Development of Ratiometric Fluorescent Biosensors for the Determination of Creatine and Creatinine in Urine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
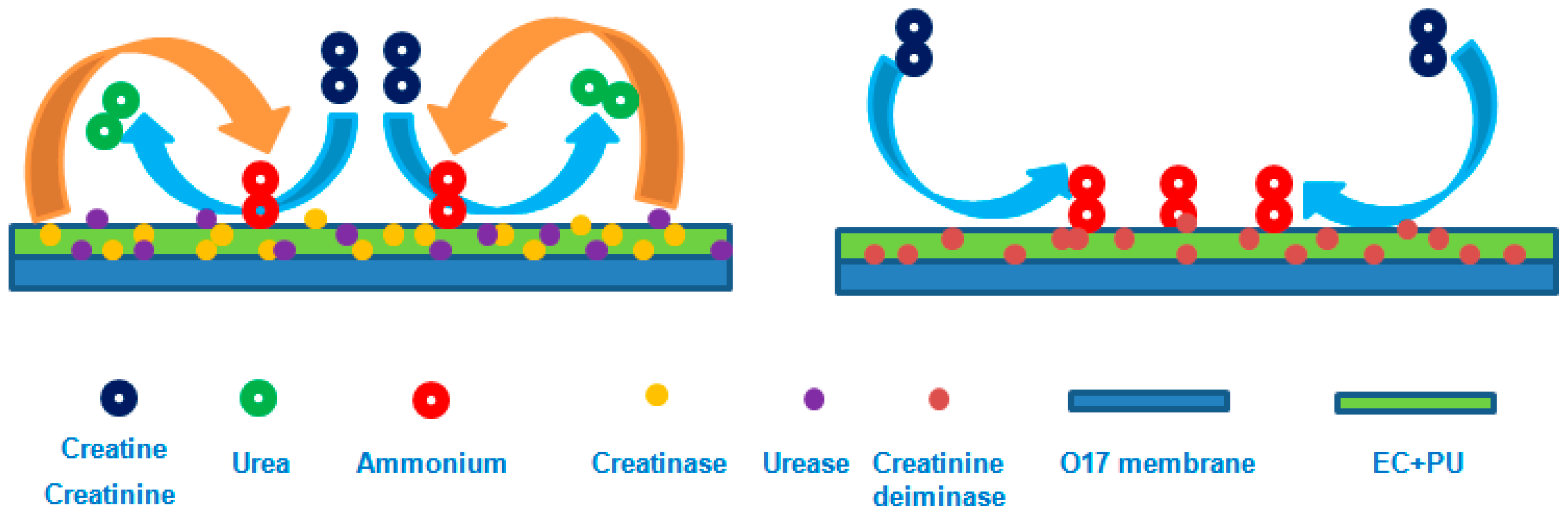
2.2. Preparation of Creatine- and Creatinine-Sensing Membranes
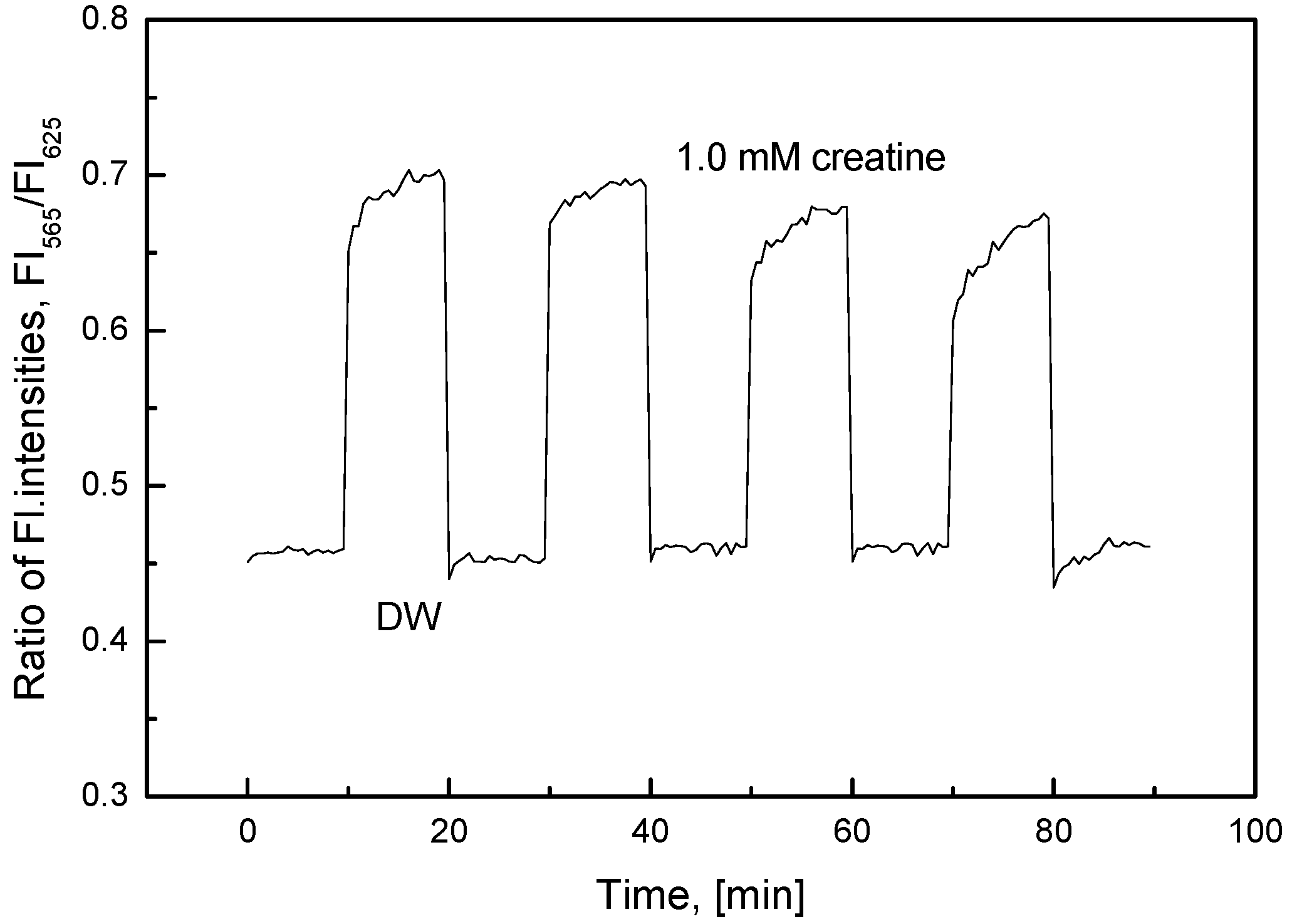
2.3. Measurements of Creatine and Creatinine
2.3.1. Creatine Measurements
2.3.2. Creatinine Measurements
2.3.3. Artificial Urine Solution (AUS)
2.4. Ratiometric Method
2.5. Data Analysis
3. Results and Discussion
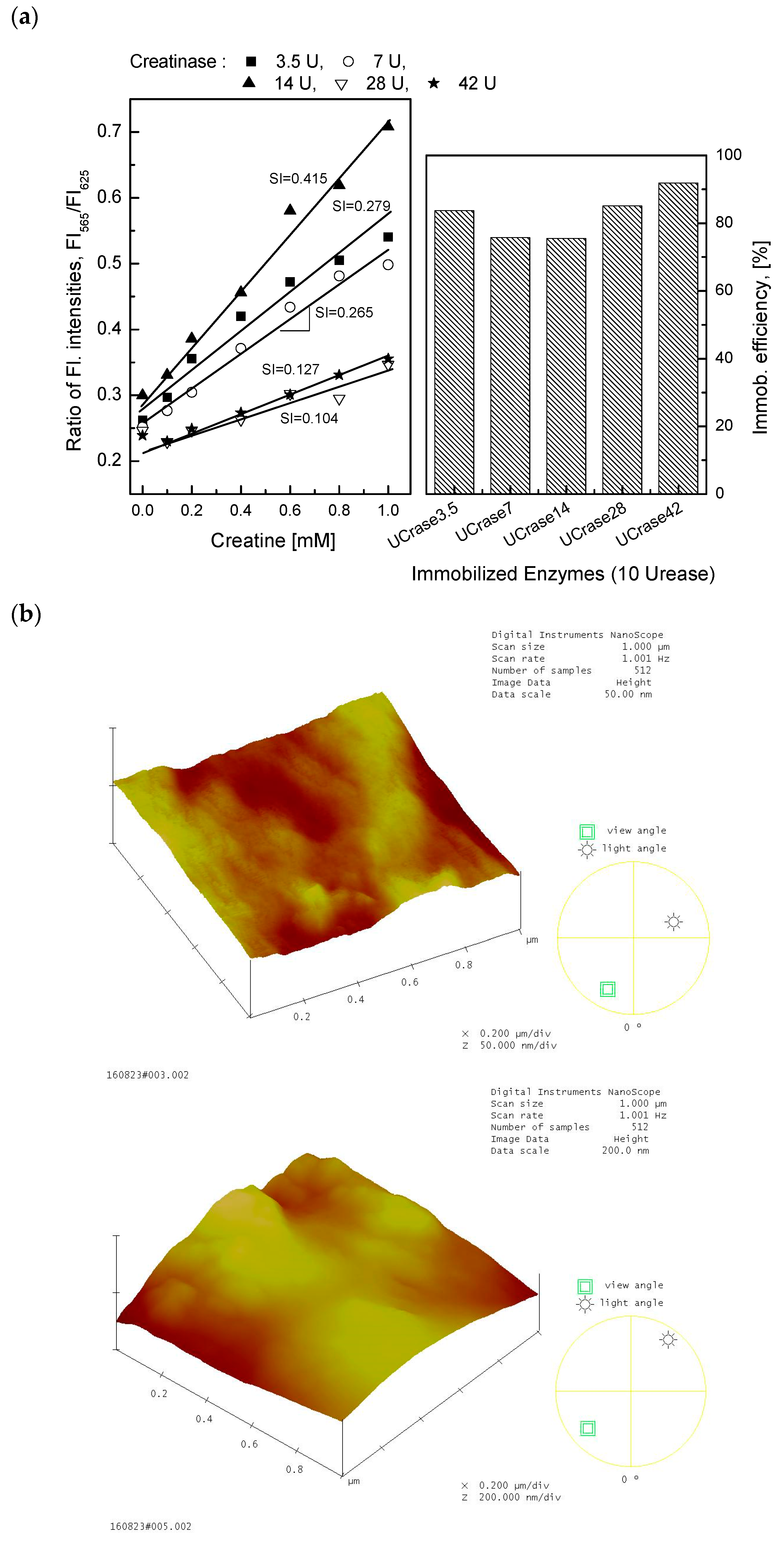
3.1. Enzyme Immobilization for Creatine-Sensing Membrane
3.2. Characterization of the Creatine-Sensing Membrane
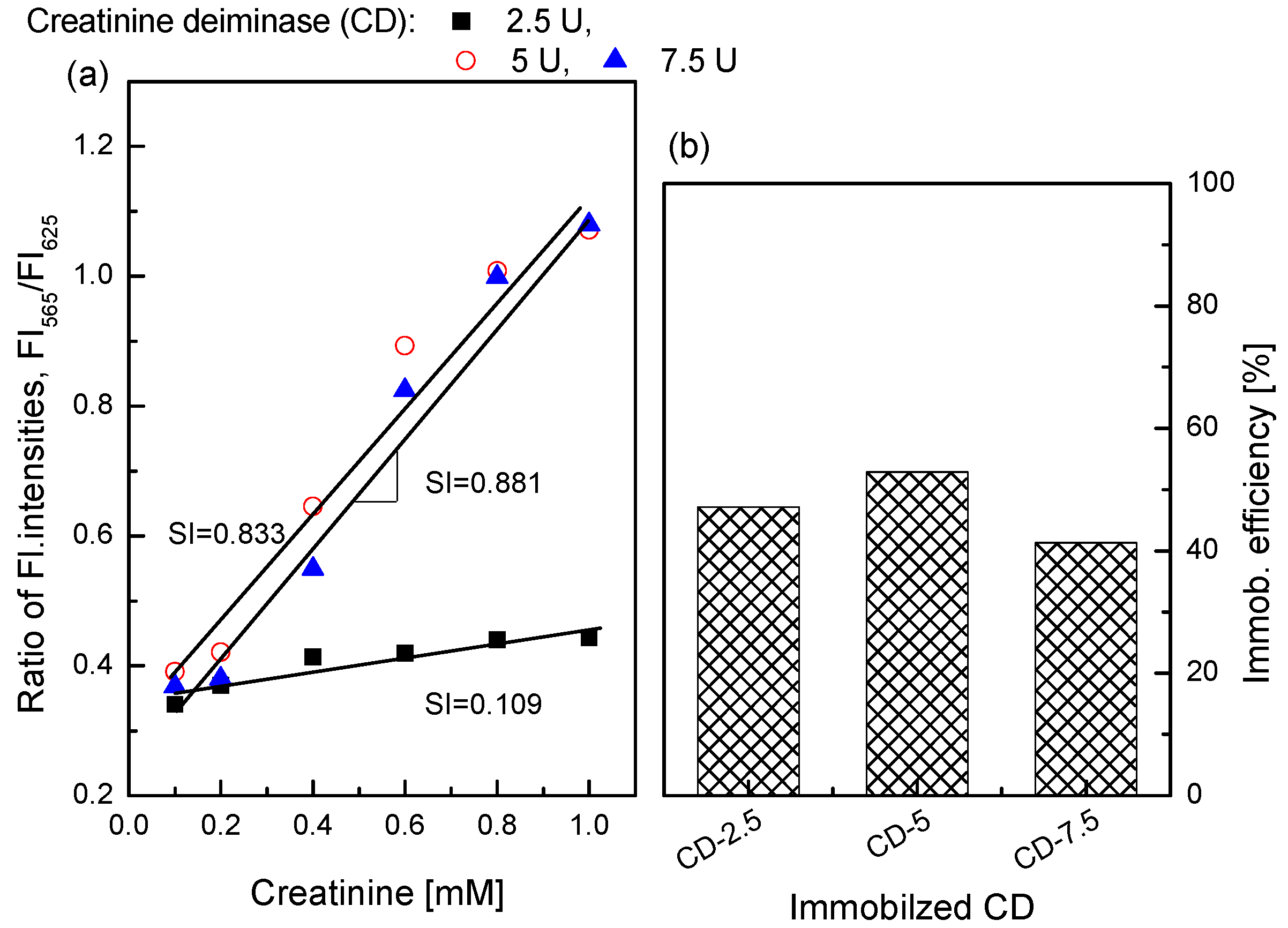
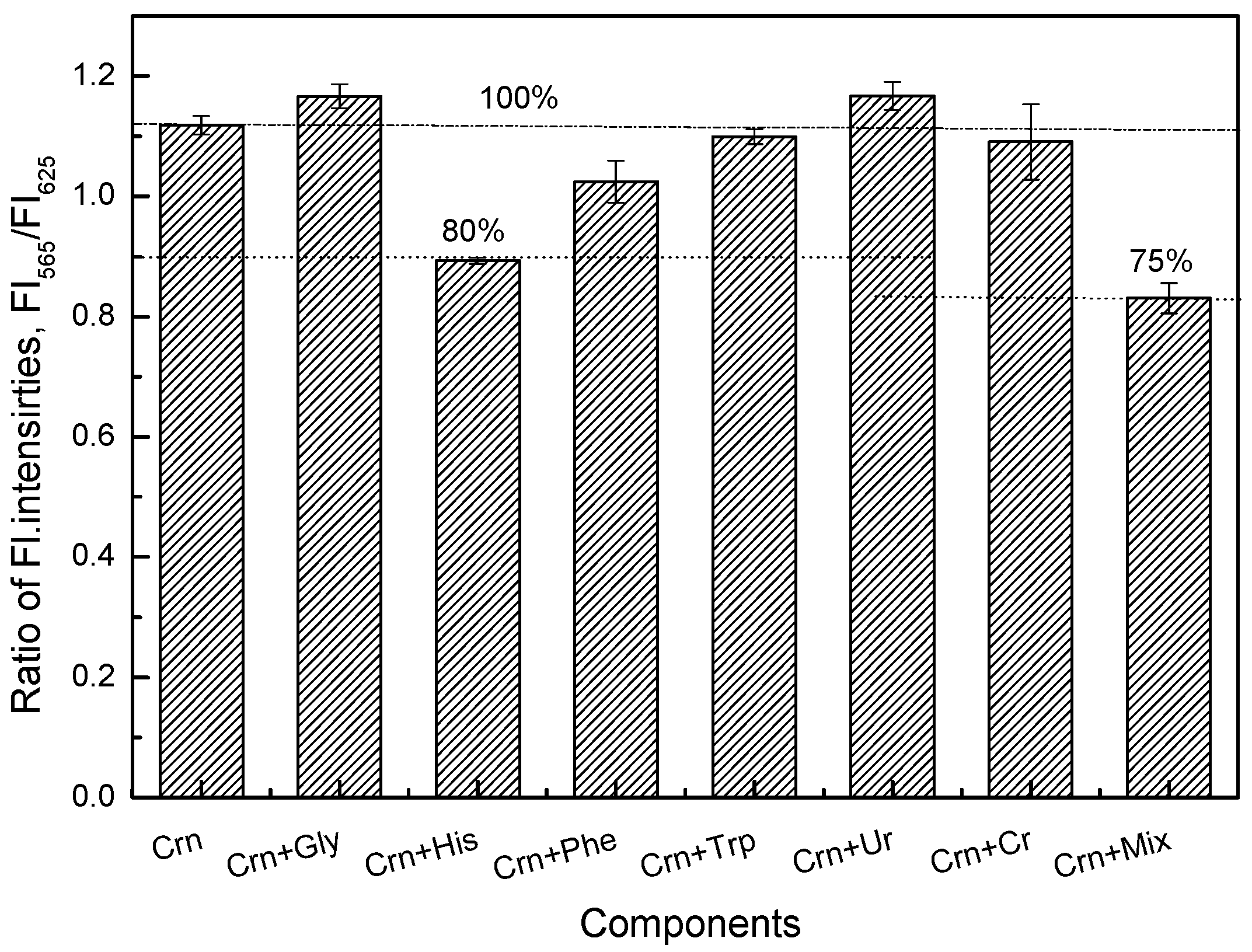
3.3. Enzyme Immobilization for the Creatinine-Sensing Membrane
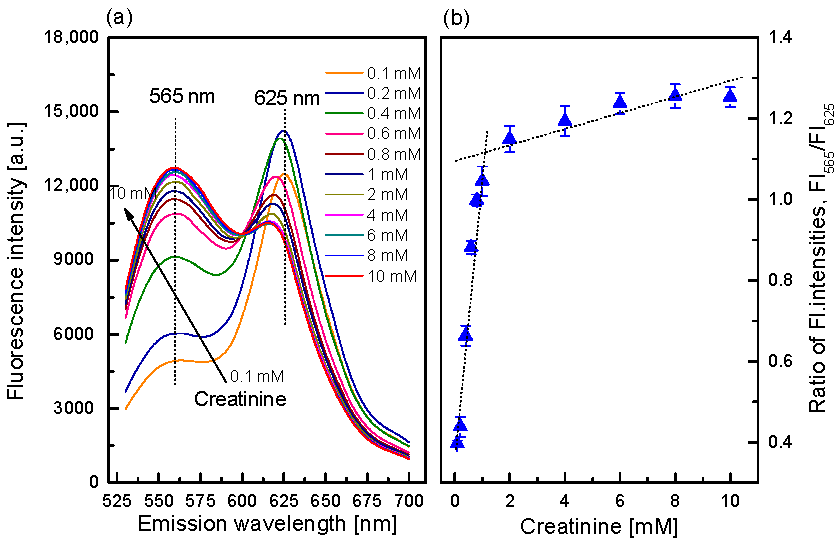
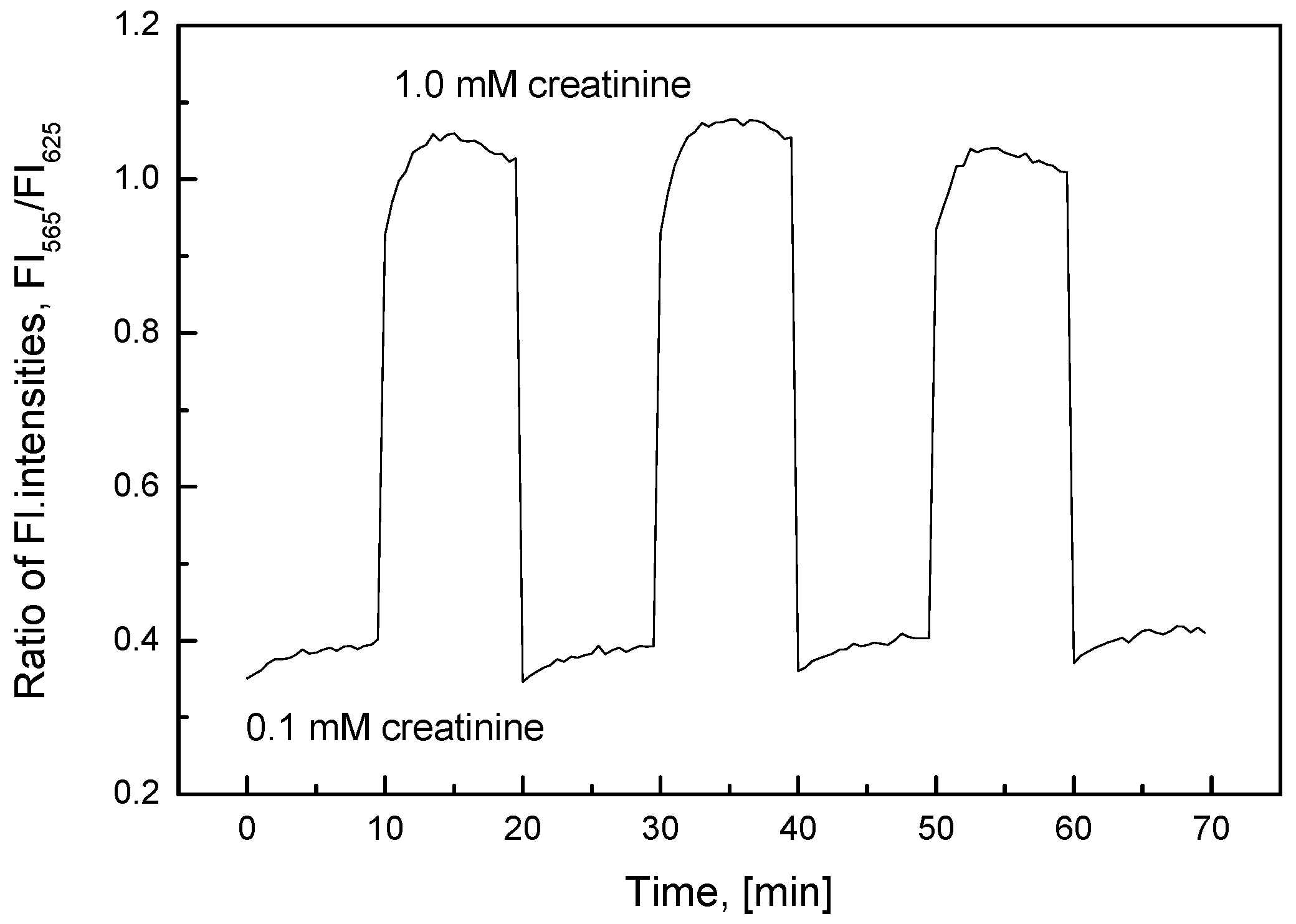
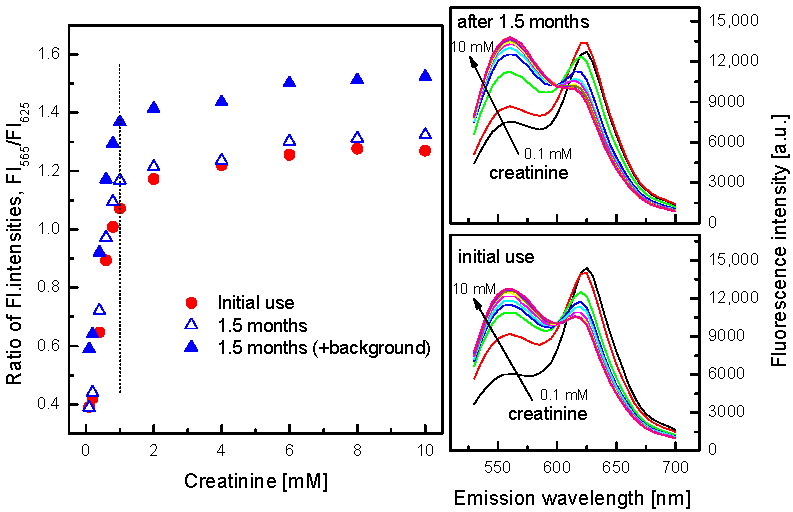
3.4. Characterization of the Creatinine-Sensing Membrane
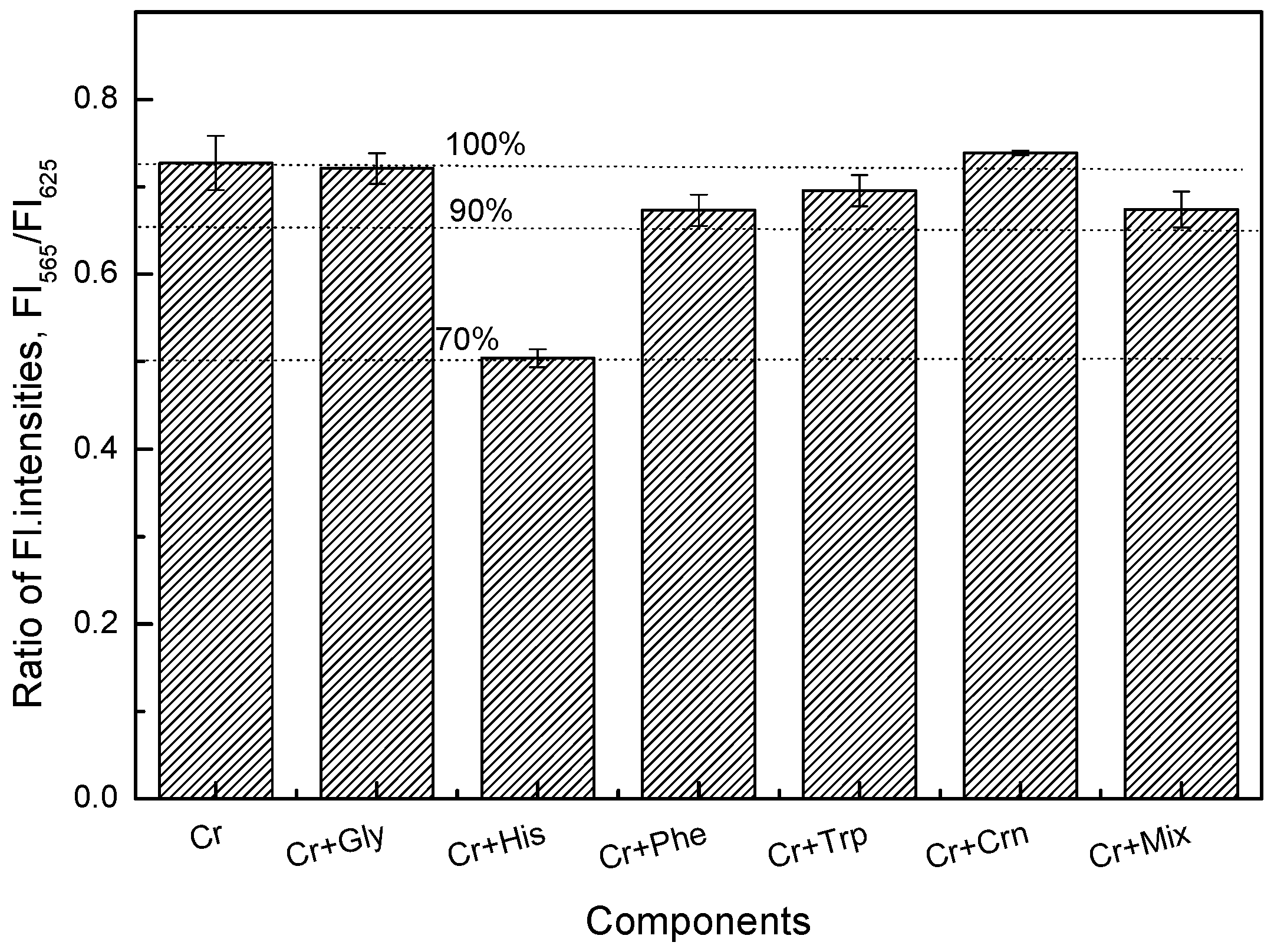
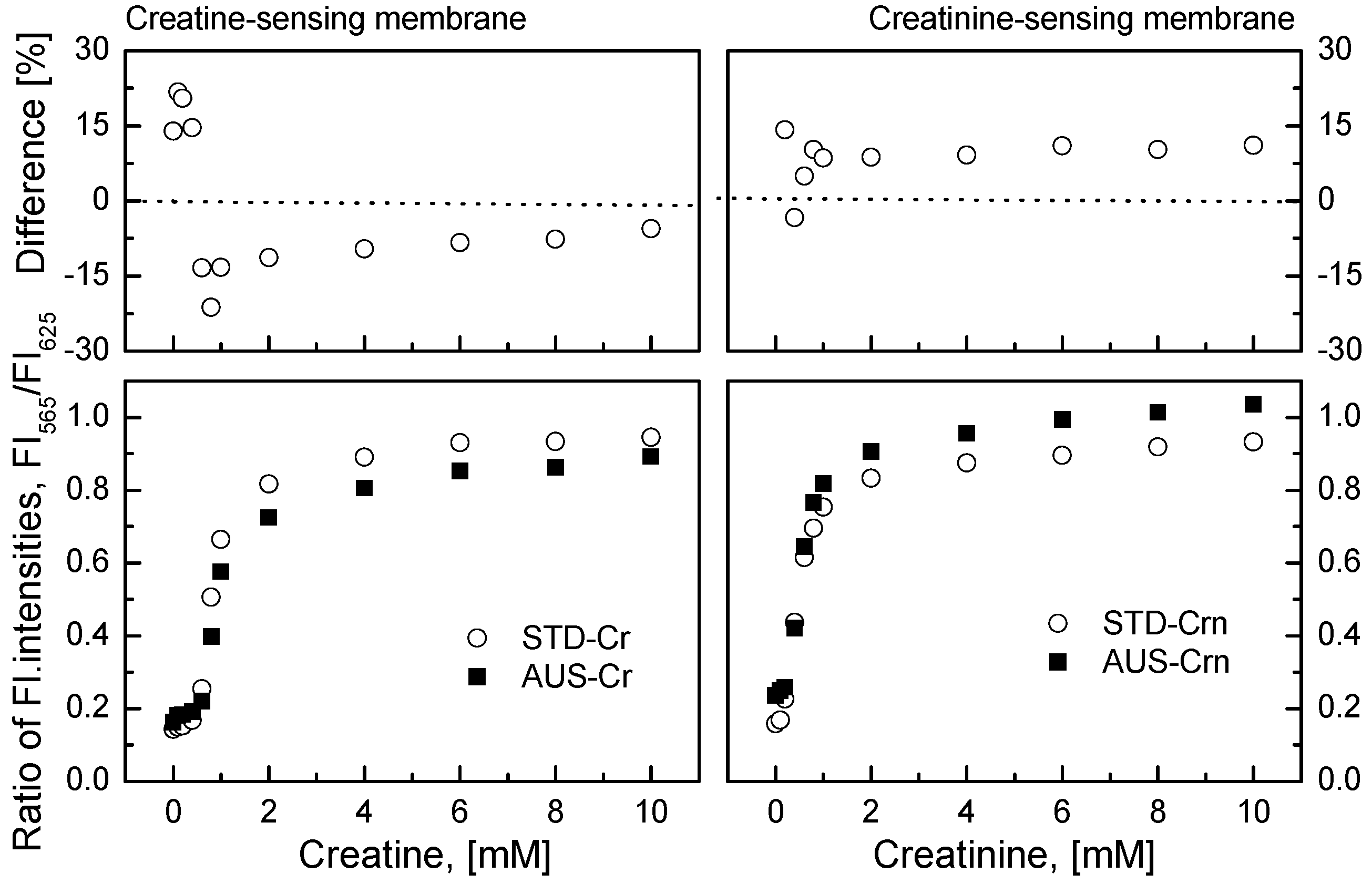
3.5. Determination of Creatine and Creatinine in Artificial Urine Solution (AUS)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wyes, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar]
- Greenhaff, P.L.; Casey, A.; Short, A.H.; Harris, R.; Soderlund, K.; Hultman, E. Influence of oral creatine supplementation of muscle torque during repeated bouts-of maximal voluntary exercise in man. Clin. Sci. 1993, 84, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.H.; Branch, J.D. Creatine supplementation and exercise performance: An update. J. Am. Coll. Nutr. 1998, 17, 216. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Sentandreu, M.A.; Toldra, F. Contents of creatine, creatinine and carnosine in porcine muscles of different metabolic types. Meat Sci. 2008, 79, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Koncki, R.; Walcerz, I.; Ruckruh, F.; Glab, S. Bienzymatic potentiometric electrodes for creatine and L-arginine Determination. Anal. Chim. Acta 1996, 333, 215–222. [Google Scholar] [CrossRef]
- Karakus, E.; Pekyardımcı, S.; Kılıc, E. Potentiometric bienzymatic biosensor based on PVC membrane containing palmitic acid for determination of creatine. Proc. Biochem. 2006, 41, 1371–1377. [Google Scholar] [CrossRef]
- Madarag, M.B.; Popescu, I.C.; Ufer, S.; Buck, R.P. Microfabricated amperometric creatine and creatinine biosensors. Anal. Chim. Acta 1996, 319, 335–345. [Google Scholar] [CrossRef]
- Ramanavicius, A. Amperometric biosensor for the determination of creatine. Anal. Bioanal. Chem. 2007, 387, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Kacar, C.; Erden, P.E.; Pekyardimci, S.; Kilic, E. An Fe3O4-nanoparticles-based amperometric biosensor for creatine determination. Artif. Cells Nanomed. Biotechnol. 2013, 41, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Kotegawa, K. Simultaneous flow-injection assay of creatinine and creatine in serum by the combined use of a 16-way switching valve, some specific enzyme reactors and a highly selective hydrogen peroxide electrode. Anal. Chim. Acta 2002, 462, 283–291. [Google Scholar] [CrossRef]
- Dash, A.K.; Sawhney, A. A simple LC method with UV detection for the analysis of creatine and creatinine and its application to several creatine Formulations. J. Pharm. Biomed. Anal. 2002, 29, 939–945. [Google Scholar] [CrossRef]
- Smith-Palmer, T. Separation methods applicable to urinary creatine and creatinine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002, 781, 93–106. [Google Scholar] [CrossRef]
- Fernandez-Fernanadez, M.; Rodríguez-Gonzalez, P.; Alvarez, M.E.A.; Rodríguez, F.; Menendez, F.V.A.; Alonso, J.I.G. Simultaneous determination of creatinine and creatine in human serum by double-spike isotope dilution liquid chromatography–tandem mass spectrometry (LC-MS/MS) and gas chromatography−mass spectrometry (GC-MS). Anal. Chem. 2015, 87, 3735–3763. [Google Scholar] [CrossRef] [PubMed]
- Liotta, E.; Gottardo, R.; Bonizzato, L.; Pascali, J.P.; Bertaso, A.; Tagliaro, F. Rapid and direct determination of creatinine in urine using capillary zone electrophoresis. Clin. Chim. Acta 2009, 409, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Miura, C.; Funaya, N.; Matsunaga, H.; Haginaka, J. Monodisperse, molecularly imprinted polymers for creatinine by modified precipitation polymerization and their applications to creatinine assays for human serum and urine. J. Pharm. Biomed. Anal. 2013, 85, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Mohabbati-Kalejahi, E.; Azimirad, V.; Bahrami, M.; Ganbari, A. A review on creatinine measurement techniques. Talanta 2012, 97, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pundir, C.S.; Yadav, S.; Kumar, A. Creatinine sensors. Trends Anal. Chem. 2013, 50, 42–52. [Google Scholar] [CrossRef]
- Randvir, E.P.; Banks, C.E. Analytical methods for quantifying creatinine within biological media. Sens. Actuators B 2013, 183, 239–252. [Google Scholar] [CrossRef]
- Lad, U.; Khokhar, S.; Kale, G.M. Electrochemical creatinine biosensors. Anal. Chem. 2008, 80, 7910–7917. [Google Scholar] [CrossRef] [PubMed]
- Killard, A.J.; Smyth, M.R. Creatinine biosensors: Principles and designs. Trends Biotechnol. 2000, 18, 433–437. [Google Scholar] [CrossRef]
- Shih, Y.T.; Huang, H.J. A creatinine deiminase modified polyaniline electrode for creatinine analysis. Anal. Chim. Acta 1999, 392, 143–150. [Google Scholar] [CrossRef]
- Yadav, S.; Kumar, A.; Pundir, C.S. Amperometric creatinine biosensor based on covalently coimmobilized enzymes onto carboxylated multiwalled carbon nanotubes/polyaniline composite film. Anal. Biochem. 2011, 419, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Radomska, A.; Bodenszac, E.; Glab, S.; Koncki, R. Creatinine biosensor based on ammonium ion selective electrode and its application in flow-injection analysis. Talanta 2004, 64, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Zinchenkoa, O.A.; Marchenkoa, S.V.; Sergeyevaa, T.A.; Kuklab, A.L.; Pavlyuchenkob, A.S.; Krasyukc, E.K.; Soldatkina, A.P.; El’skayaa, A.V. Application of creatinine-sensitive biosensor for hemodialysis control. Biosens. Bioelectron. 2012, 35, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Soldatkin, A.P.; Montoriol, J.; Sant, W.; Martelet, C.; Jaffrezic-Renault, N. Creatinine sensitive biosensor based on ISFETs and creatinine deiminase immobilised in BSA membrane. Talanta 2002, 58, 351–357. [Google Scholar] [CrossRef]
- Sant, W.; Pourciel-Gouzy, M.L.; Launay, J.; Conto, T.D.; Colin, R.; Martinez, A.; Temple-Boyer, P. Development of a creatinine-sensitive sensor for medical analysis. Sens. Actuators B 2004, 103, 260–264. [Google Scholar] [CrossRef]
- Marchenko, S.V.; Soldatkin, O.O.; Kasap, B.O.; Kurc, B.A.; Soldatkin, A.P.; Dzyadevych, S.V. Creatinine deiminase adsorption onto silicalite-modified pH-FET for creation of new creatinine-sensitive biosensor. Nanoscale Res. Lett. 2016, 11, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhu, B.; Leow, W.R.; Chen, S.; Sum, T.C.; Peng, X.; Chen, X. Colorimetric detection of creatinine based on plasmonic nanoparticles via synergistic coordination chemistry. Small 2015, 11, 4104–4110. [Google Scholar] [CrossRef] [PubMed]
- Taussky, H.H. A procedure increasing the specificity of the Jaffe reaction for the determination of creatine and creatinine in urine and plasma. Clin. Chim. Acta 1956, 1, 210–224. [Google Scholar] [CrossRef]
- Stefan, R.-L.; Bokretsion, R.G. Determination of creatine and creatinine using a diamond paste based electrode. Instum. Sci. Technol. 2003, 31, 183–188. [Google Scholar] [CrossRef]
- Duong, H.D.; Rhee, J.I. A ratiometric fluorescence sensor for the detection of ammonia. Sens. Actuators B 2014, 190, 768–774. [Google Scholar] [CrossRef]
- Duong, H.D.; Rhee, J.I. Development of a ratiometric fluorescent urea biosensor based on the urease immobilized onto the oxazine 170 perchlorate-ethyl cellulose membrane. Talanta 2015, 134, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Topics in Fluorescence Spectroscopy; Plenum Press: New York, NY, USA, 1994; Volume 4, pp. 3–6. [Google Scholar]
- Nakata, E.; Wang, H.; Hamachi, I. Ratiometric fluorescent biosensor for real-time and label-free monitoring of fine saccharide metabolic pathways. ChemBioChem 2008, 9, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zeng, F.; Wu, S. Ratiometric fluorescent biosensor for hyaluronidase with hyaluronan as both nanoparticle scaffold and substrate for enzymatic reaction. Biomacromolecules 2014, 15, 3383–3389. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.S.; Qi, L.; Zhang, R.L.; Jin, M.; Zhang, Z.Q. Ratiometric fluorescence biosensor based on CdTe quantum and carbon dots for double strand DNA detection. Sens. Actuators B 2017, 244, 585–590. [Google Scholar] [CrossRef]
- Barker, S.L.R.; Clark, H.A.; Swallen, S.F.; Kopelman, R.; Tsang, A.W.; Swanson, J.A. Ratiometric and fluorescence-lifetime-based biosensors incorporating cytochrome c and the detection of extra- and intracellular macrophage nitric oxide. Anal. Chem. 1999, 71, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Dhakate, S.R. Chitosan–SiO2–multiwall carbon nanotubes nanocomposite: A novel matrix for the immobilization of creatine amidinohydrolase. Int. J. Biol. Macromol. 2009, 44, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Weidgans, B.M.; Krause, C.; Klimant, I.; Wolfbeis, O.S. Fluorescent pH sensors with negligible sensitivity to ionic strength. Analyst 2004, 129, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Uwajima, T.; Terada, O. Properties of crystalline creatinine deiminase from Corynebacterium lilium. Agric. Biol. Chem. 1980, 44, 1787–1792. [Google Scholar] [CrossRef]
- Gottschalk, E.M.; Hippe, H.; Patzke, F. Creatinine deiminase (EC 3.5.4.21) from bacterium BN11: Purification, properties and applicability in a serum/urine creatinine assay. Clin. Chim. Acta 1991, 204, 223–238. [Google Scholar] [CrossRef]
- Zhybak, M.; Beni, V.; Vagin, M.Y.; Dempsey, E.; Turner, A.P.F.; Korpan, Y. Creatinine and urea biosensors based on a novel ammonium ion-selective copper-polyaniline nano-composite. Biosens. Bioelectron. 2016, 77, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.C.; Jana, T.; Kesavamoorthy, R.; Shi, L.; Virji, M.A.; Finegold, D.N.; Asher, S.A. A general photonic crystal sensing motif: Creatinine in bodily fluids. J. Am. Chem. Soc. 2004, 126, 2971–2977. [Google Scholar] [CrossRef] [PubMed]
- Hanif, S.; John, P.; Gao, W.; Saqib, M.; Qi, L.; Xu, G. Chemiluminescence of creatinine/H2O2/Co2+ and its application for selective creatinine detection. Biosens. Bioelect. 2016, 75, 347–351. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, X.; Yu, H. Gold nanoparticles-based colorimetric and visual creatinine-assay. Microchim. Acta 2015, 182, 2037–2043. [Google Scholar] [CrossRef]
- Talalak, K.; Noiphung, J.; Songjaroen, T.; Chailapakul, O.; Laiwattanapaisal, W. A facile low-cost enzymatic paper-based assay for the determination of urine creatinine. Talanta 2015, 144, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Ruedas-Rama, M.J.; Hall, E.A.H. Analytical nanosphere sensors using quantum dot-enzyme conjugates for urea and creatinine. Anal. Chem. 2010, 82, 9043–9049. [Google Scholar] [CrossRef] [PubMed]


| Detection Method (Enzymes Used) | Linear Detection Range | Limit of Detection | Response Time | Long-Term Stability | Ref. |
|---|---|---|---|---|---|
| Potentiometry (CTN + URS) | 0.1–30 mM | 10 µM | 1.5–4 min | 14 days | [5] |
| Potentiometry (CTN + URS) | 0.01–1 mM | 10 µM | 1–2 min | 2 months | [6] |
| Amperometry (CTN + SOD) | 0–2 mM | 3.4 µM | 3 min | 3 months | [7] |
| Amperometry (CTN + SOD) | 0–3.5 mM | n.a. | 1 min | 8 days | [8] |
| Amperometry (CTN + SOD) | 0.2–3.8 µM and 9–120 µM | 0.2 µM | 10 s | 30 days | [9] |
| Ratiometric fluorescence (CTN + URS) | 0.1–1 Mm and 1–10 mM | 0.015 mM | 3.5 min | >3 months | This work |
| Detection Method (Enzyme or Assay Used) | Linear Detection Range | Limit of Detection | Response Time | Ref. |
|---|---|---|---|---|
| Light diffraction (CD) | 0.01–0.7 mM | 6 µM | 14 min | [43] |
| Chemiluminescence (H2O2-Co2+) | 0.1–30 µM | 0.07 µM | 1 min | [44] |
| Colorimetry (uric acid-Hg2+-AuNPs) | 1–12 µM | 1.6 nM | 5 min | [28] |
| Colorimetry (citrate-AuNPs) | 0.1–20 mM | 0.72 mM | 24 min | [45] |
| Colorimetry-enzPAD (CNN + CTN + SOD) | 0.22–2.2 mM | 0.17 mM | 11 min | [46] |
| Fluorescence (QDs-CD conj.) | 0.3–5 mM | n.a. | 3–4 min | [47] |
| Ratiometric fluorescence (CD) | 0.1–1.0 mM | 0.0325 mM | 1–3 min | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duong, H.D.; Rhee, J.I. Development of Ratiometric Fluorescent Biosensors for the Determination of Creatine and Creatinine in Urine. Sensors 2017, 17, 2570. https://doi.org/10.3390/s17112570
Duong HD, Rhee JI. Development of Ratiometric Fluorescent Biosensors for the Determination of Creatine and Creatinine in Urine. Sensors. 2017; 17(11):2570. https://doi.org/10.3390/s17112570
Chicago/Turabian StyleDuong, Hong Dinh, and Jong Il Rhee. 2017. "Development of Ratiometric Fluorescent Biosensors for the Determination of Creatine and Creatinine in Urine" Sensors 17, no. 11: 2570. https://doi.org/10.3390/s17112570




