Effects of Moisture and Particle Size on Quantitative Determination of Total Organic Carbon (TOC) in Soils Using Near-Infrared Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
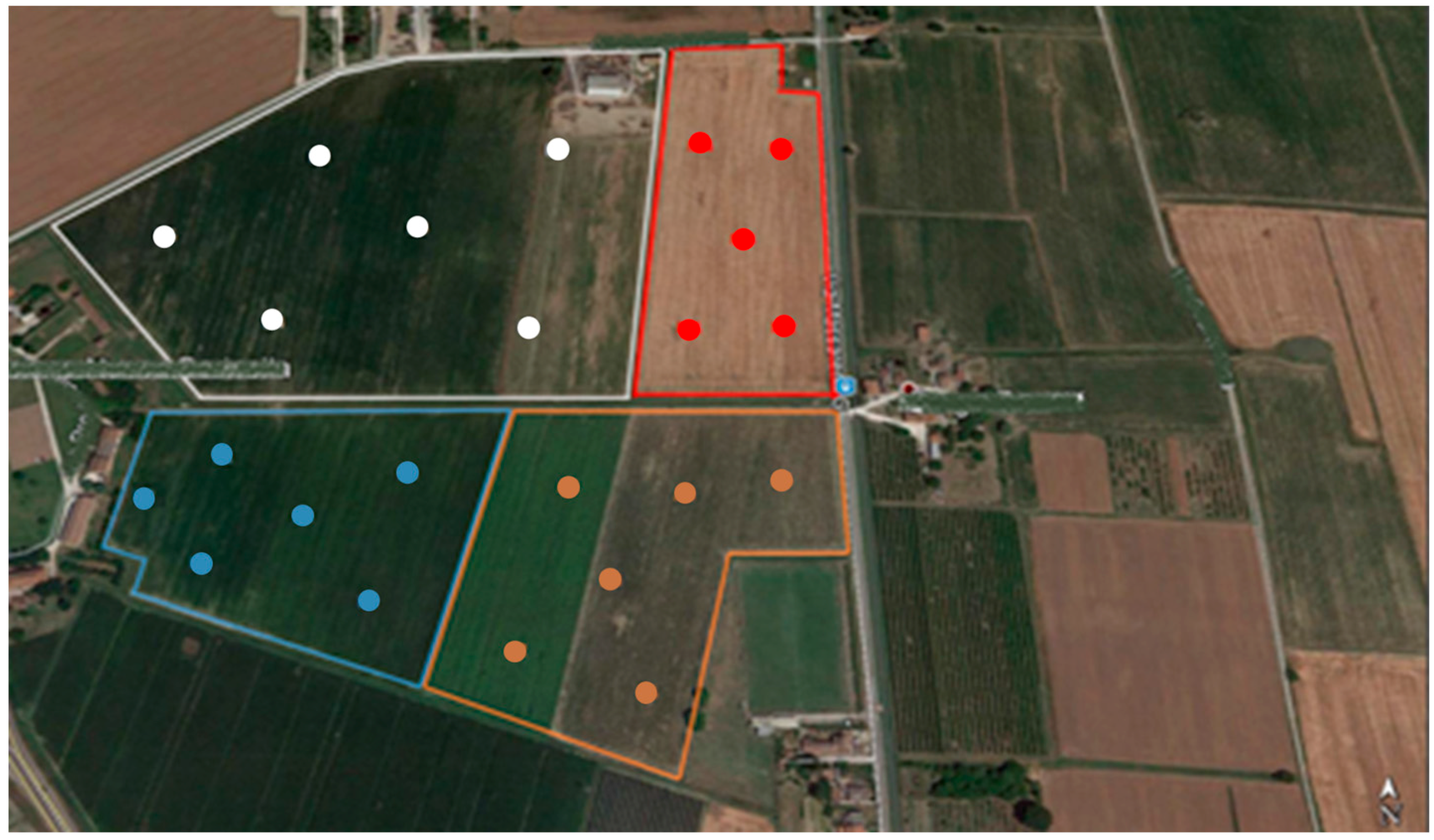
2.1. Soil, Soil Sampling and Analysis
2.2. Sample Treatments for NIR Spectral Acquisition
2.3. NIR Spectroscopy
2.4. NIR Statistics and Chemometrics
3. Results and Discussion
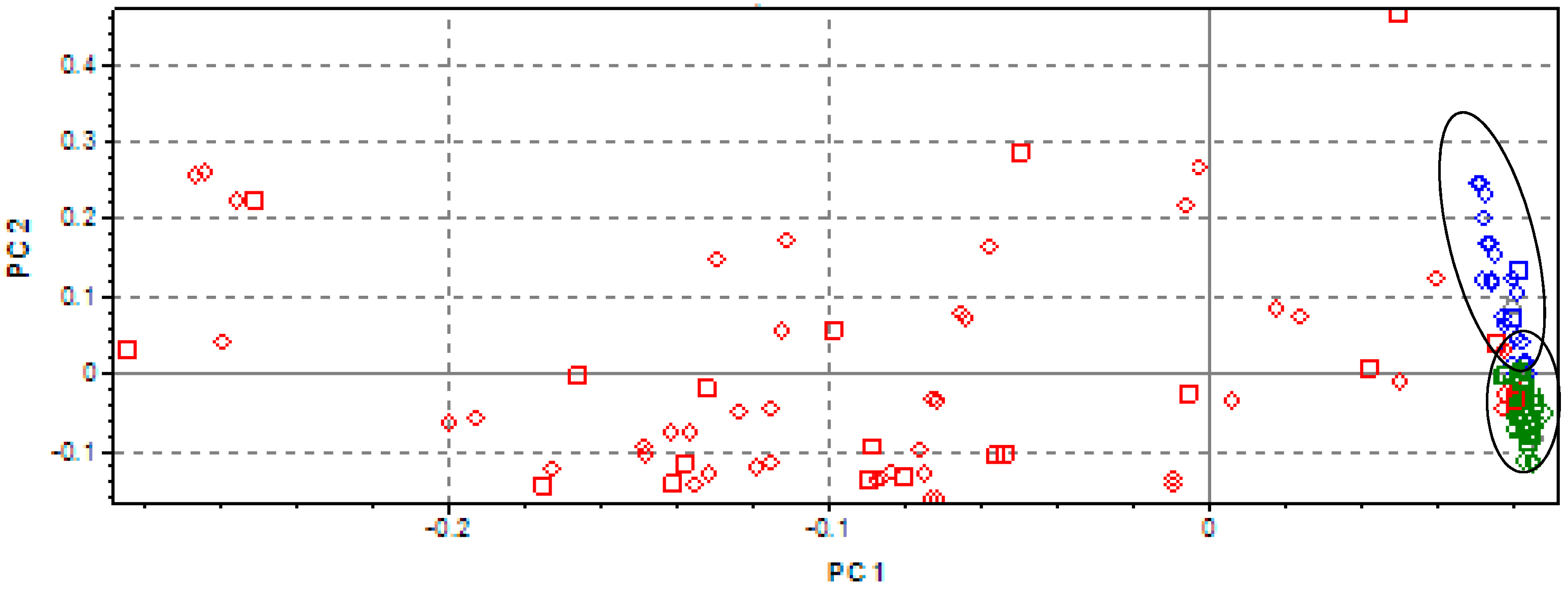
3.1. Analysis of Spectral Data
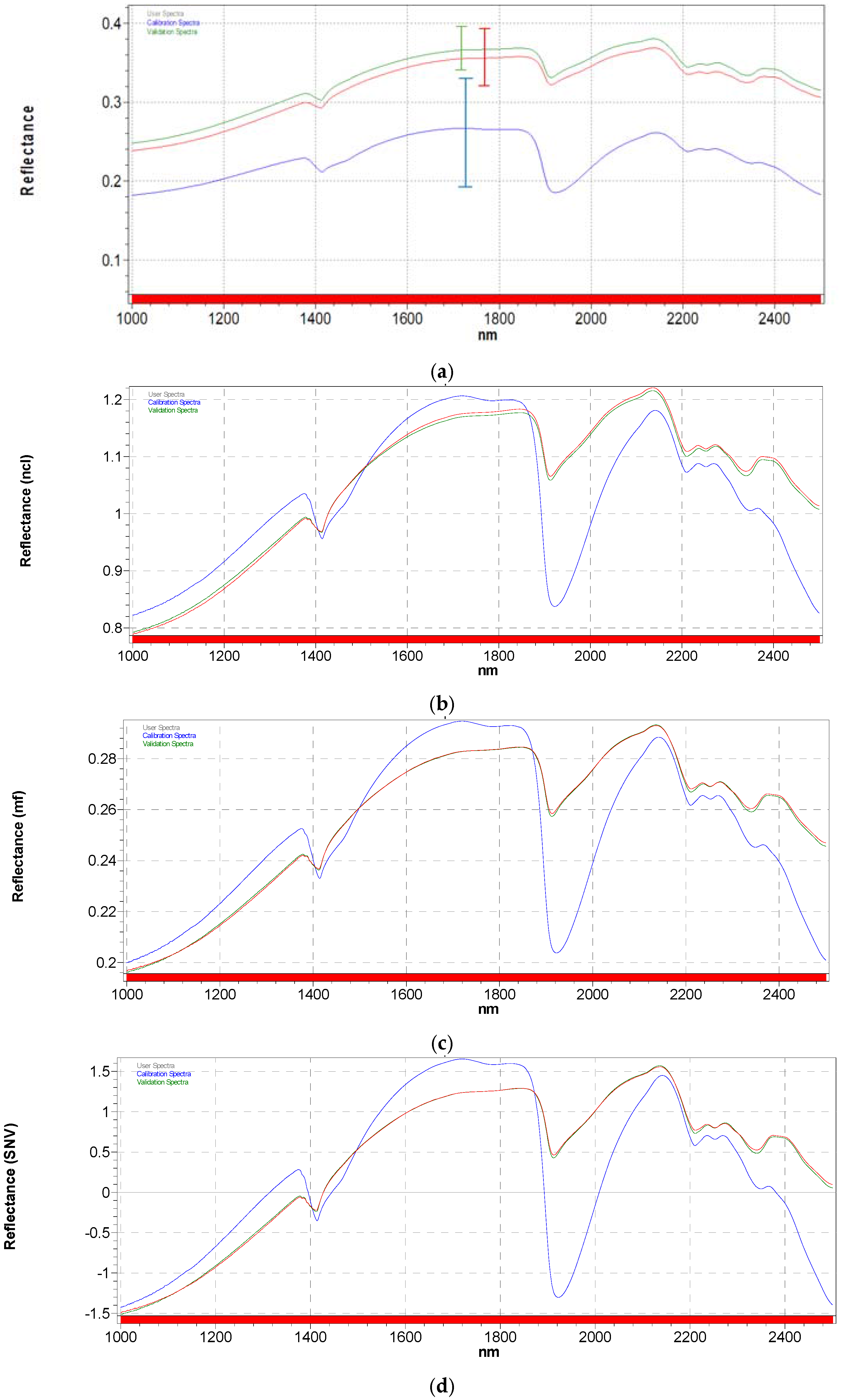
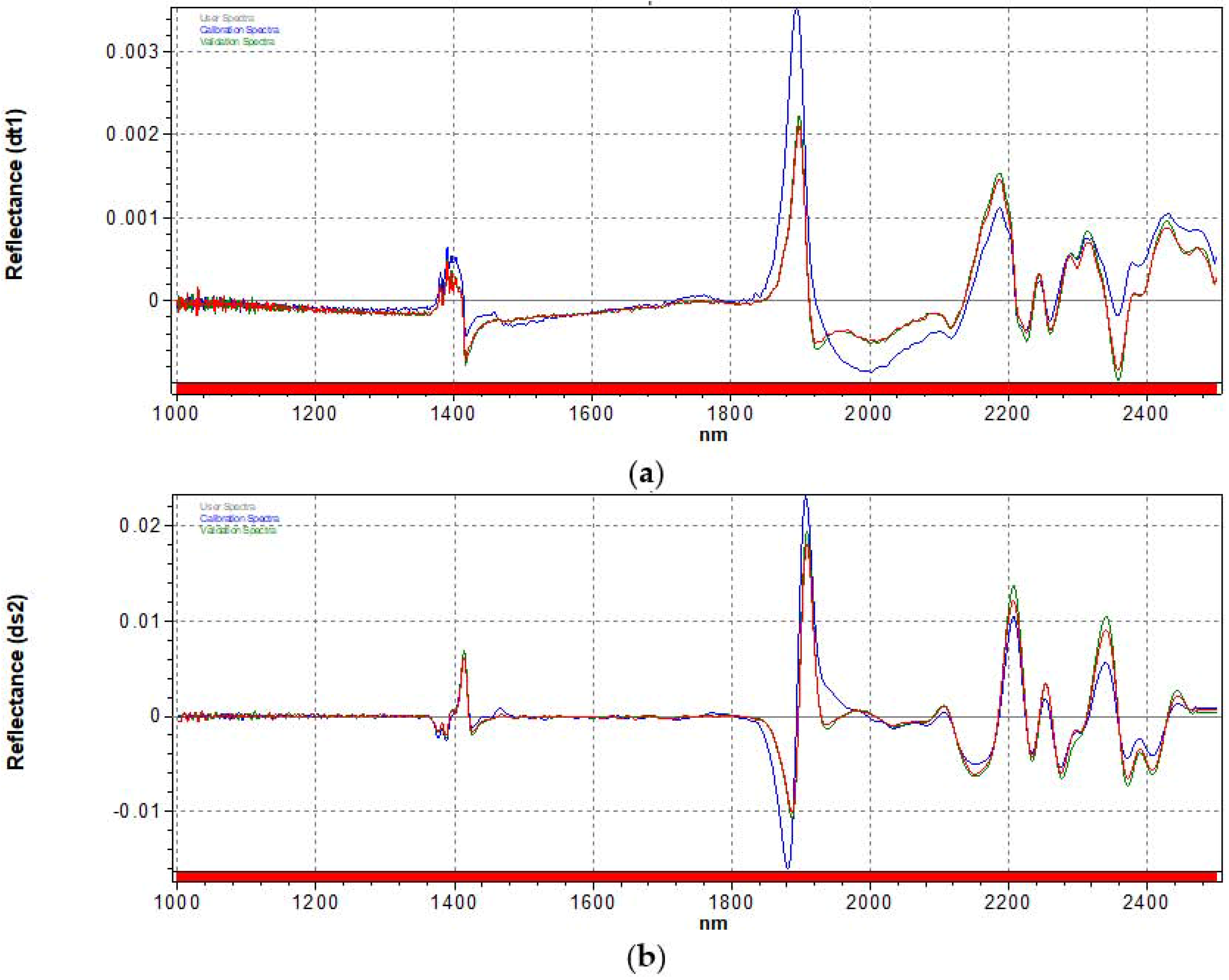
3.2. Spectra Pre-Treatments
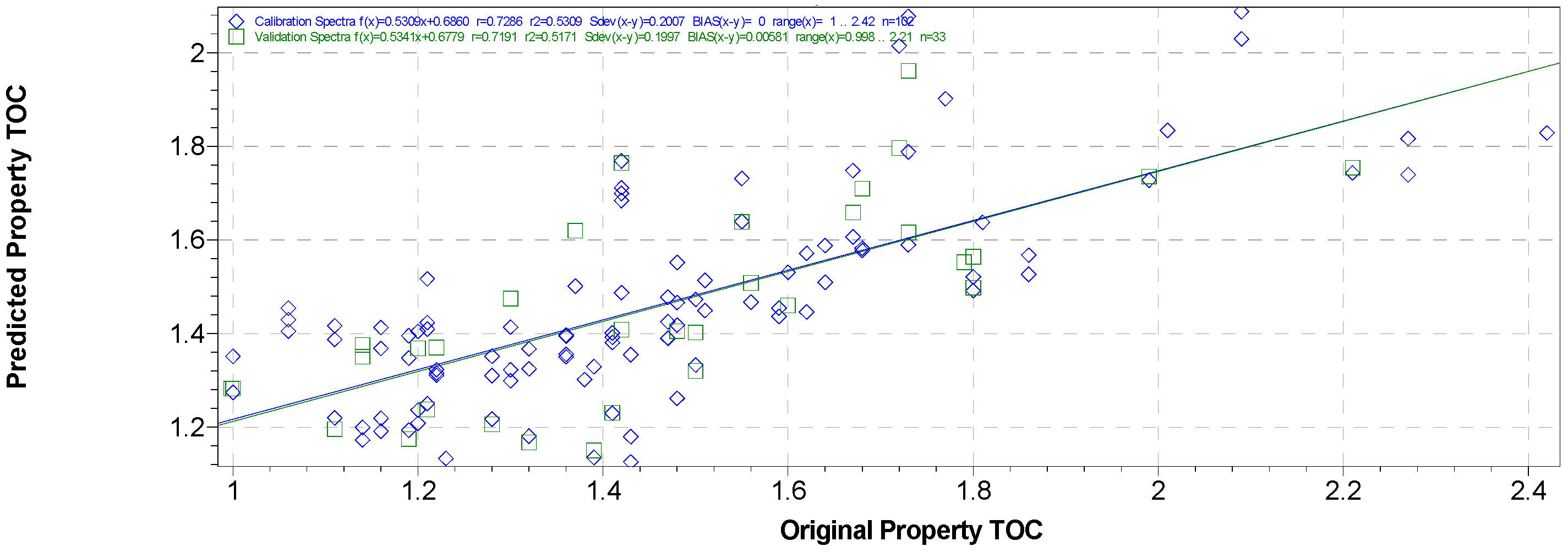
3.3. PLSR Calibration Models for TOC Predictions
3.4. External Validation
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Curry, J.P.; Good, J.A. Soil faunal degradation and restoration. Adv. Soil Sci. 1992, 17, 171–215. [Google Scholar]
- Virto, I.; Imaz, M.J.; Fernández-Ugalde, O.; Gartzia-Bengoetxea, N.; Enrique, A.; Bescansa, P. Soil Degradation and Soil Quality in Western Europe: Current Situation and Future Perspectives. Sustainability 2015, 7, 313–365. [Google Scholar] [CrossRef] [Green Version]
- Keskin, M.; Sekerli, Y.E. Awareness and adoption of precision agriculture in the Cukurova region of Turkey. Agron. Res. 2016, 14, 1307–1320. [Google Scholar]
- McBratney, A.; Whelan, B.; Ancev, T.; Bouma, J. Future directions of precision agriculture. Precis. Agric. 2015, 6, 7–23. [Google Scholar] [CrossRef]
- Stevens, A.; Nocita, M.; Tóth, G.; Montanarella, L.; van Wesemael, B. Prediction of soil organic carbon at the European scale by visible and near infrared reflectance spectroscopy. PLoS ONE 2013, 8, e66409. [Google Scholar] [CrossRef] [PubMed]
- Kogel-Knabner, I. The macromolecular organic composition of plant and microbial residue as inputs to soil organic matter. Soil Biol. Biochem. 2002, 109, 139–162. [Google Scholar] [CrossRef]
- Loveland, P.; Webb, J. Is there a critical level of organic matter in the agricultural soils of temperate regions: A review. Soil Till. Res. 2003, 70, 1–18. [Google Scholar] [CrossRef]
- Schumacher, B.A. Methods for the Determination of Total Organic Carbon (TOC) in Soils and Sediments; United States Environmental Protection Agency, Environmental Sciences Division National, Exposure Research Laboratory: Las Vegas, NV, USA, 2002.
- Chatterjee, A.; Lal, R.; Wielopolski, L.; Martin, M.Z.; Ebinger, M.H. Evaluation of different soil carbon determination methods. Crit. Rev. Plant Sci. 2009, 28, 164–178. [Google Scholar] [CrossRef]
- Jimenez, R.R.; Ladha, J.K. Automated elemental analysis: A rapid and reliable but expensive measurement of total carbon and nitrogen in plant and soil samples. Commun. Soil Sci. Plant Anal. 1993, 24, 1897–1924. [Google Scholar] [CrossRef]
- Tamburini, E.; Marchetti, M.G.; Pedrini, P. Monitoring key parameters in bioprocesses using near-infrared technology. Sensors 2014, 14, 18941–18959. [Google Scholar] [CrossRef] [PubMed]
- Siesler, H.W.; Ozaki, Y.; Kawata, S.; Heise, H.M. (Eds.) Near-Infrared Spectroscopy: Principles, Instruments, Applications; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Schellberg, J.; Hill, M.J.; Gerhards, R.; Rothmund, M.; Braun, M. Precision agriculture on grassland: Applications, perspectives and constraints. Eur. J. Agron. 2008, 29, 59–71. [Google Scholar] [CrossRef]
- Shi, Z.; Huang, J.; Li, S. Correction: In Situ Measurement of Some Soil Properties in Paddy Soil Using Visible and Near-Infrared Spectroscopy. PLoS ONE 2016, 11, e0159785. [Google Scholar] [CrossRef]
- Luce, M.S.; Ziadi, N.; Zebarth, B.J.; Grant, C.A.; Tremblay, G.F.; Gregorich, E.G. Rapid determination of soil organic matter quality indicators using visible near infrared reflectance spectroscopy. Geoderma 2014, 232, 449–458. [Google Scholar] [CrossRef]
- Shi, T.; Cui, L.; Wang, J.; Fei, T.; Chen, Y.; Wu, G. Comparison of multivariate methods for estimating soil total nitrogen with visible/near-infrared spectroscopy. Plant Soil 2013, 366, 363–375. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, T.; Liu, J.; Lin, Z.; Li, S.; Wang, R.; Ge, Y. Soil pH value, organic matter and macronutrients contents prediction using optical diffuse reflectance spectroscopy. Comput. Electron. Agric. 2015, 111, 69–77. [Google Scholar] [CrossRef]
- Dalal, R.C.; Henry, R.J. Simultaneous determination of moisture, organic carbon, and total nitrogen by near infrared reflectance spectrophotometry. Soil Sci. Soc. Am. J. 1986, 50, 120–123. [Google Scholar] [CrossRef]
- Gehl, R.J.; Rice, C.W. Emerging technologies for in situ measurement of soil carbon. Clim. Chang. 2007, 80, 43–54. [Google Scholar] [CrossRef]
- Beyer, L. The chemical composition of soil organic matter in classical humic compound fractions and in bulk samples—A review. J. Soil Sci. Plant Nutr. 1996, 159, 527–539. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Borůvka, L.; Saberioon, M.; Vašát, R. Visible, near-infrared, and mid-infrared spectroscopy applications for soil assessment with emphasis on soil organic matter content and quality: State-of-the-art and key issues. Appl. Spectrosc. 2013, 67, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Weidong, L.; Baret, F.; Xingfa, G.; Qingxi, T.; Lanfen, Z.; Bing, Z. Relating soil surface moisture to reflectance. Remote Sens. Environ. 2002, 81, 238–246. [Google Scholar] [CrossRef]
- Martens, H.; Nielsen, J.P.; Engelsen, S.B. Light scattering and light absorbance separated by extended multiplicative signal correction. Application to near-infrared transmission analysis of powder mixtures. Anal. Chem. 2003, 75, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Castaldelli, G.; Colombani, N.; Vincenzi, F.; Mastrocicco, M. Linking dissolved organic carbon, acetate and denitrification in agricultural soil. Environ. Earth Sci. 2013, 68, 939–945. [Google Scholar] [CrossRef]
- Potter, B.B.; Wimsatt, J.C. Method 415.3. Determination of Total Organic Carbon and Specific UV Absorbance at 254 nm in Source Water and Drinking Water; U.S. Environmental Protection Agency: Washington, DC, USA, 2005.
- Rinnan, A.; van den Berg, F.; Engelsen, B.S. Review of the most common pre-processing techniques for near-infrared spectra. Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Martens, H.; Naes, T. Multivariate Calibration; Wiley and Sons: Chichester, NY, USA, 1988. [Google Scholar]
- Williams, P.C. Near-Infrared Technology in the Agricultural and Food Industries, 2nd ed.; American Association of Cereal Chemists: Eagan, MN, USA, 2001. [Google Scholar]
- Durbin, J.; Watson, G.S. Testing for serial correlation in least squares regression. Biometrika 1950, 37, 409–428. [Google Scholar] [PubMed]
- Rocke, D.M.; Woodruff, D.L. Identification of Outliers in Multivariate Data. J. Am. Stat. Assoc. 1996, 91, 1047–1061. [Google Scholar] [CrossRef]
- Dardenne, P. Some Considerations about NIR Spectroscopy: Closing Speech at NIR-2009. NIR News 2010, 21, 8–14. [Google Scholar] [CrossRef]
- Reeves, J.B.; McCarty, G.W., III; Reeves, V.B. Mid-infrared and diffuse reflectance spectroscopy for the quantitative analysis of agricultural soils. J. Agric. Food Chem. 2001, 49, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, D.; Moron, A. Potential of near-infrared reflectance spectroscopy and chemometrics to predict soil organic carbon fractions. Soil Till. Res. 2006, 85, 78–85. [Google Scholar] [CrossRef]
- Fidencio, P.H.; Poppi, R.J.; De Andrade, J.C.; Cantarella, H. Determination of organic matter in soil using near-infrared spectroscopy and partial least squares regression. Commun. Soil Sci. Plant Anal. 2002, 33, 1607–1615. [Google Scholar] [CrossRef]
- Stenberg, B.; Rossel, R.A.V.; Mouazen, A.M.; Wetterlind, J. Chapter five-visible and near infrared spectroscopy in soil science. Adv. Agron. 2010, 107, 163–215. [Google Scholar]
- Viscarra Rossel, R.A.; Walvoort, D.J.J.; McBratney, A.B.; Janik, L.J.; Skjemstad, J.O. Visible, near-infrared, mid-infrared or combined diffuse reflectance spectroscopy for simultaneous assessment of various soil properties. Geoderma 2006, 131, 59–75. [Google Scholar] [CrossRef]
- Daughtry, C.S.T. Discriminating crop residues from soil by shortwave infrared reflectance. Agron. J. 2001, 93, 125–131. [Google Scholar] [CrossRef]
- Stoner, E.R.; Baumgardner, M.F. Characteristic variations in reflectance of surface soils. Soil Sci. Soc. Am. J. 1981, 45, 1161–1165. [Google Scholar] [CrossRef]
- Bach, H.; Mauser, W. Modelling and model verification of the spectral reflectance of soils under varying moisture conditions. In Proceedings of the IEEE Geoscience and Remote Sensing Symposium, IGARSS ’94, Pasadena, CA, USA, 8–12 August 1994; Volume 4, pp. 2354–2356. [Google Scholar]
- Somers, B.; Gysels, W.; Verstraeten, W.W.; Delalieux, S.; Coppin, P. Modeling moisture-induced soil reflectance changes in cultivated sandy soils: A case study in citrus orchards. Eur. J. Soil Sci. 2010, 61, 1091–1105. [Google Scholar] [CrossRef]
- Lobell, D.; Asner, G. Moisture effects on soil reflectance. Soil Sci. Soc. Am. J. 2002, 66, 722–727. [Google Scholar] [CrossRef]
- Chen, H.; Song, Q.; Tang, G.; Feng, Q.; Lin, L. The combined optimization of Savitzky-Golay smoothing and multiplicative scatter correction for FT-NIR PLS models. ISRN Spectrosc. 2013, 9. [Google Scholar] [CrossRef]
- Bazar, G.; Kovacs, Z.; Tsenkova, R. Evaluating spectral signals to identify spectral error. PLoS ONE 2016, 11, e0146249. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Coello, J.; Montoliu, I.; Romero, M.A. Orthogonal signal correction in near infrared calibration. Anal. Chim. Acta 2001, 434, 125–132. [Google Scholar] [CrossRef]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Dhanoa, M.S.; Lister, S.J.; Sanderson, R.; Barnes, R.J. The link between multiplicative scatter correction (MSC) and standard normal variate (SNV) transformations of NIR spectra. JNIRS 1994, 2, 43–47. [Google Scholar] [CrossRef]
- Czarnecki, M.A. Resolution enhancement in second-derivative spectra. Appl. Spectrosc. 2015, 69, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, C. Near infrared spectroscopy: Fundamentals, practical aspects and analytical applications. J. Braz. Chem. Soc. 2002, 14, 198–219. [Google Scholar] [CrossRef]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.A. Primary and Secondary Effects of Water Content on the Spectral Reflectance of Leaves. Am. J. Bot. 1991, 78, 916–924. [Google Scholar] [CrossRef]
- Salguero-Chaparro, L.; Baeten, V.; Abbas, O.; Peña-Rodríguez, F. On-line analysis of intact olive fruits by vis–NIR spectroscopy: Optimisation of the acquisition parameters. J. Food Eng. 2012, 112, 152–157. [Google Scholar] [CrossRef]
- Brown, D.J.; Shepherd, K.D.; Walsh, M.G.; Dewayne Mays, M.; Reinsch, T.G. Global soil characterization with VNIR diffuse reflectance spectroscopy. Geoderma 2006, 132, 273–290. [Google Scholar] [CrossRef]
- Shepherd, K.D.; Walsh, M.G. Development of reflectance spectral libraries for characterization of soil properties. Soil Sci. Soc. Am. J. 2002, 66, 988–998. [Google Scholar] [CrossRef]
- Saiano, F.; Oddo, G.; Scalenghe, R.; La Mantia, T.; Ajmone-Marsan, F. DRIFTS sensor: Soil carbon validation at large scale (Pantelleria, Italy). Sensors 2013, 13, 5603–5613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minasny, B.; McBratney, A.B.; Bellon-Maurel, V.; Roger, J.M.; Gobrecht, A.; Ferrand, L.; Joalland, S. Removing the effect of soil moisture from NIR diffuse reflectance spectra for the prediction of soil organic carbon. Geoderma 2011, 167, 118–124. [Google Scholar] [CrossRef]
- Nocita, M.; Stevens, A.; Noon, C.; van Wesemael, B. Prediction of soil organic carbon for different levels of soil moisture using Vis-NIR spectroscopy. Geoderma 2013, 199, 37–42. [Google Scholar] [CrossRef]
- Górecki, T.; Smaga, Ł. A comparison of tests for the one-way ANOVA problem for functional data. Computat. Stat. 2015, 30, 987–1010. [Google Scholar] [CrossRef]



| Pre-Treatment Applied | Outliers | F | R2CAL | SEC | R2CV | SECV | RPDCAL | RPDCV | DW | Q-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| NCL | 1 | 5 | 0.91 | 0.13 | 0.88 | 0.15 | 2.50 | 2.15 | 1.6 | 0.76 |
| MSC | 1 | 4 | 0.91 | 0.14 | 0.91 | 0.14 | 2.43 | 2.31 | 1.4 | 0.69 |
| SNV | 1 | 6 | 0.92 | 0.13 | 0.92 | 0.15 | 2.64 | 2.23 | 1.5 | 0.68 |
| DV1 | 1 | 6 | 0.82 | 0.19 | 0.76 | 0.16 | 0.34 | 2.04 | 1.9 | 0.52 |
| DV2 | 1 | 3 | 0.92 | 0.15 | 0.92 | 0.18 | 2.25 | 1.89 | 1.8 | 0.88 |
| SNV + DV1 | 1 | 4 | 0.91 | 0.11 | 0.91 | 0.13 | 3.04 | 2.48 | 1.6 | 0.71 |
| SNV + DV2 | 1 | 3 | 0.96 | 0.06 | 0.93 | 0.09 | 5.31 | 3.52 | 2.0 | 0.89 |
| Pre-Treatment Applied | Outliers | F | R2CAL | SEC | R2CV | SECV | RPDCAL | RPDCV | DW | Q-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| NCL | 1 | 7 | 0.89 | 0.12 | 0.74 | 0.16 | 2.81 | 2.12 | 0.7 | 2.32 |
| MSC | 1 | 2 | 0.90 | 0.11 | 0.89 | 0.14 | 3.16 | 2.34 | 0.5 | 2.45 |
| SNV | 1 | 3 | 0.95 | 0.11 | 0.93 | 0.10 | 3.13 | 3.28 | 0.7 | 1.91 |
| DV1 | 1 | 4 | 0.90 | 0.10 | 0.87 | 0.15 | 3.38 | 2.21 | 0.7 | 1.87 |
| DV2 | 1 | 2 | 0.96 | 0.09 | 0.92 | 0.10 | 3.45 | 3.25 | 0.8 | 2.54 |
| SNV + DV1 | 1 | 2 | 0.92 | 0.10 | 0.90 | 0.11 | 3.28 | 3.01 | 0.7 | 2.18 |
| SNV + DV2 | 1 | 2 | 0.99 | 0.09 | 0.93 | 0.10 | 3.52 | 3.31 | 0.8 | 2.64 |
| Pre-Treatment Applied | Outliers | F | R2CAL | SEC | R2CV | SECV | RPDCAL | RPDCV | DW | Q-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| NCL | 2 | 3 | 0.79 | 0.16 | 0.73 | 0.23 | 2.13 | 1.53 | 0.5 | 1.21 |
| MSC | 2 | 5 | 0.74 | 0.19 | 0.69 | 0.17 | 1.70 | 1.93 | 0.5 | 1.31 |
| SNV | 2 | 3 | 0.80 | 0.16 | 0.78 | 0.21 | 2.07 | 1.57 | 0.5 | 1.62 |
| DV1 | 1 | 4 | 0.74 | 0.14 | 0.74 | 0.17 | 2.31 | 1.93 | 0.6 | 1.65 |
| DV2 | 1 | 4 | 0.89 | 0.13 | 0.85 | 0.18 | 2.50 | 1.90 | 0.6 | 1.58 |
| SNV + DV1 | 2 | 4 | 0.93 | 0.08 | 0.91 | 0.10 | 4.03 | 3.64 | 0.6 | 1.83 |
| SNV + DV2 | 1 | 3 | 0.96 | 0.11 | 0.92 | 0.12 | 3.07 | 2.81 | 0.7 | 2.01 |
| Sample Set | Range | SD | MAE ± SDMAE | Outliers | R2EX.VAL. | RMSEP | Bias | Slope |
|---|---|---|---|---|---|---|---|---|
| WS | 1.21%–2.05% | 0.32% | 0.11 ± 0.08 | 0 | 0.76 | 0.25 | 0.38 | 0.72 |
| DS | 1.16%–1.99% | 0.33% | 0.10 ± 0.09 | 0 | 0.79 | 0.27 | 0.21 | 0.87 |
| GSS | 1.00%–2.16% | 0.27% | 0.13 ± 0.08 | 0 | 0.83 | 0.26 | 0.20 | 0.88 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamburini, E.; Vincenzi, F.; Costa, S.; Mantovi, P.; Pedrini, P.; Castaldelli, G. Effects of Moisture and Particle Size on Quantitative Determination of Total Organic Carbon (TOC) in Soils Using Near-Infrared Spectroscopy. Sensors 2017, 17, 2366. https://doi.org/10.3390/s17102366
Tamburini E, Vincenzi F, Costa S, Mantovi P, Pedrini P, Castaldelli G. Effects of Moisture and Particle Size on Quantitative Determination of Total Organic Carbon (TOC) in Soils Using Near-Infrared Spectroscopy. Sensors. 2017; 17(10):2366. https://doi.org/10.3390/s17102366
Chicago/Turabian StyleTamburini, Elena, Fabio Vincenzi, Stefania Costa, Paolo Mantovi, Paola Pedrini, and Giuseppe Castaldelli. 2017. "Effects of Moisture and Particle Size on Quantitative Determination of Total Organic Carbon (TOC) in Soils Using Near-Infrared Spectroscopy" Sensors 17, no. 10: 2366. https://doi.org/10.3390/s17102366






