org/10.3390/s17112594
Chicago/Turabian StyleHu, Jingfang, Yueqi Li, Guowei Gao, and Shanhong Xia. 2017. "A Mediated BOD Biosensor Based on Immobilized B. Subtilis on Three-Dimensional Porous Graphene-Polypyrrole Composite" Sensors 17, no. 11: 2594. https://doi.org/10.3390/s17112594




