Doping Ag in ZnO Nanorods to Improve the Performance of Related Enzymatic Glucose Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Hydrothermal Synthesis of ZnO-Ag Nanorods on the ITO Electrodes
2.3. Immobilization of GOx on the ZnO/ITO and ZnO-Ag/ITO Electrodes
2.4. Characterization of the ZnO and ZnO-Ag Nanorods
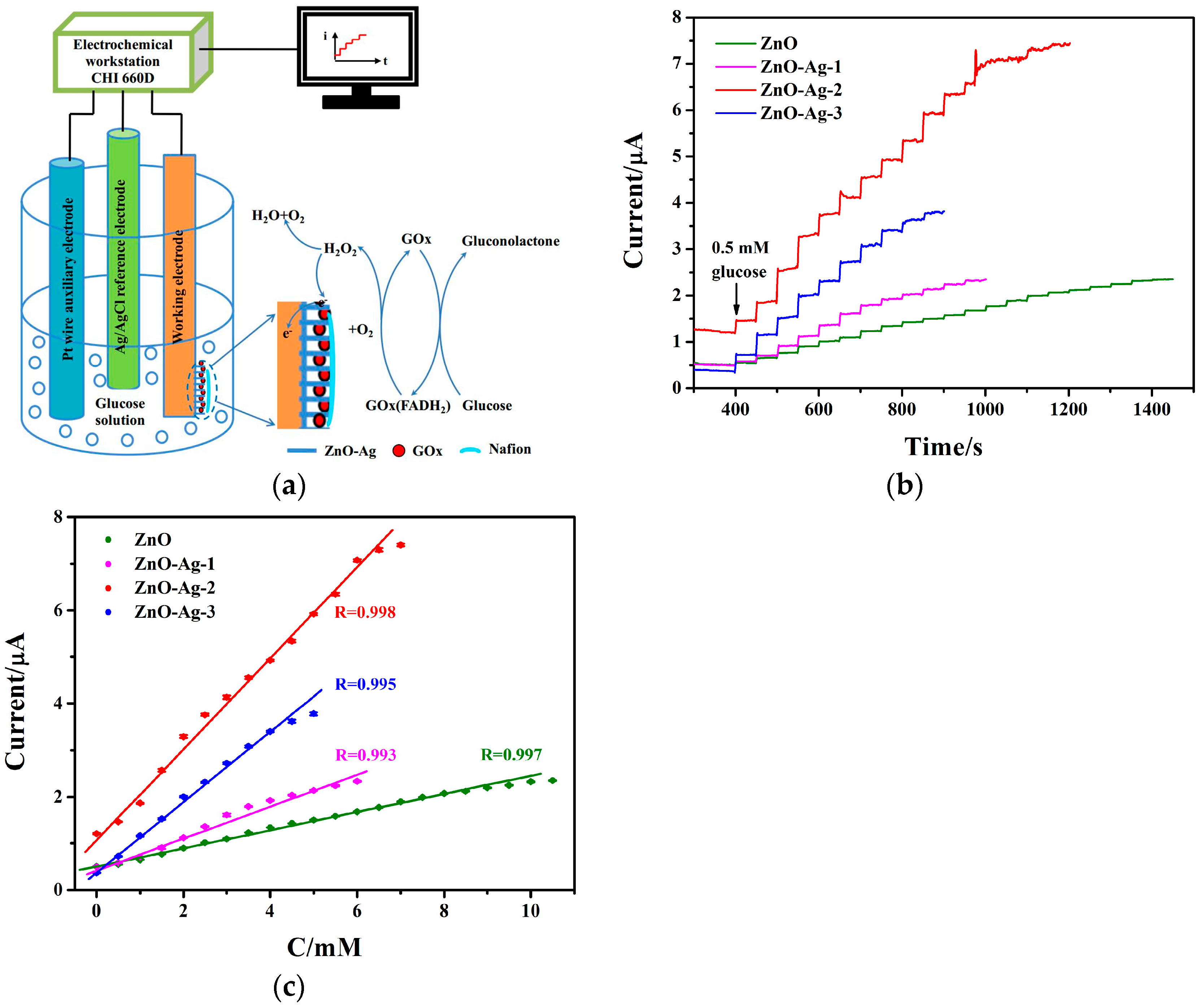
2.5. Electrochemical Measurements of the Glucose Sensors
3. Results and Discussion
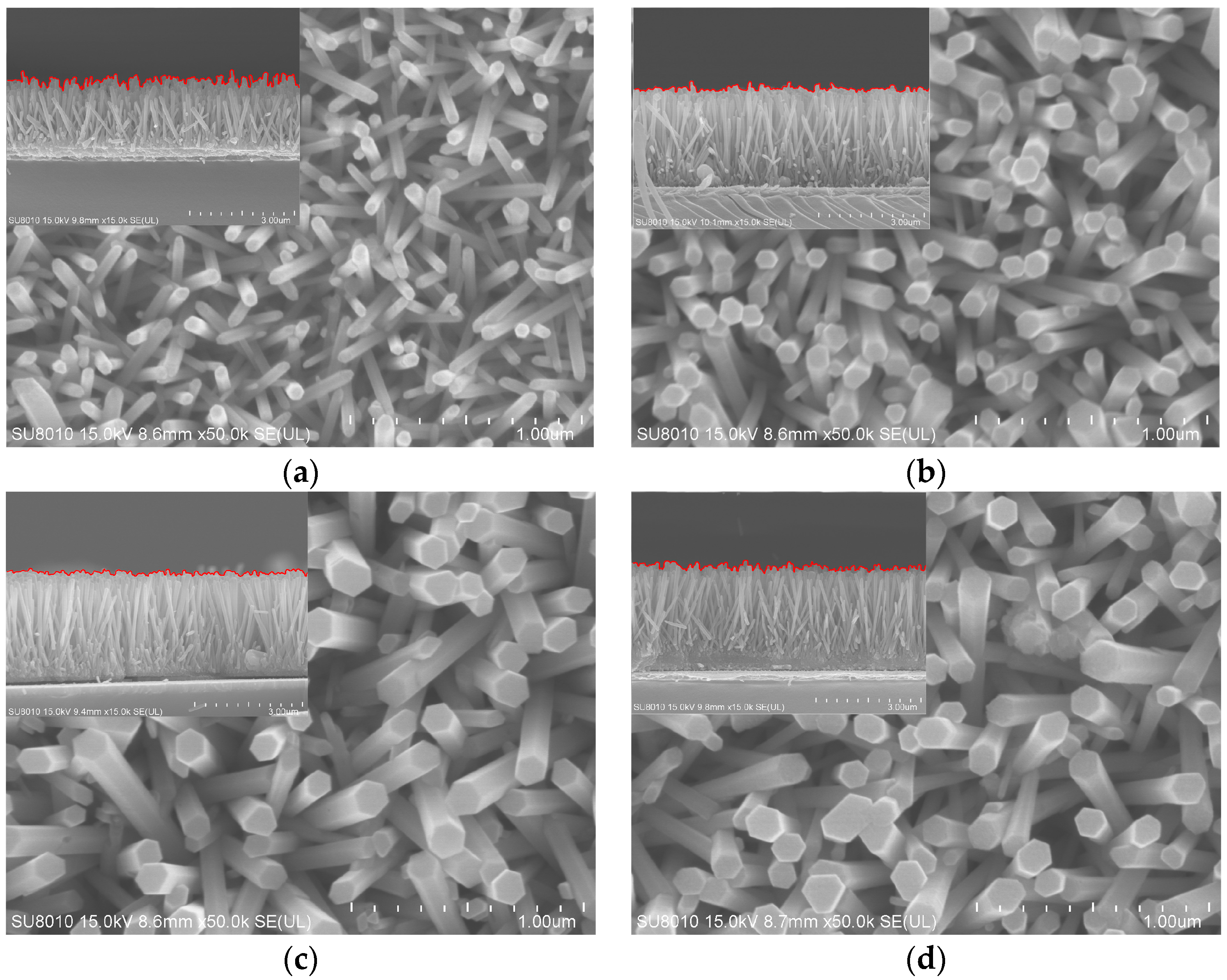
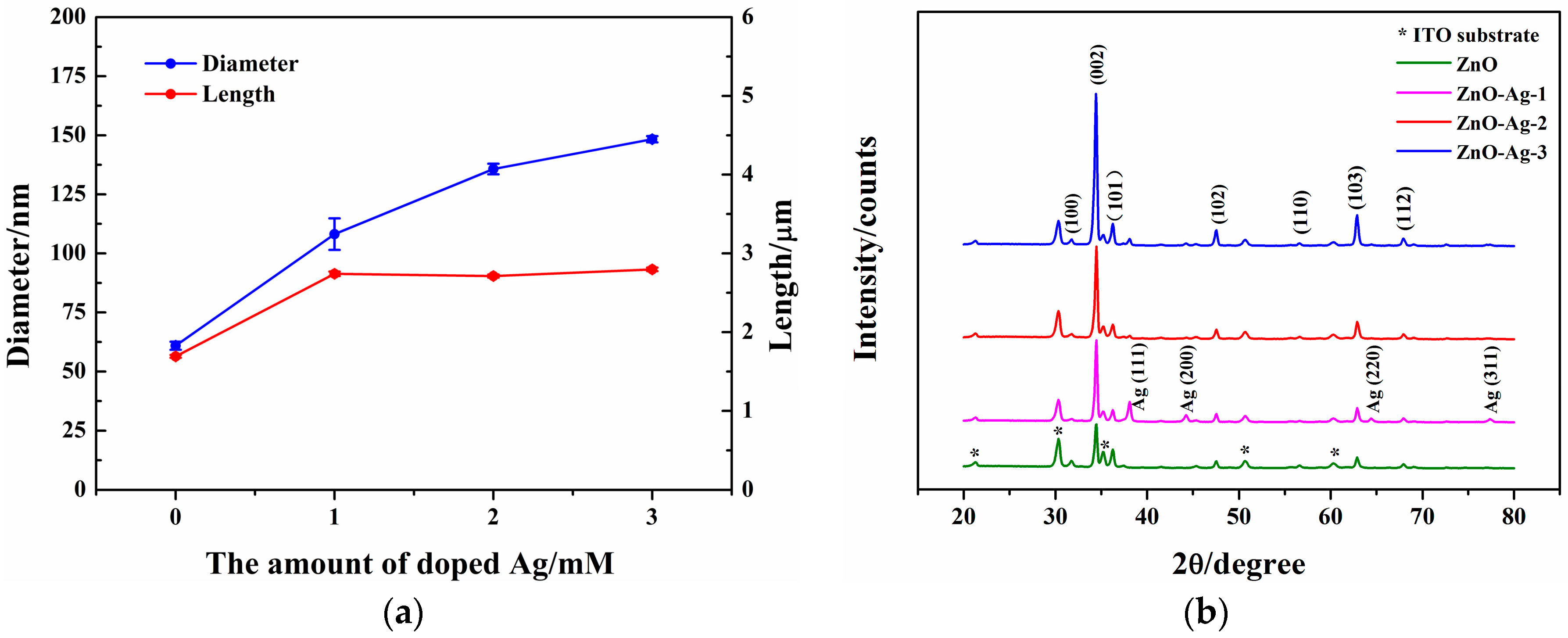
3.1. Characterization of the ZnO and ZnO-Ag Nanorods
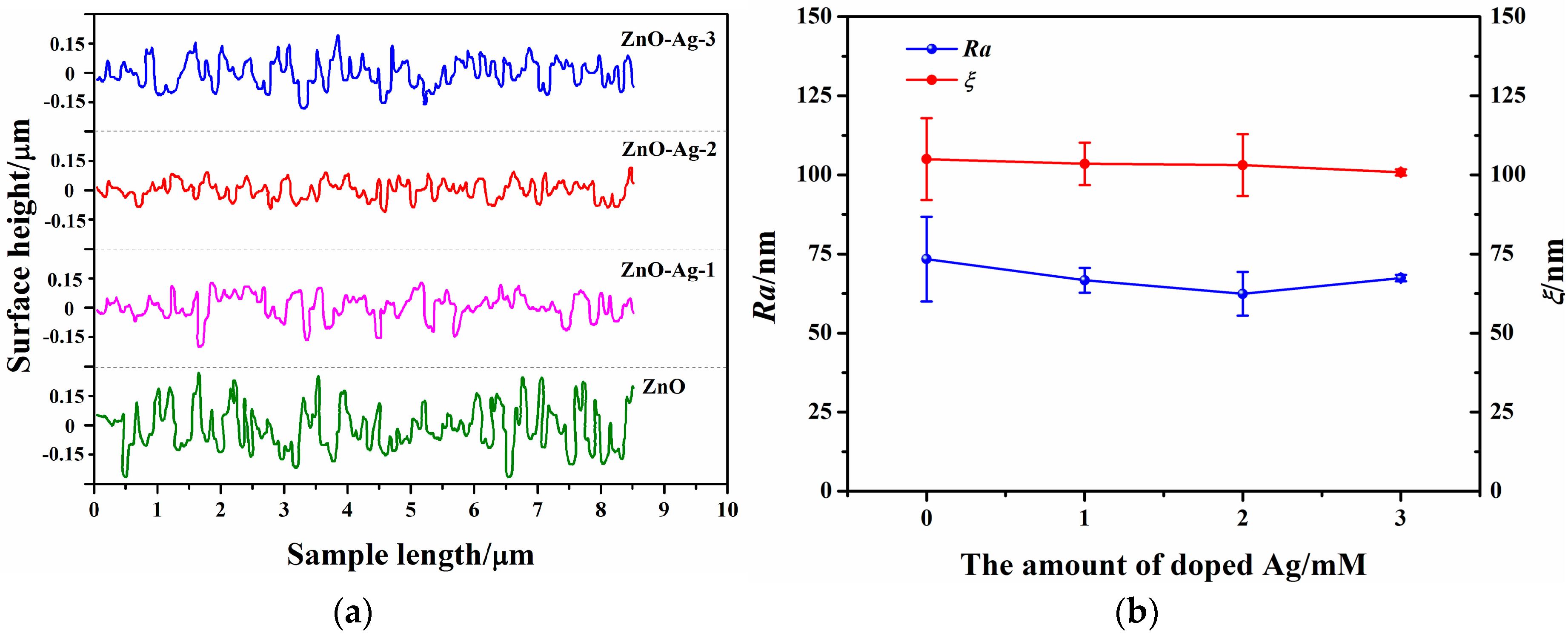
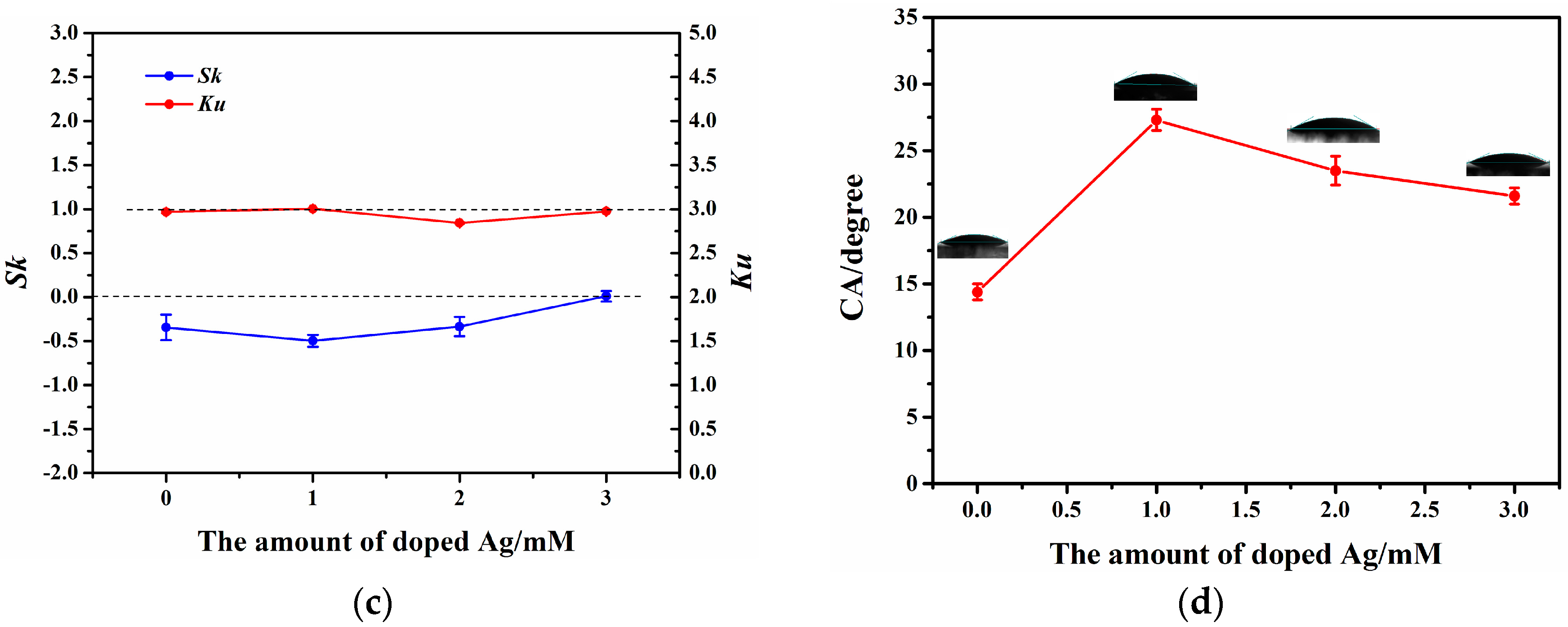
3.2. Surface Morphologies and Wettability of the ZnO and ZnO-Ag Nanorods
3.3. Electrochemical Properties of the Glucose Sensors
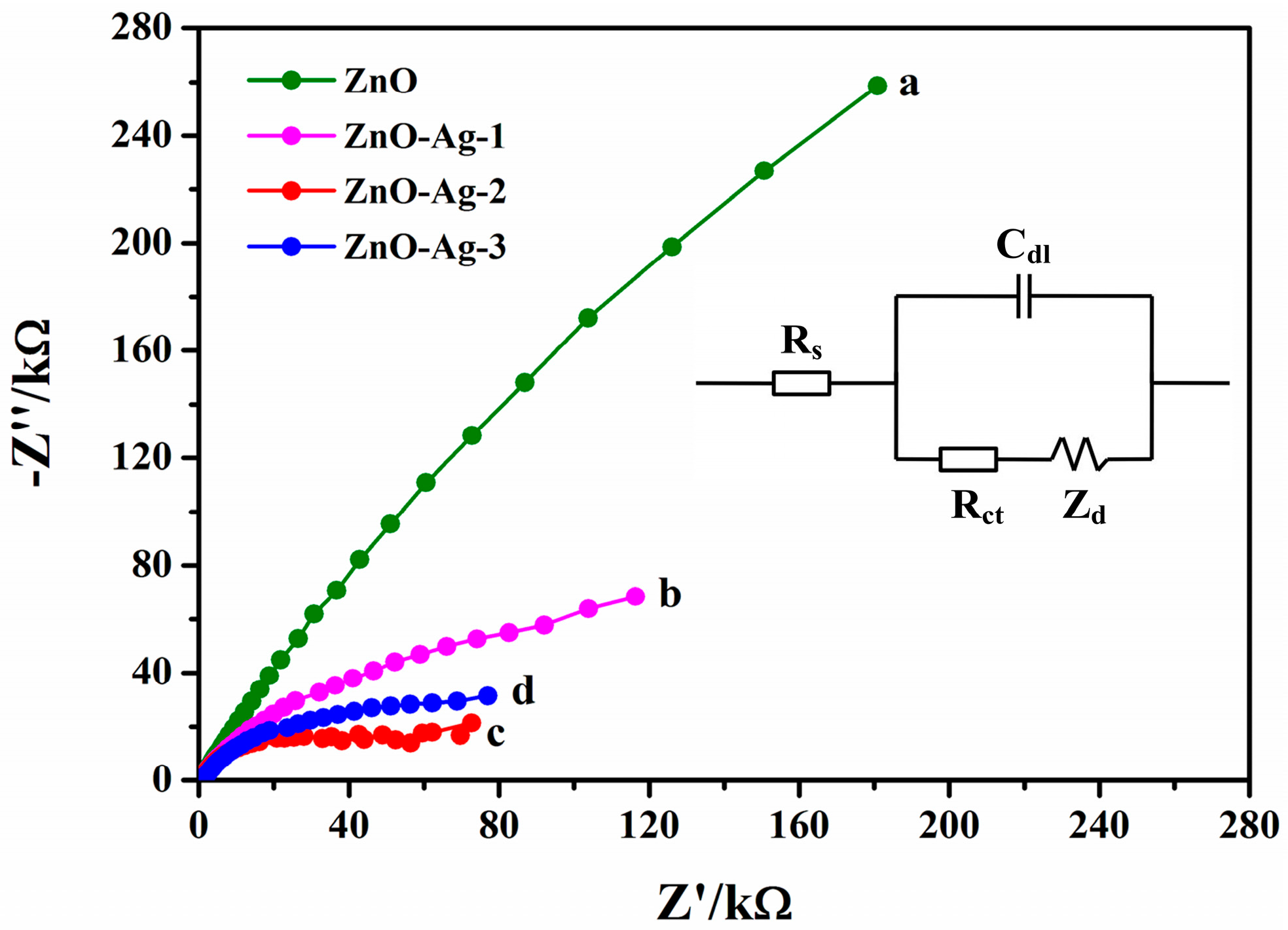
3.3.1. Electrochemical Impedance Spectroscopy
3.3.2. Cyclic Voltammogram
3.3.3. Amperometric Response
3.3.4. Comparison of the Glucose Sensors in This and Previous Works
3.3.5. Selectivity and Storage Stability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Khun, K.; Ibupoto, Z.H.; Lu, J.; Alsalhi, M.S.; Atif, M.; Ansari, A.A.; Willander, M. Potentiometric glucose sensor based on the glucose oxidase immobilized iron ferrite magnetic particle/chitosan composite modified gold coated glass electrode. Sens. Actuators B Chem. 2012, 173, 698–703. [Google Scholar] [CrossRef]
- Zhao, Z.; Lei, W.; Zhang, X.; Wang, B.; Jiang, H. ZnO-based amperometric enzyme biosensors. Sensors 2010, 10, 1216–1231. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.; Pan, L.; Huang, W. Recent progress in the ZnO nanostructure-based sensors. Mater. Sci. Eng. B 2011, 176, 1409–1421. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Jin, H.K.; Hahn, Y.B. Highly selective wide linear-range detecting glucose biosensors based on aspect-ratio controlled ZnO nanorods directly grown on electrodes. Sens. Actuators B Chem. 2012, 174, 195–201. [Google Scholar] [CrossRef]
- Yang, K.; She, G.W.; Wang, H.; Ou, X.M.; Zhang, X.H.; Lee, C.S.; Lee, S.T. ZnO nanotube arrays as biosensors for glucose. J. Phys. Chem. C 2009, 113, 20169–20172. [Google Scholar] [CrossRef]
- Wang, J.X.; Sun, X.W.; Wei, A.; Lei, Y.; Cai, X.P.; Li, C.M.; Dong, Z.L. Zinc oxide nanocomb biosensor for glucose detection. Appl. Phys. Lett. 2006, 88, 34. [Google Scholar] [CrossRef]
- Xu, C.X.; Sun, X.W.; Dong, Z.L.; Yu, M.B. Zinc oxide nanodisk. Appl. Phys. Lett. 2004, 85, 3878–3880. [Google Scholar] [CrossRef]
- Tereshchenko, A.; Bechelany, M.; Viter, R.; Khranovskyy, V.; Smyntyna, V.; Starodub, N.; Yakimova, R. Optical biosensors based on ZnO nanostructures: advantages and perspectives. A review. Sens. Actuators B Chem. 2016, 229, 664–677. [Google Scholar] [CrossRef]
- Fang, L.; Liu, B.; Liu, L.; Li, Y.; Huang, K.; Zhang, Q. Direct electrochemistry of glucose oxidase immobilized on Au nanoparticles-functionalized 3D hierarchically ZnO nanostructures and its application to bioelectrochemical glucose sensor. Sens. Actuators B Chem. 2016, 222, 1096–1102. [Google Scholar] [CrossRef]
- Tian, K.; Alex, S.; Siegel, G.; Tiwari, A. Enzymatic glucose sensor based on Au nanoparticle and plant-like ZnO film modified electrode. Mater. Sci. Eng. C 2015, 46, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Anusha, J.R.; Kim, H.J.; Fleming, A.T.; Das, S.J.; Yu, K.H.; Kim, B.C.; Raj, C.J. Simple fabrication of ZnO/Pt/chitosan electrode for enzymatic glucose biosensor. Sens. Actuators B Chem. 2014, 202, 827–833. [Google Scholar] [CrossRef]
- Chu, X.; Zhu, X.; Dong, Y.; Chen, T.; Ye, M.; Sun, W. An amperometric glucose biosensor based on the immobilization of glucose oxidase on the platinum electrode modified with NiO doped ZnO nanorods. J. Electroanal. Chem. 2012, 676, 20–26. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Chen, X.J.; Tay, B.K.; Chen, J.S.; Han, Z.J.; Khor, K.A. A novel amperometric biosensor based on ZnO:Co nanoclusters for biosensing glucose. Biosens. Bioelectron. 2007, 23, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, Y.; Liu, X.; Xian, Y.; Shi, G.; Jin, L. ZnO nanorods/Au hybrid nanocomposites for glucose biosensor. Biosens. Bioelectron. 2010, 26, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, C.; Li, C.M.; Li, Y.; Chi, Q.; Huang, X.; Liao, L.; Yu, T. Carbon-decorated ZnO nanowire array: A novel platform for direct electrochemistry of enzymes and biosensing applications. Electrochem. Commun. 2009, 11, 202–205. [Google Scholar] [CrossRef]
- Fidal, V.T.K.P.; Inguva, S.; Krishnamurthy, S.; Marsili, E.; Mosnier, J.P.; Chandra, T.S. Mediator-free interaction of glucose oxidase, as model enzyme for immobilization, with Al-doped and undoped ZnO thin films laser-deposited on polycarbonate supports. Enzyme Microb. Technol. 2017, 96, 67–74. [Google Scholar]
- Patil, S.S.; Mali, M.G.; Tamboli, M.S.; Patil, D.R.; Kulkarni, M.V.; Yoon, H.; Kim, H.; Al-Deyab, S.S.; Yoon, S.S.; Kolekar, S.S.; et al. Green approach for hierarchical nanostructured Ag-ZnO and their photocatalytic performance under sunlight. Catal. Today 2016, 260, 126–134. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, L.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K. Ag/ZnO heterostructure nanocrystals: Synthesis, characterization, and photocatalysis. Inorg. Chem. 2007, 46, 6980–6986. [Google Scholar] [CrossRef] [PubMed]
- Xian, F.; Miao, K.; Bai, X.; Ji, Y.; Chen, F.; Li, X. Characteraction of Ag-doped ZnO thin film synthesized by sol–gel method and its using in thin film solar cells. Optik 2013, 124, 4876–4879. [Google Scholar] [CrossRef]
- Mu-Hsiang, H.; Chi-Jung, C. Ag-doped ZnO nanorods coated metal wire meshes as hierarchical photocatalysts with high visible-light driven photoactivity and photostability. J. Hazard. Mater. 2014, 278, 444–453. [Google Scholar]
- Zhou, F.; Jing, W.; Wu, Q.; Gao, W.; Jiang, Z.; Shi, J.; Cui, Q.B. Effects of the surface morphologies of ZnO nanotube arrays on the performance of amperometric glucose sensors. Mater. Sci. Semicond. Process. 2016, 56, 137–144. [Google Scholar] [CrossRef]
- Quéré, D. Wetting and roughness. Annu. Rev. Mater. Res. 2008, 38, 71–99. [Google Scholar] [CrossRef]
- Baszkin, A.; Lyman, D.J. The interaction of plasma proteins with polymers. I. Relationship between polymer surface energy and protein adsorption/desorption. J. Biomed. Mater. Res. 1980, 14, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Michiardi, A.; Aparicio, C.; Ratner, B.D.; Planell, J.A.; Gil, J. The influence of surface energy on competitive protein adsorption on oxidized NiTi surfaces. Biomaterials 2007, 28, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wen, L.; Li, F.; Cheng, H.M. Nanosized Li4Ti5O12/graphene hybrid materials with low polarization for high rate lithium ion batteries. J. Power Sources 2011, 196, 8610–8617. [Google Scholar] [CrossRef]


| Nanostructures | Sensitivity μA/(mM·cm2) | Detection Limit μM | Linear Range mM | Reference |
|---|---|---|---|---|
| ZnO | 0.75 | 2.1 | 2.1 × 10−3–10.0 | This work |
| ZnO-Ag-1 | 1.34 | 1.9 | 1.9 × 10−3–6.0 | This work |
| ZnO-Ag-2 | 3.85 | 1.5 | 1.5 × 10−3–6.5 | This work |
| ZnO-Ag-3 | 2.85 | 1.4 | 1.4 × 10−3–5.0 | This work |
| ZnO-Ni | 61.78 | 2.5 | 0.5–8 | [13] |
| ZnO-Co | 13.3 | 20 | 0–4 | [14] |
| ZnO-Al | 5.5 | 167 | 0.28–28 | [17] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, F.; Jing, W.; Liu, P.; Han, D.; Jiang, Z.; Wei, Z. Doping Ag in ZnO Nanorods to Improve the Performance of Related Enzymatic Glucose Sensors. Sensors 2017, 17, 2214. https://doi.org/10.3390/s17102214
Zhou F, Jing W, Liu P, Han D, Jiang Z, Wei Z. Doping Ag in ZnO Nanorods to Improve the Performance of Related Enzymatic Glucose Sensors. Sensors. 2017; 17(10):2214. https://doi.org/10.3390/s17102214
Chicago/Turabian StyleZhou, Fan, Weixuan Jing, Pengcheng Liu, Dejun Han, Zhuangde Jiang, and Zhengying Wei. 2017. "Doping Ag in ZnO Nanorods to Improve the Performance of Related Enzymatic Glucose Sensors" Sensors 17, no. 10: 2214. https://doi.org/10.3390/s17102214





