Particle Accumulation in a Microchannel and Its Reduction by a Standing Surface Acoustic Wave (SSAW)
Abstract
:1. Introduction
2. Methodology
2.1. Numerical Simulation
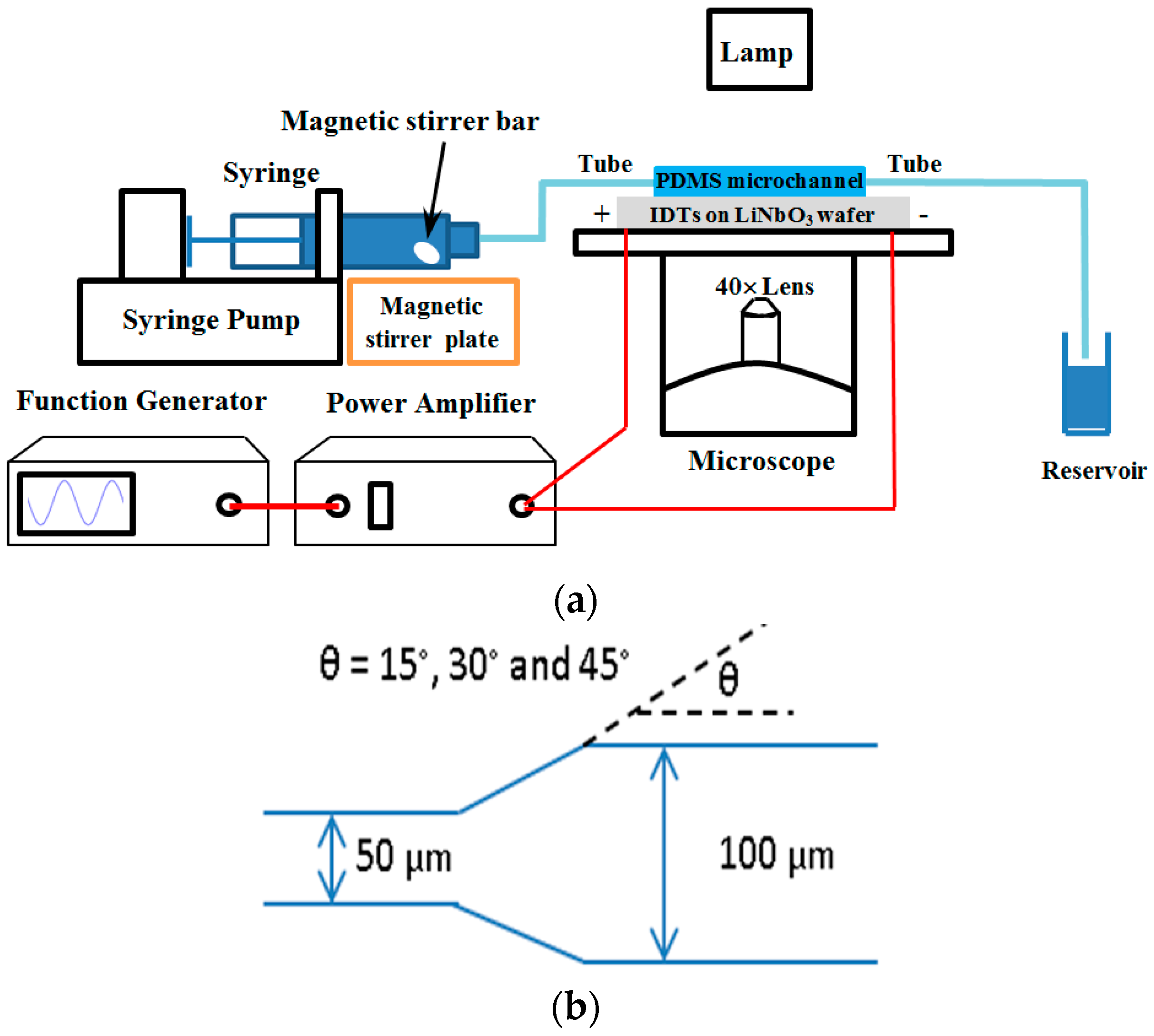
2.2. Experiment Setup
2.3. Statistical Analysis
3. Results
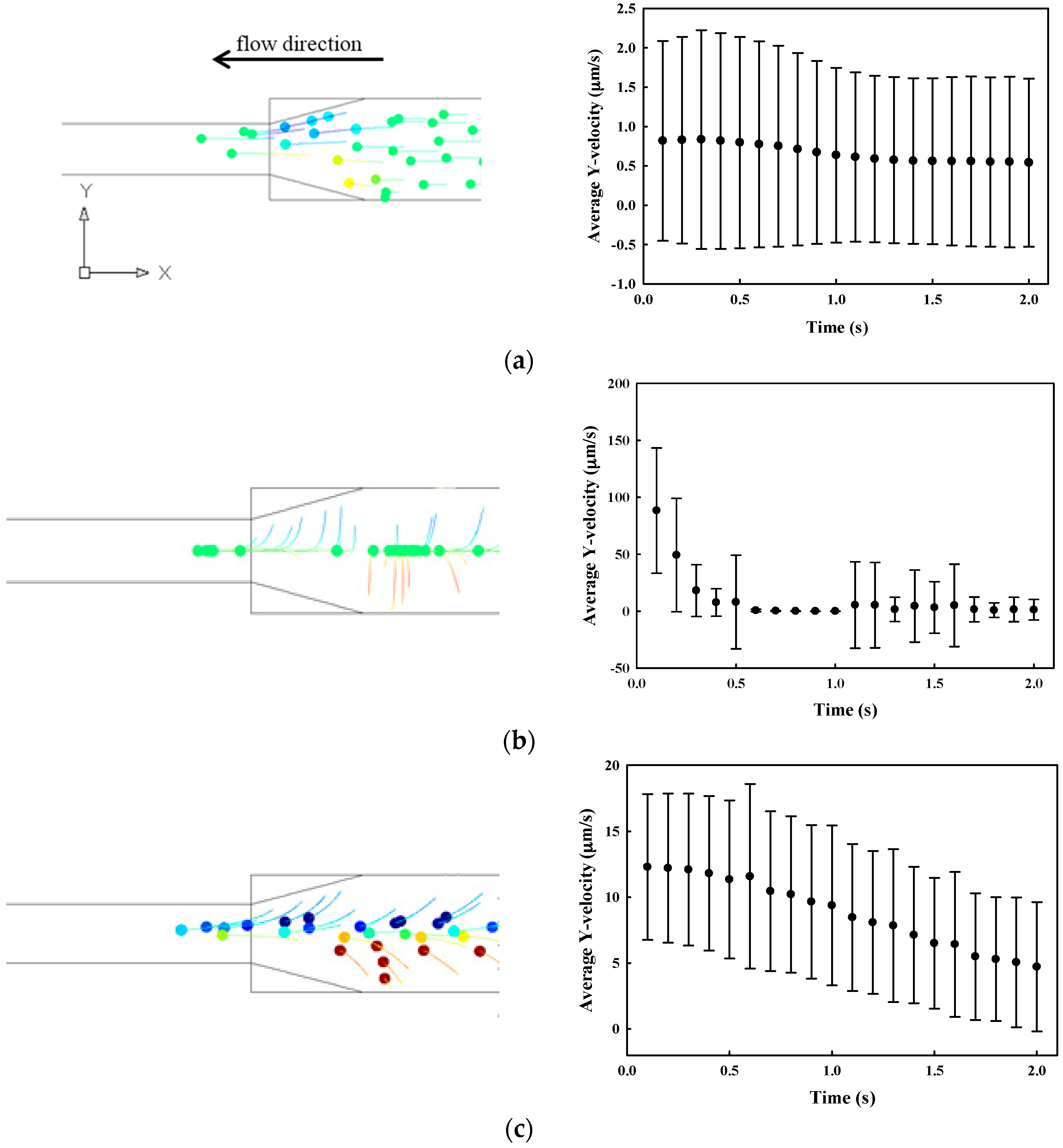
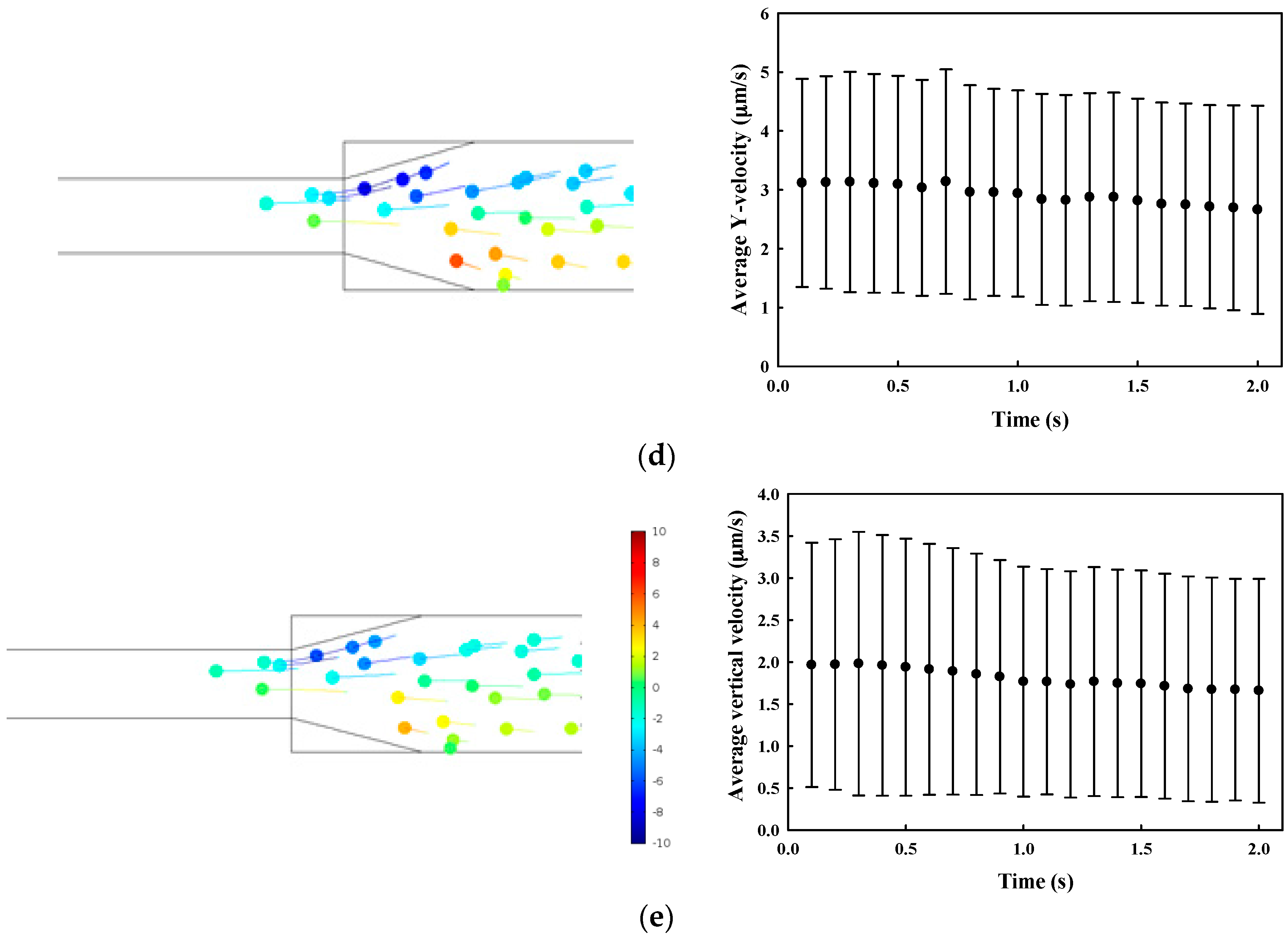
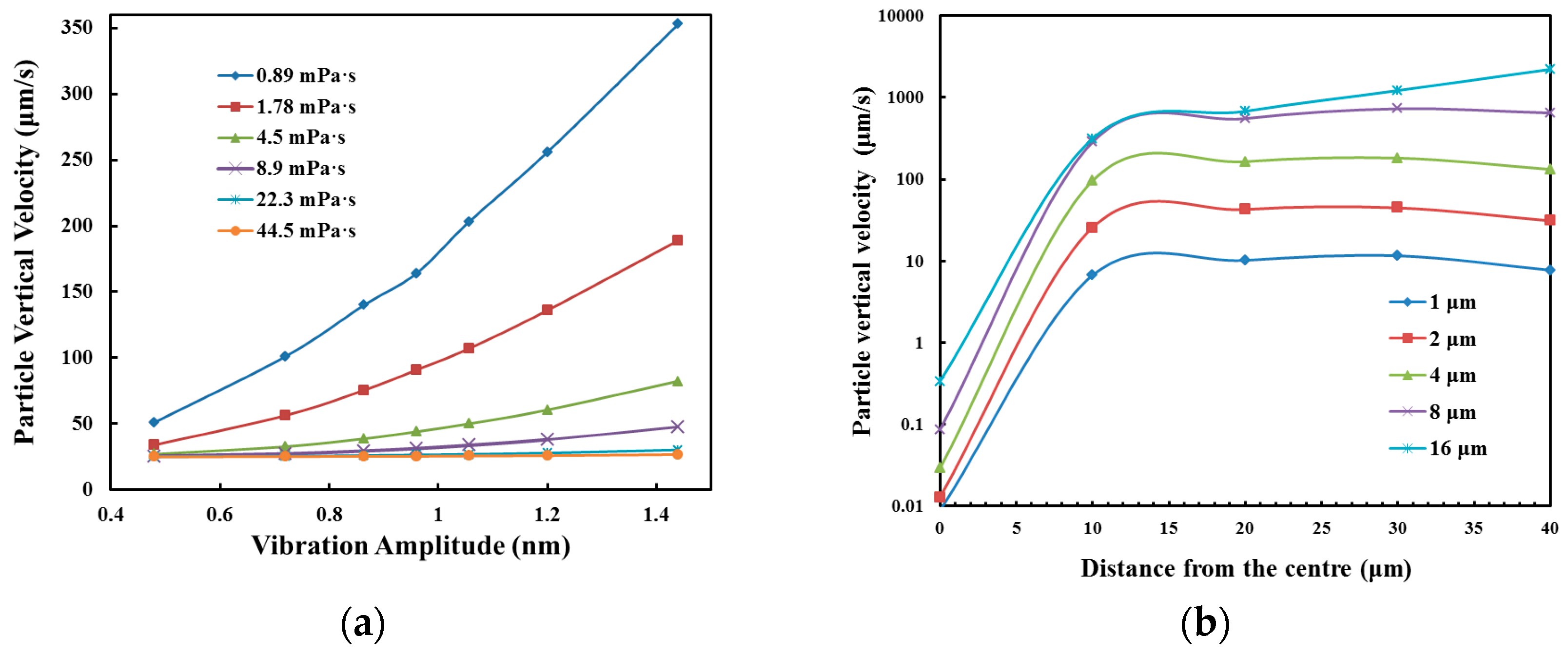
3.1. Numerical Simulation
3.2. Experiment Results
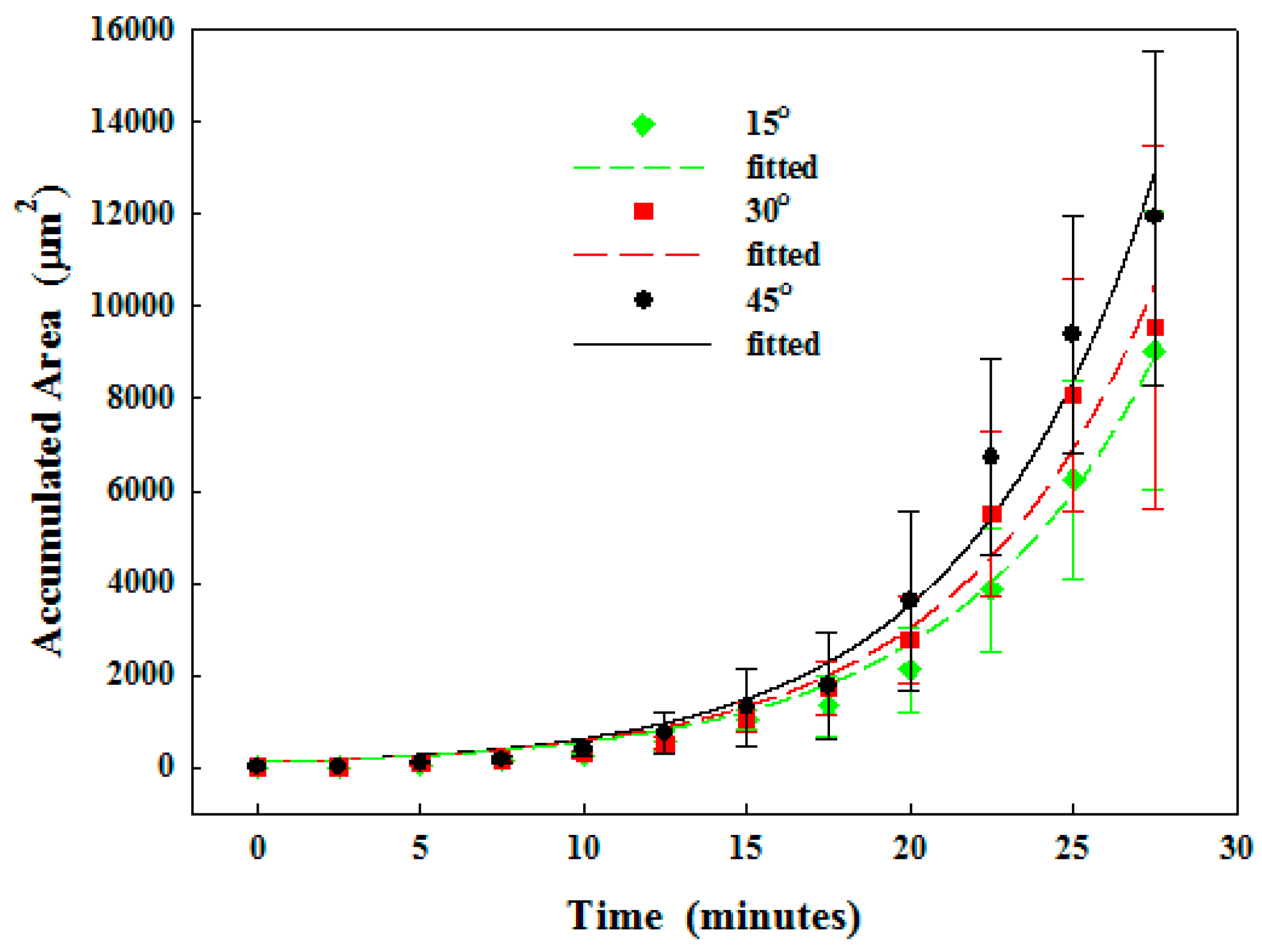
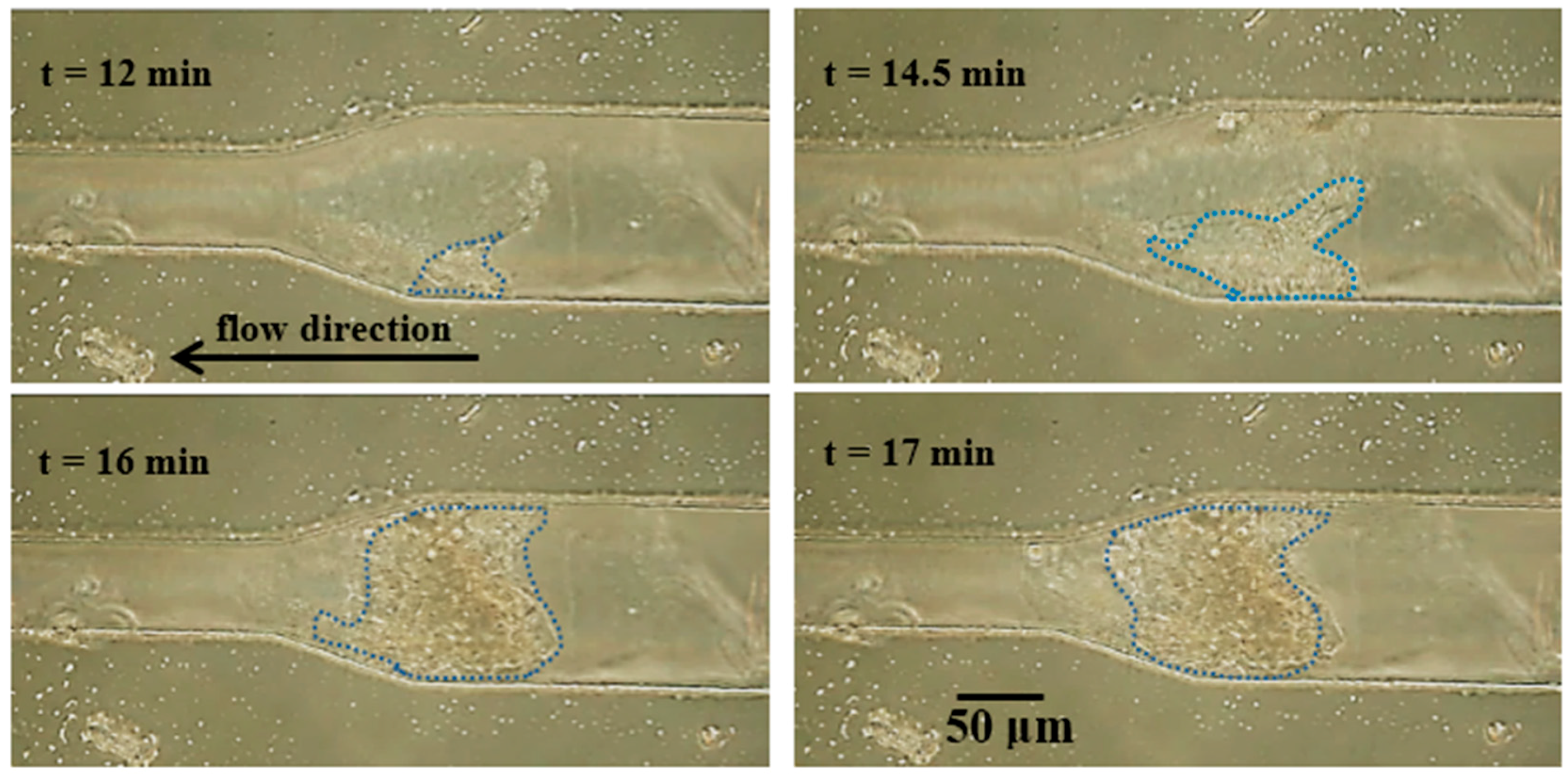
3.2.1. Particle Clogging
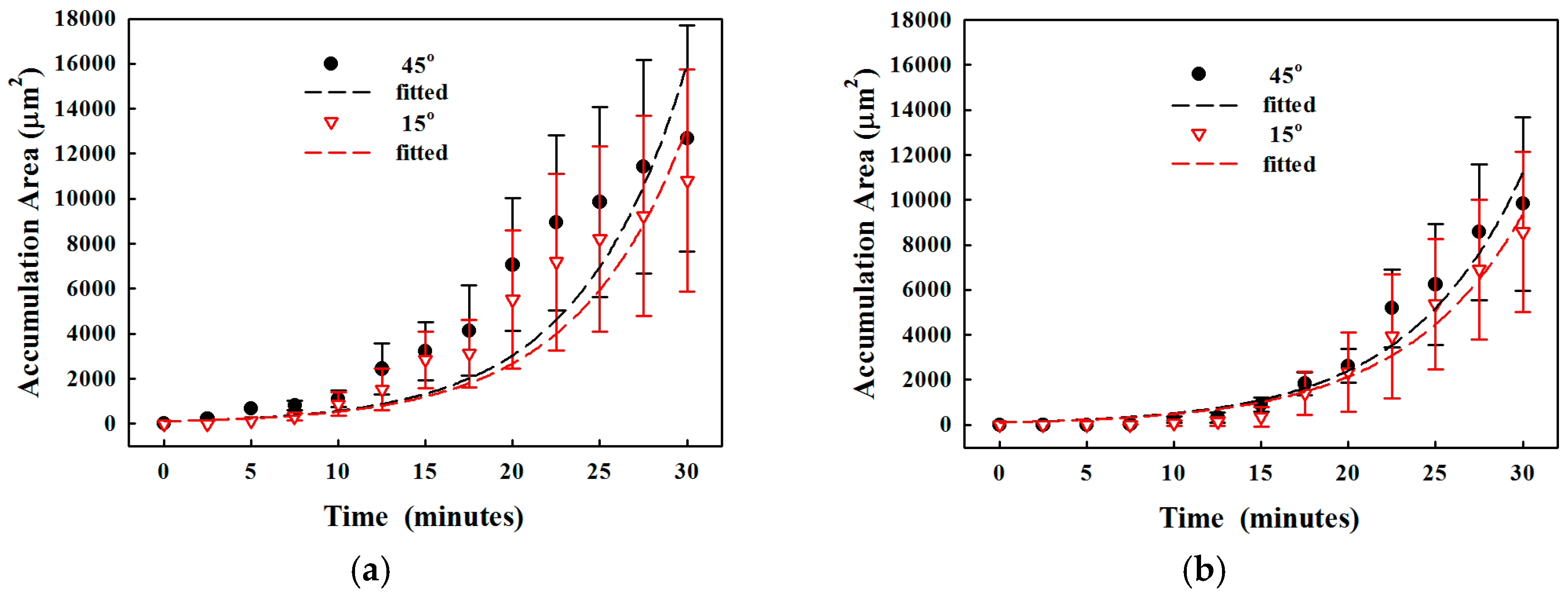
3.2.2. Reduction of Clogging by SSAW
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kamphoefner, F.J. Ink jet printing. IEEE Trans. Electron. Devices 1972, 19, 584–593. [Google Scholar] [CrossRef]
- Derby, B. Inkjet printing of functional and structural materials: Fluid property requirements, feature stability, and resolution. Annu. Rev. Mater. Res. 2010, 40, 395–414. [Google Scholar] [CrossRef]
- Sirringhaus, H.; Kawase, T.; Friend, R.; Shimoda, T.; Inbasekaran, M.; Wu, W.; Woo, E. High-resolution inkjet printing of all-polymer transistor circuits. Science 2000, 290, 2123–2126. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.; Boland, T.; Trusk, T.; Forgacs, G.; Markwald, R.R. Organ printing: Computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003, 21, 157–161. [Google Scholar] [CrossRef]
- MacBeath, G.; Schreiber, S.L. Printing proteins as microarrays for high-throughput function determination. Science 2000, 289, 1760–1763. [Google Scholar] [PubMed]
- Agbangla, G.C.; Climent, É.; Bacchin, P. Experimental investigation of pore clogging by microparticles: Evidence for a critical flux density of particle yielding arches and deposits. Sep. Purif. Technol. 2012, 101, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Bacchin, P.; Derekx, Q.; Veyret, D.; Glucina, K.; Moulin, P. Clogging of microporous channels networks: Role of connectivity and tortuosity. Microfluid. Nanofluid. 2014, 17, 85–96. [Google Scholar] [CrossRef]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef] [PubMed]
- Meacham, J.M.; O’Rourke, A.; Yang, Y.; Fedorov, A.G.; Degertekin, F.L.; Rosen, D.W. Micromachined ultrasonic print-head for deposition of high-viscosity materials. J. Manuf. Sci. Eng. 2010, 132, 030905. [Google Scholar] [CrossRef]
- Dersoir, B.; de Saint Vincent, M.R.; Abkarian, M.; Tabuteau, H. Clogging of a single pore by colloidal particles. Microfluid. Nanofluid. 2015, 19, 953–961. [Google Scholar] [CrossRef]
- Hassan, I.B.; Lafforgue, C.; Ayadi, A.; Schmitz, P. In situ 3D characterization of bidisperse cakes using confocal laser scanning microscopy. J. Membr. Sci. 2014, 466, 103–113. [Google Scholar] [CrossRef]
- Sharp, K.; Adrian, R. On flow-blocking particle structures in microtubes. Microfluid. Nanofluid. 2005, 1, 376–380. [Google Scholar] [CrossRef]
- Wyss, H.M.; Blair, D.L.; Morris, J.F.; Stone, H.A.; Weitz, D.A. Mechanism for clogging of microchannels. Phys. Rev. E 2006, 74, 061402. [Google Scholar] [CrossRef] [PubMed]
- Parsa, S.; Gupta, M.; Loizeau, F.; Cheung, K.C. Effects of surfactant and gentle agitation on inkjet dispensing of living cells. Biofabrication 2010, 2, 025003. [Google Scholar] [CrossRef] [PubMed]
- Lavers, J.; Kadar, L. Application of electromagnetic forces to reduce tundish nozzle clogging. Appl. Math. Model. 2004, 28, 29–45. [Google Scholar] [CrossRef]
- Ding, X.; Lin, S.-C.S.; Kiraly, B.; Yue, H.; Li, S.; Chiang, I.-K.; Shi, J.; Benkovic, S.J.; Huang, T.J. On-chip manipulation of single microparticles, cells, and organisms using surface acoustic waves. Proc. Natl. Acad. Sci. USA 2012, 109, 11105–11109. [Google Scholar] [CrossRef] [PubMed]
- Franke, T.; Braunmüller, S.; Schmid, L.; Wixforth, A.; Weitz, D. Surface acoustic wave actuated cell sorting (SAWACS). Lab Chip 2010, 10, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Friend, J.R.; Yeo, L.Y. Surface acoustic wave concentration of particle and bioparticle suspensions. Biomed. Microdevices 2007, 9, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Destgeer, G.; Lee, K.H.; Jung, J.H.; Alazzam, A.; Sung, H.J. Continuous separation of particles in a PDMS microfluidic channel via travelling surface acoustic waves (TSAW). Lab Chip 2013, 13, 4210–4216. [Google Scholar] [CrossRef] [PubMed]
- Kapishnikov, S.; Kantsler, V.; Steinberg, V. Continuous particle size separation and size sorting using ultrasound in a microchannel. J. Stat. Mech. 2006, 2006, P01012. [Google Scholar] [CrossRef]
- Coakley, W.T. Ultrasonic separations in analytical biotechnology. Trends Biotechnol. 1997, 15, 506–511. [Google Scholar] [CrossRef]
- Lenshof, A.; Magnusson, C.; Laurell, T. Acoustofluidics 8: Applications of acoustophoresis in continuous flow microsystems. Lab Chip 2012, 12, 1210–1223. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.J.; Alan, T.; Helmerson, K.; Neild, A. Surface acoustic waves for on-demand production of picoliter droplets and particle encapsulation. Lab Chip 2013, 13, 3225–3231. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Finley, T.D.; Turkaydin, M.; Sung, Y.; Gurkan, U.A.; Yavuz, A.S.; Guldiken, R.O.; Demirci, U. The assembly of cell-encapsulating microscale hydrogels using acoustic waves. Biomaterials 2011, 32, 7847–7855. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, P.; Lin, S.-C.S.; Stratton, Z.S.; Nama, N.; Guo, F.; Slotcavage, D.; Mao, X.; Shi, J.; Costanzo, F. Surface acoustic wave microfluidics. Lab Chip 2013, 13, 3626–3649. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ahmed, D.; Mao, X.; Lin, S.-C.S.; Lawit, A.; Huang, T.J. Acoustic tweezers: Patterning cells and microparticles using standing surface acoustic waves (SSAW). Lab Chip 2009, 9, 2890–2895. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Mao, X.; Ahmed, D.; Colletti, A.; Huang, T.J. Focusing microparticles in a microfluidic channel with standing surface acoustic waves (SSAW). Lab Chip 2008, 8, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Peng, Z.; Lin, S.-C.S.; Geri, M.; Li, S.; Li, P.; Chen, Y.; Dao, M.; Suresh, S.; Huang, T.J. Cell separation using tilted-angle standing surface acoustic waves. Proc. Natl. Acad. Sci. USA 2014, 111, 12992–12997. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.J.; Morahan, B.; Garcia-Bustos, J.; Doerig, C.; Plebanski, M.; Neild, A. Two-dimensional single-cell patterning with one cell per well driven by surface acoustic waves. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wu, M.; Guo, F.; Li, P.; Chan, C.Y.; Mao, Z.; Li, S.; Ren, L.; Zhang, R.; Huang, T.J. Rapid formation of size-controllable multicellular spheroids via 3D acoustic tweezers. Lab Chip 2016, 16, 2636–2643. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Mao, Z.; Chen, Y.; Xie, Z.; Lata, J.P.; Li, P.; Ren, L.; Liu, J.; Yang, J.; Dao, M. Three-dimensional manipulation of single cells using surface acoustic waves. Proc. Natl. Acad. Sci. USA 2016, 113, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, G.K. An Introduction to Fluid Dynamics; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Glynne-Jones, P.; Hill, M. Acoustofluidics 23: Acoustic manipulation combined with other force fields. Lab Chip 2013, 13, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Bruus, H. Acoustofluidics 1: Governing equations in microfluidics. Lab Chip 2011, 11, 3742–3751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruus, H. Acoustofluidics 7: The acoustic radiation force on small particles. Lab Chip 2012, 12, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications; Academic Press: San Diego, CA, USA, 2013. [Google Scholar]
- Sriphutkiat, Y. Particles in the Microchannel. Available online: https://www.dropbox.com/sh/uv01q29cq9zla5l/AAD9VlTBxVmY_thlJ8x0IBtca?dl=0 (accessed on 7 June 2016).
- Sendekie, Z.B.; Bacchin, P. Colloidal jamming dynamics in microchannel bottlenecks. Langmuir 2016, 32, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Whittle, M.; Murray, B.S.; Chen, J.; Dickinson, E. Simulation and experiments on colloidal particle capture in a shear field. Langmuir 2000, 16, 9784–9791. [Google Scholar] [CrossRef]
- Whittle, M.; Murray, B.S.; Dickinson, E. Simulation of colloidal particle scattering: Sensitivity to attractive forces. J. Colloid Interface Sci. 2000, 225, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Mustin, B.; Stoeber, B. Deposition of particles from polydisperse suspensions in microfluidic systems. Microfluid. Nanofluid. 2010, 9, 905–913. [Google Scholar] [CrossRef]
- Bergendahl, J.; Grasso, D. Prediction of colloid detachment in a model porous media: Hydrodynamics. Chem. Eng. Sci. 2000, 55, 1523–1532. [Google Scholar] [CrossRef]
- Bacchin, P.; Marty, A.; Duru, P.; Meireles, M.; Aimar, P. Colloidal surface interactions and membrane fouling: Investigations at pore scale. Adv. Colloid Interface Sci. 2011, 164, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Mustin, B.; Stoeber, B. Single layer deposition of polystyrene particles onto planar Polydimethylsiloxane substrates. Langmuir 2015, 32, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Mi, B.; Elimelech, M. Chemical and physical aspects of organic fouling of forward osmosis membranes. J. Membr. Sci. 2008, 320, 292–302. [Google Scholar] [CrossRef]
- Colosi, C.; Shin, S.R.; Manoharan, V.; Massa, S.; Costantini, M.; Barbetta, A.; Dokmeci, M.R.; Dentini, M.; Khademhosseini, A. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv. Mater. 2016, 28, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Gumbiner, B.M. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell 1996, 84, 345–357. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Academic Press: San Diego, CA, USA, 2011. [Google Scholar]
- Ninham, B. On progress in forces since the DLVO theory. Adv. Colloid Interface Sci. 1999, 83, 1–17. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Weroński, P. Application of the DLVO theory for particle deposition problems. Adv. Colloid Interface Sci. 1999, 83, 137–226. [Google Scholar] [CrossRef]
- Ahmed, D.; Ozcelik, A.; Bojanala, N.; Nama, N.; Upadhyay, A.; Chen, Y.; Hanna-Rose, W.; Huang, T.J. Rotational manipulation of single cells and organisms using acoustic waves. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Anhäuser, A.; Wunenburger, R.; Brasselet, E. Acoustic rotational manipulation using orbital angular momentum transfer. Phys. Rev. Lett. 2012, 109, 034301. [Google Scholar] [CrossRef] [PubMed]
- Seager, C.R.; Mason, T.G. Slippery diffusion-limited aggregation. Phys. Rev. E 2007, 75, 011406. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, L.A.; Coakley, W.T. Applications of ultrasound streaming and radiation force in biosensors. Biosens. Bioelectron. 2007, 22, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Settnes, M.; Bruus, H. Forces acting on a small particle in an acoustical field in a viscous fluid. Phys. Rev. E 2012, 85, 016327. [Google Scholar] [CrossRef] [PubMed]
- Nama, N.; Barnkob, R.; Mao, Z.; Kähler, C.J.; Costanzo, F.; Huang, T.J. Numerical study of acoustophoretic motion of particles in a PDMS microchannel driven by surface acoustic waves. Lab Chip 2015, 15, 2700–2709. [Google Scholar] [CrossRef] [PubMed]
- Devendran, C.; Albrecht, T.; Brenker, J.; Alan, T.; Neild, A. The importance of travelling wave components in standing surface acoustic wave (SSAW) systems. Lab Chip 2016, 16, 3756–3766. [Google Scholar] [CrossRef] [PubMed]
- Mengwei, L.; Junhong, L.; Jun, M.; Chenghao, W. Design and fabrication of a MEMS Lamb wave device based on ZnO thin film. J. Semicond. 2011, 32, 044006. [Google Scholar]
- Zhou, X.; Zhao, C.; Hou, R.; Zhang, J.; Kirk, K.; Hutson, D.; Guo, Y.; Hu, P.; Peng, S.; Zu, X. Sputtered ZnO film on aluminium foils for flexible ultrasonic transducers. Ultrasonics 2014, 54, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; El-Hady, A.M.; Zhao, C.; Du, J.; Liu, Y.; Fu, Y. Flexible and bendable acoustofluidics based on ZnO film coated aluminium foil. Sens. Actuators B Chem. 2015, 221, 230–235. [Google Scholar] [CrossRef]






| Medium | Parameters | Values |
|---|---|---|
| Water | density, ρw | 997 kg/m |
| speed of sound, cw | 1497 m/s | |
| viscosity, μw | 0.890 mPa·s | |
| compressibility, κw | 448 TPa−1 | |
| Microparticle | density, ρp | 1050 kg/m |
| speed of sound, cp | 2350 m/s | |
| Poisson’s ratio, εp | 0.35 | |
| compressibility, κp | 249 TPa−1 | |
| Poly-dimethylsiloxane (PDMS, 10:1) | density, ρPDMS | 920 kg/m |
| speed of sound, cPDMS | 1076.5 m/s | |
| Lithium niobate (LiNbO3) | speed of sound, cLNB | 3990 m/s |
| wavelength, λ | 200 μm | |
| frequency, f | 19.95 MHz |
| Angle | SSAW | Alginate | Accumulation Area (μm2) | A | B | R2 |
|---|---|---|---|---|---|---|
| 15° | No | 0% | 6.3 × 103 ± 2.2 × 103 | 6.2 × 101 | 1.8 × 10−1 | 1.00 |
| 30° | No | 0% | 8.1 × 103 ± 2.5 × 103 | 1.3 × 102 | 1.6 × 10−1 | 0.97 |
| 45° | No | 0% | 9.4 × 103 ± 2.6 × 103 | 1.5 × 102 | 1.6 × 10−1 | 0.98 |
| 15° | Yes | 0% | 1.7 × 103 ± 2.7 × 102 | 5.0 × 101 | 1.4 × 10−1 | 0.99 |
| 30° | Yes | 0% | 2.2 × 103 ± 5.0 × 102 | 5.0 × 101 | 1.5 × 10−1 | 0.99 |
| 45° | Yes | 0% | 2.6 × 103 ± 5.7 × 102 | 6.1 × 101 | 1.5 × 10−1 | 1.00 |
| 15° | No | 3% | 5.4 × 103 ± 2.9 × 103 | 1.5 × 102 | 1.4 × 10−1 | 0.97 |
| 15° | No | 5% | 8.2 × 103 ± 4.1 × 103 | 6.3 × 102 | 9.8 × 10−2 | 0.95 |
| 15° | Yes | 3% | 2.0 × 103 ± 5.2 × 102 | 3.9 × 101 | 1.6 × 10−1 | 0.99 |
| 15° | Yes | 5% | 4.1 × 103 ± 2.0 × 103 | 2.7 × 102 | 1.1 × 10−1 | 0.97 |
| 45° | No | 3% | 6.2 × 103 ± 2.7 × 103 | 2.1 × 102 | 1.3 × 10−1 | 0.96 |
| 45° | No | 5% | 9.9 × 103 ± 4.2 × 103 | 9.0 × 102 | 9.2 × 10−2 | 0.95 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sriphutkiat, Y.; Zhou, Y. Particle Accumulation in a Microchannel and Its Reduction by a Standing Surface Acoustic Wave (SSAW). Sensors 2017, 17, 106. https://doi.org/10.3390/s17010106
Sriphutkiat Y, Zhou Y. Particle Accumulation in a Microchannel and Its Reduction by a Standing Surface Acoustic Wave (SSAW). Sensors. 2017; 17(1):106. https://doi.org/10.3390/s17010106
Chicago/Turabian StyleSriphutkiat, Yannapol, and Yufeng Zhou. 2017. "Particle Accumulation in a Microchannel and Its Reduction by a Standing Surface Acoustic Wave (SSAW)" Sensors 17, no. 1: 106. https://doi.org/10.3390/s17010106






