Selectivity Enhancement by Using Double-Layer MOX-Based Gas Sensors Prepared by Flame Spray Pyrolysis (FSP)
Abstract
:1. Introduction
- (1)
- (2)
- Chemical filters can either eliminate the interfering gas by catalytic conversion into an inert product (for example, by depositing metallic films such as Pd, Pt, or Rh [10] or porous layers of metal oxides [8] on top of the sensing layer) or can influence the chemical reaction of the analyte in the sensing layer to enhance the desired response. In [7,14,15], an increase of sensitivity to a certain target gas was reported, which was achieved by the presence of a second noble metal-loaded Al2O3 layer.
2. Materials and Methods
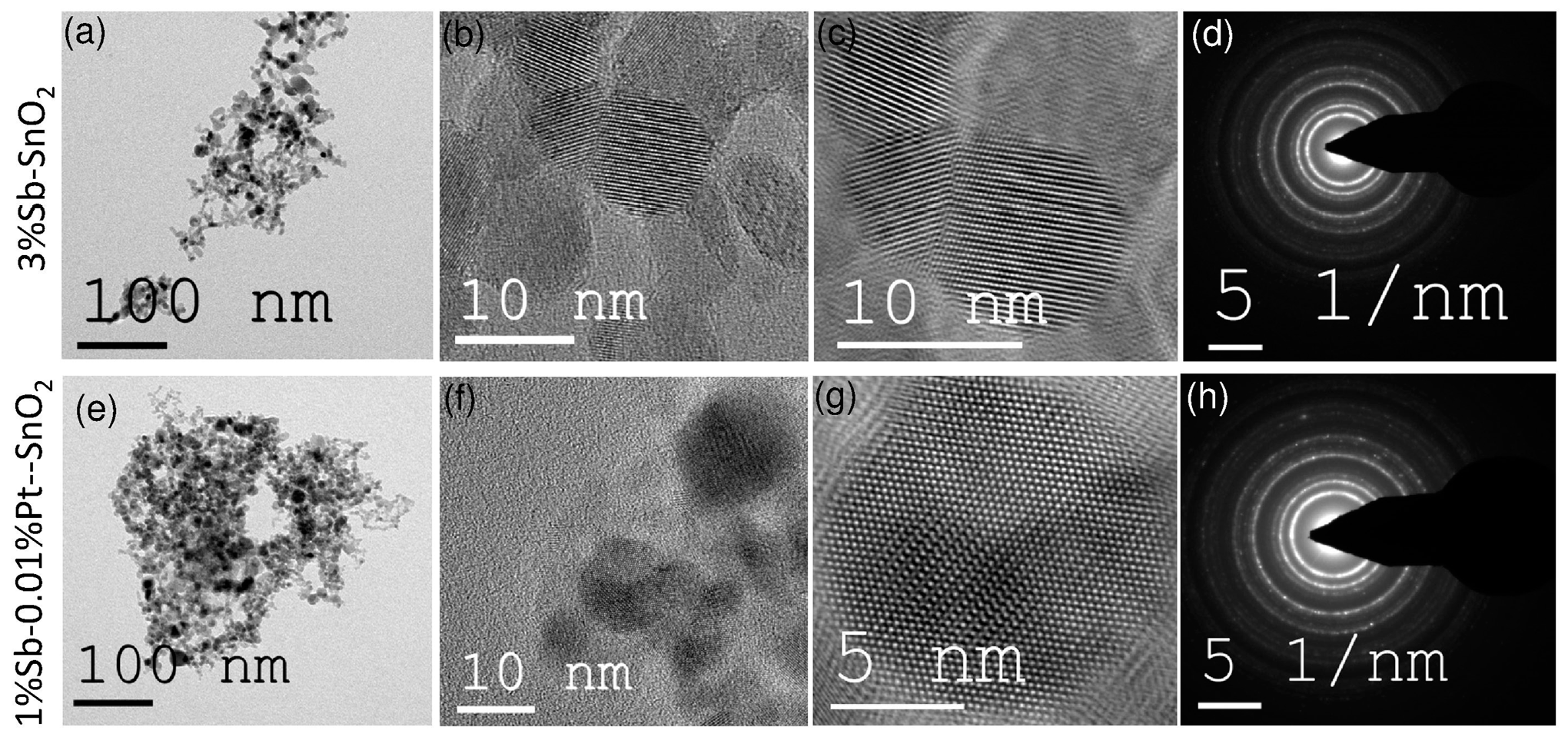
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ihokura, K.; Watson, J. The Stannic Oxide Gas Sensor Principles and Applications; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Williams, D.E. Semiconducting oxides as gas-sensitive resistors. Sens. Actuators B Chem. 1999, 57, 1–16. [Google Scholar] [CrossRef]
- Bârsan, N.; Schweizer-Berberich, M.; Göpel, W. Fundamental and practical aspects in the design of nanoscaled SnO2 gas sensors: A status report. Fresenius J. Anal. Chem. 1999, 365, 287–304. [Google Scholar] [CrossRef]
- Fleischer, M.; Meixner, H. Selectivity in high-temperature operated semiconductor gas-sensors. Sens. Actuators B Chem. 1998, 52, 179–187. [Google Scholar] [CrossRef]
- Moore, S.W.; Gardner, J.W.; Hines, E.L.; Göpel, W.; Weimar, U. A modified multilayer perceptron model for gas mixture analysis. Sens. Actuators B Chem. 1993, 16, 344–348. [Google Scholar] [CrossRef]
- Gardner, J.W. Detection of vapours and odours from a multisensor array using pattern recognition Part 1. Principal component and cluster analysis. Sens. Actuators B Chem. 1991, 4, 109–115. [Google Scholar] [CrossRef]
- Sahm, T.; Rong, W.; Barsan, N.; Mädler, L.; Weimar, U. Sensing of CH4, CO and ethanol with in situ nanoparticle aerosol-fabricated multilayer sensors. Sens. Actuators B Chem. 2007, 127, 63–68. [Google Scholar] [CrossRef]
- Fleischer, M.; Kornely, S.; Weh, T.; Frank, J.; Meixner, H. Selective gas detection with high-temperature operated metal oxides using catalytic filters. Sens. Actuators B Chem. 2000, 69, 205–210. [Google Scholar] [CrossRef]
- Cabot, A.; Arbiol, J.; Cornet, A.; Morante, J.R.; Chen, F.; Liu, M. Mesoporous catalytic filters for semiconductor gas sensors. Thin Solid Films 2003, 436, 64–69. [Google Scholar] [CrossRef]
- Pijolat, C.; Viricelle, J.P.; Tournier, G.; Montmeat, P. Application of membranes and filtering films for gas sensors improvements. Thin Solid Films 2005, 490, 7–16. [Google Scholar] [CrossRef]
- Kwon, C.H.; Yun, D.H.; Hong, H.K.; Kim, S.R.; Lee, K.; Lim, H.Y.; Yoon, K.H. Multi-layered thick-film gas sensor array for selective sensing by catalytic filtering technology. Sens. Actuators B Chem. 2000, 65, 327–330. [Google Scholar] [CrossRef]
- Ryzhikov, A.; Labeau, M.; Gaskov, A. Al2O3 (M = Pt, Ru) catalytic membranes for selective semiconductor gas sensors. Sens. Actuators B Chem. 2005, 109, 91–96. [Google Scholar] [CrossRef]
- Tournier, G.; Pijolat, C. Selective filter for SnO2-based gas sensor: Application to hydrogen trace detection. Sens. Actuators B Chem. 2005, 106, 553–562. [Google Scholar] [CrossRef]
- Hubálek, J.; Malysz, K.; Prášek, J.; Vilanova, X.; Ivanov, P.; Llobet, E.; Brezmes, J.; Correig, X.; Svěrák, Z. Pt-loaded Al2O3 catalytic filters for screen-printed WO3 sensors highly selective to benzene. Sens. Actuators B Chem. 2004, 101, 277–283. [Google Scholar] [CrossRef]
- Su, P.G.; Chen, I.C. Laminating two-layer thick films structure tin oxide-based butane gas sensor operating at low temperature. Sens. Actuators B Chem. 2004, 99, 304–309. [Google Scholar] [CrossRef]
- Takao, Y. High ammonia sensitive semiconductor gas sensors with double-layer structure and interface electrodes. J. Electrochem. Soc. 1994, 141, 1028. [Google Scholar] [CrossRef]
- Yeh, M.-H.; Hwang, W.-S.; Lee, G.-B.; Lu, Y.-M. Characterization of SnO2/TiO2 Double-Layer Films as Alcohol Sensing Materials. Mater. Trans. 2004, 45, 3318–3323. [Google Scholar] [CrossRef]
- Sahm, T.; Rong, W.; Bârsan, N.; Mädler, L.; Friedlander, S.K.; Weimar, U. Formation of multilayer films for gas sensing by in situ thermophoretic deposition of nanoparticles from aerosol phase. J. Mater. Res. 2007, 22, 850–857. [Google Scholar] [CrossRef]
- Sahm, T.; Mädler, L.; Gurlo, A.; Bârsan, N.; Weimar, U.; Roessler, A.; Pratsinis, S.E. High performance porous metal oxide sensors via single step fabrication. In Proceedings of the Eurosensors XIX, Barcelona, Spain, 11–14 September 2005; pp. 850–857.
- Mädler, L.; Roessler, A.; Pratsinis, S.E.; Sahm, T.; Gurlo, A.; Barsan, N.; Weimar, U. Direct formation of highly porous gas-sensing films by in situ thermophoretic deposition of flame-made Pt/SnO2 nanoparticles. Sens. Actuators B Chem. 2006, 114, 283–295. [Google Scholar] [CrossRef]
- Großmann, K.; Kovács, K.E.; Pham, D.K.; Mädler, L.; Bârsan, N.; Weimar, U. Enhancing performance of FSP SnO2-based gas sensors through Sb-doping and Pd-functionalization. Sens. Actuators B Chem. 2011, 158, 388–392. [Google Scholar] [CrossRef]
- Kemmler, J.; Pokhrel, S.; Mädler, L.; Weimar, U.; Barsan, N. Flame spray pyrolysis for sensing at the nanoscale. Nanotechnology 2013, 24, 442001. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.J.; Fischer, R.X. Profile agreement indices in Rietveld and pattern-fitting analysis. J. Appl. Crystallogr. 1990, 23, 462–468. [Google Scholar] [CrossRef]
- Pokhrel, S.; Birkenstock, J.; Schowalter, M.; Rosenauer, A.; Mädler, L. Growth of ultrafine single crystalline WO3 nanoparticles using flame spray pyrolysis. Cryst. Growth Des. 2010, 10, 632–639. [Google Scholar] [CrossRef]
- Koziej, D.; Barsan, N.; Shimanoe, K.; Yamazoe, N.; Szuber, J.; Weimar, U. Spectroscopic insights into CO sensing of undoped and palladium doped tin dioxide sensors derived from hydrothermally treated tin oxide sol. Sens. Actuators B Chem. 2006, 118, 98–104. [Google Scholar] [CrossRef]
- Degler, D.; de Pereira Carvalho, H.W.; Weimar, U.; Barsan, N.; Pham, D.; Mädler, L.; Grunwaldt, J.-D. Structure-function relationships of concentrionally and flame made Pd-doped sensors studied by X-ray absorption spectroscopy and DC-resistance. Sens. Actuators B Chem. 2015, 219, 315–323. [Google Scholar] [CrossRef]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebholz, J.; Grossmann, K.; Pham, D.; Pokhrel, S.; Mädler, L.; Weimar, U.; Barsan, N. Selectivity Enhancement by Using Double-Layer MOX-Based Gas Sensors Prepared by Flame Spray Pyrolysis (FSP). Sensors 2016, 16, 1437. https://doi.org/10.3390/s16091437
Rebholz J, Grossmann K, Pham D, Pokhrel S, Mädler L, Weimar U, Barsan N. Selectivity Enhancement by Using Double-Layer MOX-Based Gas Sensors Prepared by Flame Spray Pyrolysis (FSP). Sensors. 2016; 16(9):1437. https://doi.org/10.3390/s16091437
Chicago/Turabian StyleRebholz, Julia, Katharina Grossmann, David Pham, Suman Pokhrel, Lutz Mädler, Udo Weimar, and Nicolae Barsan. 2016. "Selectivity Enhancement by Using Double-Layer MOX-Based Gas Sensors Prepared by Flame Spray Pyrolysis (FSP)" Sensors 16, no. 9: 1437. https://doi.org/10.3390/s16091437




