Stable and Fast-Response Capacitive Humidity Sensors Based on a ZnO Nanopowder/PVP-RGO Multilayer
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Sensors
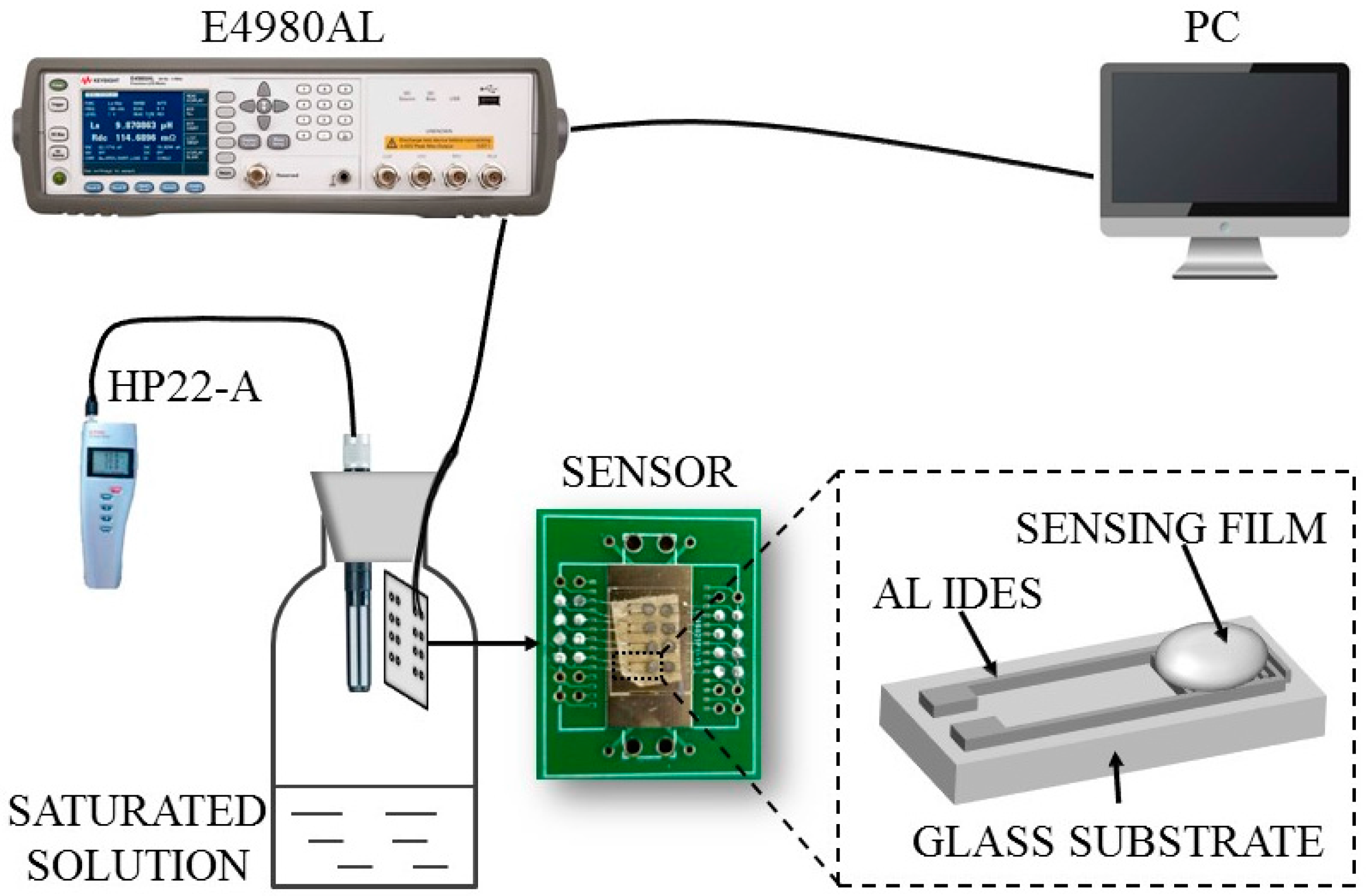
2.3. Material Characterization and Humidity Sensing Measurements
3. Results and Discussion
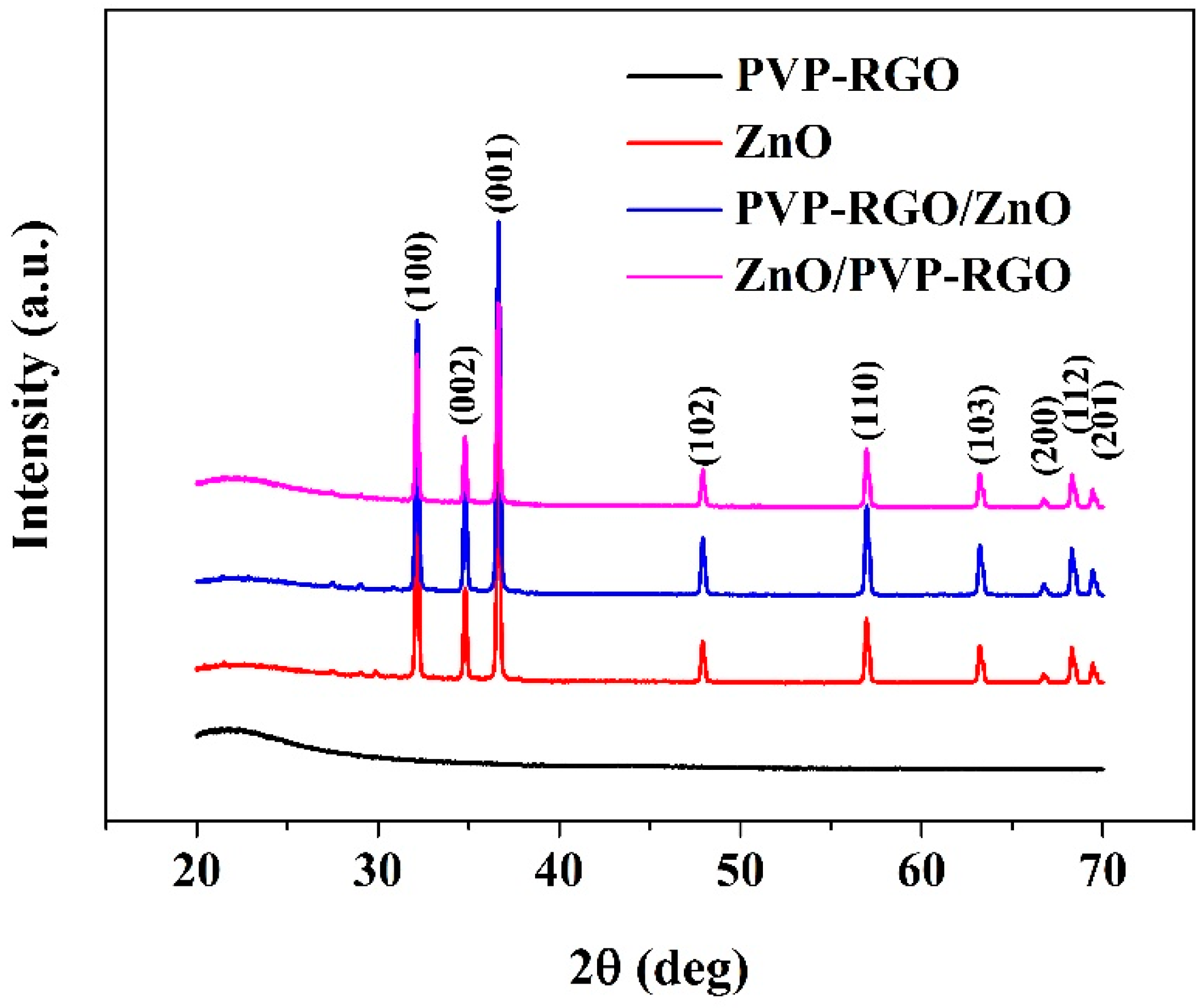
3.1. Microstructure and Morphological Characterization
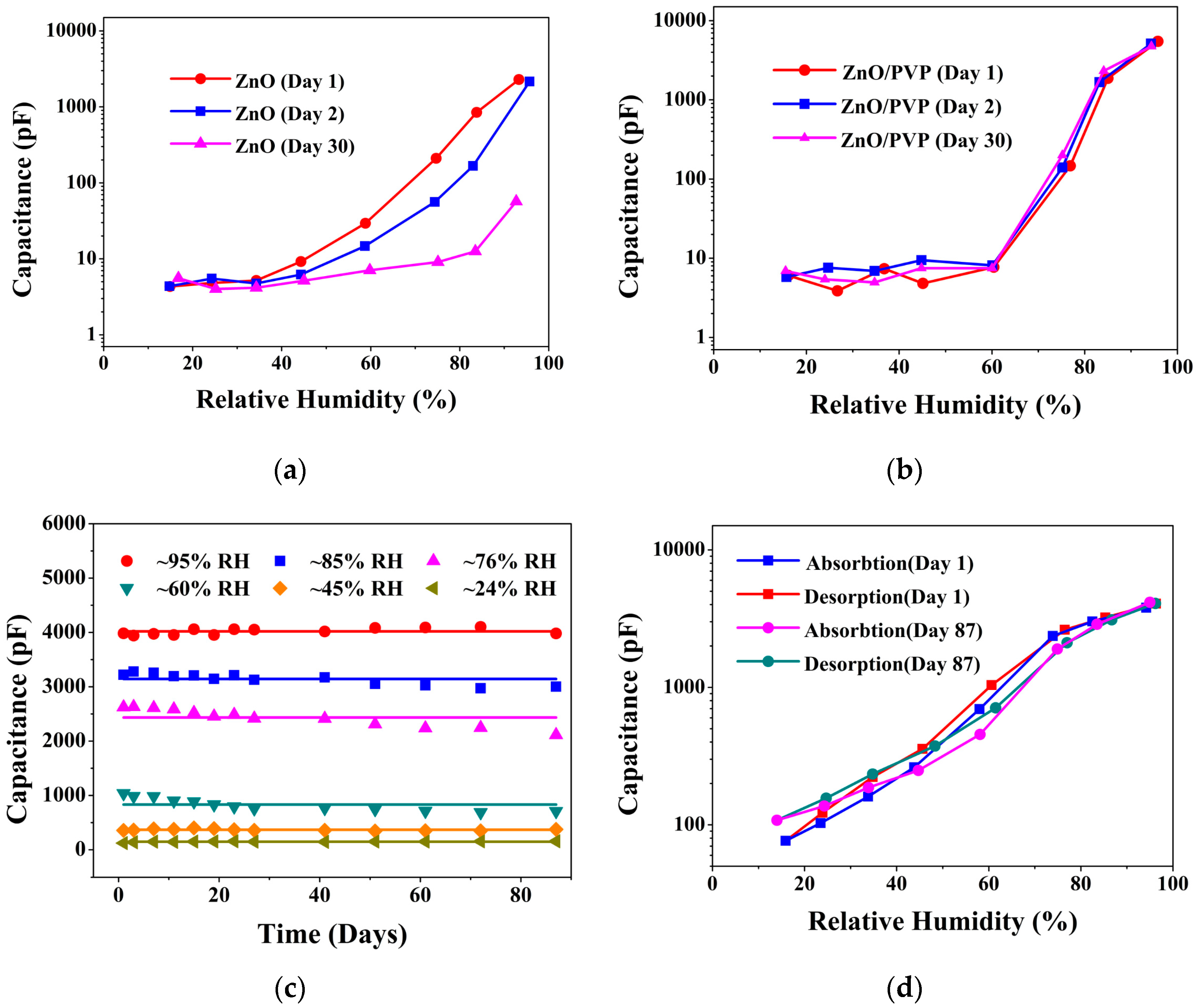
3.2. Humidity Sensing Properties
3.3. Discussion of the Sensing Mechanism
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Herrán, J.; Fernández, I.; Ochoteco, E.; Cabañero, G.; Grande, H. The role of water vapour in ZnO nanostructures for humidity sensing at room temperature. Sens. Actuators B Chem. 2014, 198, 239–242. [Google Scholar] [CrossRef]
- Wang, L.L.; Wang, H.Y.; Wang, W.C.; Li, K.; Wang, X.C.; Li, X.J. Capacitive humidity sensing properties of ZnO cauliflowers grown on silicon nanoporous pillar array. Sens. Actuators B Chem. 2013, 177, 740–744. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Zhu, Z.; Zhu, J. Humidity sensing properties of Pd2+-doped ZnO nanotetrapods. Appl. Surf. Sci. 2007, 253, 3168–3173. [Google Scholar] [CrossRef]
- Jung, D.; Kim, J.; Lee, G.S. Enhanced humidity-sensing response of metal oxide coated carbon nanotube. Sens. Actuators A Phys. 2015, 223, 11–17. [Google Scholar] [CrossRef]
- Su, P.G.; Shiu, W.L.; Tsai, M.S. Flexible humidity sensor based on Au nanoparticles/graphene oxide/thiolated silica sol-gel film. Sens. Actuators B Chem. 2015, 216, 467–475. [Google Scholar] [CrossRef]
- Reddy, A.S.G.; Narakathu, B.B.; Atashbar, M.Z.; Rebros, M.; Rebrosova, E.; Joyce, M.K. Fully Printed Flexible Humidity Sensor. Procedia Eng. 2011, 25, 120–123. [Google Scholar] [CrossRef]
- Mathew, J.; Semenova, Y.; Farrell, G. Relative humidity sensor based on an agarose-infiltrated photonic crystal fiber interferometer. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1553–1559. [Google Scholar] [CrossRef]
- Gao, Z.; Wu, Y.; Wang, X. High performance of enhanced mode field effect transistor and ultraviolet sensor based on ZnO nanosheets. Chin. J. Chem. Phys. 2015, 28, 1–10. [Google Scholar] [CrossRef]
- Xuan, W.; He, M.; Meng, N.; He, X.; Wang, W.; Chen, J.; Shi, T.; Hasan, T.; Xu, Z.; Xu, Y.; et al. Fast response and high sensitivity ZnO/glass surface acoustic wave humidity sensors using graphene oxide sensing layer. Sci. Rep. 2014, 4, 07206. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, X.; Guo, H.; Wu, Z. Graphene oxide thin film coated quartz crystal microbalance for humidity detection. Appl. Surf. Sci. 2011, 257, 7778–7782. [Google Scholar] [CrossRef]
- Narimani, K.; Nayeri, F.D.; Kolahdouz, M.; Ebrahimi, P. Fabrication, modeling and simulation of high sensitivity capacitive humidity sensors based on ZnO nanorods. Sens. Actuators B Chem. 2016, 224, 338–343. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, G.; Li, R.; Pan, T. Photopatternable PEDOT: PSS/PEG hybrid thin film with moisture stability and sensitivity. Microsyst. Nanoeng. 2017, 3, 17004. [Google Scholar] [CrossRef]
- Pandey, N.K.; Tiwari, K.; Akash, R. ZnO-TiO2 nanocomposite: Characterization and moisture sensing studies. Bull. Mater. Sci. 2012, 35, 347–352. [Google Scholar] [CrossRef]
- Chang, S.P.; Chang, S.J.; Lu, C.Y.; Li, M.J.; Hsu, C.L.; Chiou, Y.Z.; Hsueh, T.J.; Chen, I.C. A ZnO nanowire-based humidity sensor. Superlattices Microstruct. 2010, 47, 772–778. [Google Scholar] [CrossRef]
- Popov, V.I.; Nikolaev, D.; Timofeev, V.B.; Smagulova, S.A.; Antonova, I.V. Graphene based humidity sensors: The origin of alternating resistance change. Nanotechnology 2017, 28, 355501. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Yin, K.; Xie, X.; Ji, J.; Wan, S.; Sun, L.; Terrones, M.; Dresselhaus, M.S. Ultrahigh humidity sensitivity of graphene oxide. Sci. Rep. 2013, 3, 2714. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, S.; Priolkar, K.R. Evaluation of ZnO nanoparticles and study of ZnO-TiO2 composites for lead free humidity sensor. Sens. Actuators B Chem. 2013, 183, 411–418. [Google Scholar] [CrossRef]
- Erol, A.; Okur, S.; Comba, B.; Mermer, Ö.; Aikan, M.Ç. Humidity sensing properties of ZnO nanoparticles synthesized by sol-gel process. Sens. Actuators B Chem. 2010, 145, 174–180. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, T.; Yu, Q.; Wang, R.; Zeng, Y.; Liu, L.; Yang, H. Properties of humidity sensing ZnO nanorods-base sensor fabricated by screen-printing. Sens. Actuators B Chem. 2008, 133, 638–643. [Google Scholar] [CrossRef]
- Peng, X.; Chu, J.; Yang, B.; Feng, P.X. Mn-doped zinc oxide nanopowders for humidity sensors. Sens. Actuators B Chem. 2012, 174, 258–262. [Google Scholar] [CrossRef]
- Sang, L.; Liao, M.; Sumiya, M. A comprehensive review of semiconductor ultraviolet photodetectors: From thin film to one-dimensional nanostructures. Sensors 2013, 13, 10482–10518. [Google Scholar] [CrossRef] [PubMed]
- Ates, T.; Tatar, C.; Yakuphanoglu, F. Preparation of semiconductor ZnO powders by sol-gel method: Humidity sensors. Sens. Actuators A Phys. 2013, 190, 153–160. [Google Scholar] [CrossRef]
- Imran, Z.; Rasool, K.; Batool, S.S.; Ahmad, M.; Rafiq, M.A. Effect of different electrodes on the transport properties of ZnO nanofibers under humid environment. AIP Adv. 2015, 5, 117214. [Google Scholar] [CrossRef]
- Misra, S.K.; Pandey, N.K.; Shakya, V.; Roy, A. Application of undoped and Al2O3-doped ZnO nanomaterials as solid-state humidity sensor and its characterization studies. IEEE Sens. J. 2015, 15, 3582–3589. [Google Scholar] [CrossRef]
- Tiwari, K.; Tripathi, A.; Pandey, N.K. A resistive humidity sensor based on nanostructured WO3-ZnO composites. Sens. Trans. 2011, 134, 65–75. [Google Scholar]
- Ismail, A.S.; Mamat, M.H.; Sin, N.D.M.; Malek, M.F.; Zoolfakar, A.S.; Suriani, A.B.; Mohamed, A.; Ahmad, M.K.; Rusop, M. Fabrication of hierarchical Sn-doped ZnO nanorod arrays through sonicated sol−gel immersion for room temperature, resistive-type humidity sensor applications. Ceram. Int. 2016, 42, 9785–9795. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, T.; Zeng, Y.; Yang, H. Humidity sensing properties of KCl-doped Cu-Zn/CuO-ZnO nanoparticles. Sens. Actuators B Chem. 2009, 137, 21–26. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Xia, B. Layer-by-layer self-assembly of zinc oxide/graphene oxide hybrid toward ultrasensitive humidity sensing. IEEE Electron. Device Lett. 2016, 37, 916–919. [Google Scholar] [CrossRef]
- Yuan, Z.; Tai, H.; Bao, X.; Liu, C.; Ye, Z.; Jiang, J. Enhanced humidity-sensing properties of novel graphene oxide/Zinc oxide nanoparticles layered thin film QCM sensor. Mater. Lett. 2016, 174, 28–31. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, Y.; Cui, X.; Cheng, P.; Wang, B.; Gao, Y.; Li, X.; Yang, T.; Zhang, T.; Lu, G. Humidity-sensing properties of urchinlike CuO nanostructures modified by reduced graphene oxide. ACS Appl. Mater. Interface 2014, 6, 3888–3895. [Google Scholar] [CrossRef] [PubMed]
- Pustelny, T.; Setkiewicz, M.; Drewniak, S.; Maciak, E.; Stolarczyk, A.; Procek, M.; Urbanczyk, M.; Gut, K.; Opilski, Z.; et al. The influence of humidity on the resistance structures with graphene sensor layer. Acta Phys. Pol. 2012, 122, 870–873. [Google Scholar] [CrossRef]
- Chen, M.C.; Hsu, C.L.; Hsueh, T.J. Fabrication of humidity sensor based on bilayer graphene. IEEE Electron. Device Lett. 2014, 35, 590–592. [Google Scholar] [CrossRef]
- Zhang, D.; Chang, H.; Li, P.; Liu, R.; Xue, Q. Fabrication and characterization of an ultrasensitive humidity sensor based on metal oxide/graphene hybrid nanocomposite. Sens. Actuators B Chem. 2016, 225, 233–240. [Google Scholar] [CrossRef]
- Li, Y.; Fan, K.; Ban, H.; Yang, M. Detection of very low humidity using polyelectrolyte/graphene bilayer humidity sensors. Sens. Actuators B Chem. 2016, 222, 151–158. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, G.; Wang, W.; Zhou, X.; Guo, S. Individual nanocomposite sheets of chemically reduced graphene oxide and poly(N-vinyl pyrrolidone): Preparation and humidity sensing characteristics. J. Mater. Chem. 2010, 20, 10824–10828. [Google Scholar] [CrossRef]
- Lin, W.D.; Chang, H.M.; Wu, R.J. Applied novel sensing material graphene/polypyrrole for humidity sensor. Sens. Actuators B Chem. 2013, 181, 326–331. [Google Scholar] [CrossRef]
- Wang, T.; Sun, Z.; Huang, D.; Yang, Z.; Ji, Q.; Hu, N.; Yin, G.; He, D.; Wei, H.; Zhang, Y. Studies on NH3 gas sensing by zinc oxide nanowire-reduced graphene oxide nanocomposites. Sens. Actuators B Chem. 2017, 252, 284–294. [Google Scholar] [CrossRef]
- Hjiri, M.; Zahmouli, N.; Dhahri, R.; Leonardi, S.G.; Mir, L.E.; Neri, G. Doped-ZnO nanoparticles for selective gas sensors. J. Mater. Sci. 2017, 28, 9667–9674. [Google Scholar] [CrossRef]
- Bin, D.; Ren, F.; Wang, H.; Zhang, K.; Yang, B.; Zhai, C.; Zhu, M.; Yang, P.; Du, Y. Facile synthesis of PVP-assisted PtRu/RGO nanocomposites with high electrocatalytic performance for methanol oxidation. RSC Adv. 2014, 4, 39612–39618. [Google Scholar] [CrossRef]
- Chou, K.S.; Lee, T.K.; Liu, F.J. Sensing mechanism of a porous ceramic as humidity sensor. Sens. Actuators B Chem. 1999, 56, 106–111. [Google Scholar] [CrossRef]
- Ahmad, Z.; Zafar, Q.; Sulaiman, K.; Akram, R.; Karimov, K.S. A humidity sensing origanic-inoriganic composite for environmental monitoring. Sensors 2013, 13, 3615–3624. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Song, C.; Yin, H.; Zhang, J. Capacitive humidity sensors based on zinc oxide nanorods grown on silicon nanowires array at room temperature. Sens. Actuators A Phys. 2015, 235, 234–239. [Google Scholar] [CrossRef]
- Fratoddi, I.; Bearzotti, A.; Venditti, I.; Cametti, C.; Russo, M.V. Role of nanostructured polymers on the improvement of electrical response-based relative humidity sensors. Sens. Actuators B Chem. 2016, 225, 96–108. [Google Scholar] [CrossRef]





| Sensing Material | Fabrication Method | Measurement Range | Response/Recovery Time | Hysteresis | Reference |
|---|---|---|---|---|---|
| ZnO/PVP-RGO | Drop-coating | 15–95% RH | 12 s/3 s | ~3.9% RH | This work |
| ZnO | Sol-gel method | 55–90% RH | 250 s/- | - | [11] |
| GO | Drop-casting | 23–86% RH | 10.5 s/41 s | - | [16] |
| PEPC+NiPC+Cu2O | Spin-coating | 40–97% RH | 13 s/15 s | ~12% RH | [41] |
| ZnO/Si | Sol-gel method | 11.3–97.7% RH | 26 s/7 s | ~0.79% RH | [42] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Ye, Q.; Zeng, R.; Zhang, J.; Yue, L.; Xu, M.; Qiu, Z.-J.; Wu, D. Stable and Fast-Response Capacitive Humidity Sensors Based on a ZnO Nanopowder/PVP-RGO Multilayer. Sensors 2017, 17, 2415. https://doi.org/10.3390/s17102415
Yang H, Ye Q, Zeng R, Zhang J, Yue L, Xu M, Qiu Z-J, Wu D. Stable and Fast-Response Capacitive Humidity Sensors Based on a ZnO Nanopowder/PVP-RGO Multilayer. Sensors. 2017; 17(10):2415. https://doi.org/10.3390/s17102415
Chicago/Turabian StyleYang, Hui, Qiangqiang Ye, Ruixue Zeng, Junkai Zhang, Lei Yue, Ming Xu, Zhi-Jun Qiu, and Dongping Wu. 2017. "Stable and Fast-Response Capacitive Humidity Sensors Based on a ZnO Nanopowder/PVP-RGO Multilayer" Sensors 17, no. 10: 2415. https://doi.org/10.3390/s17102415




