An H2S Sensor Based on Electrochemistry for Chicken Coops
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrochemistry
2.2. Materials and Experiments
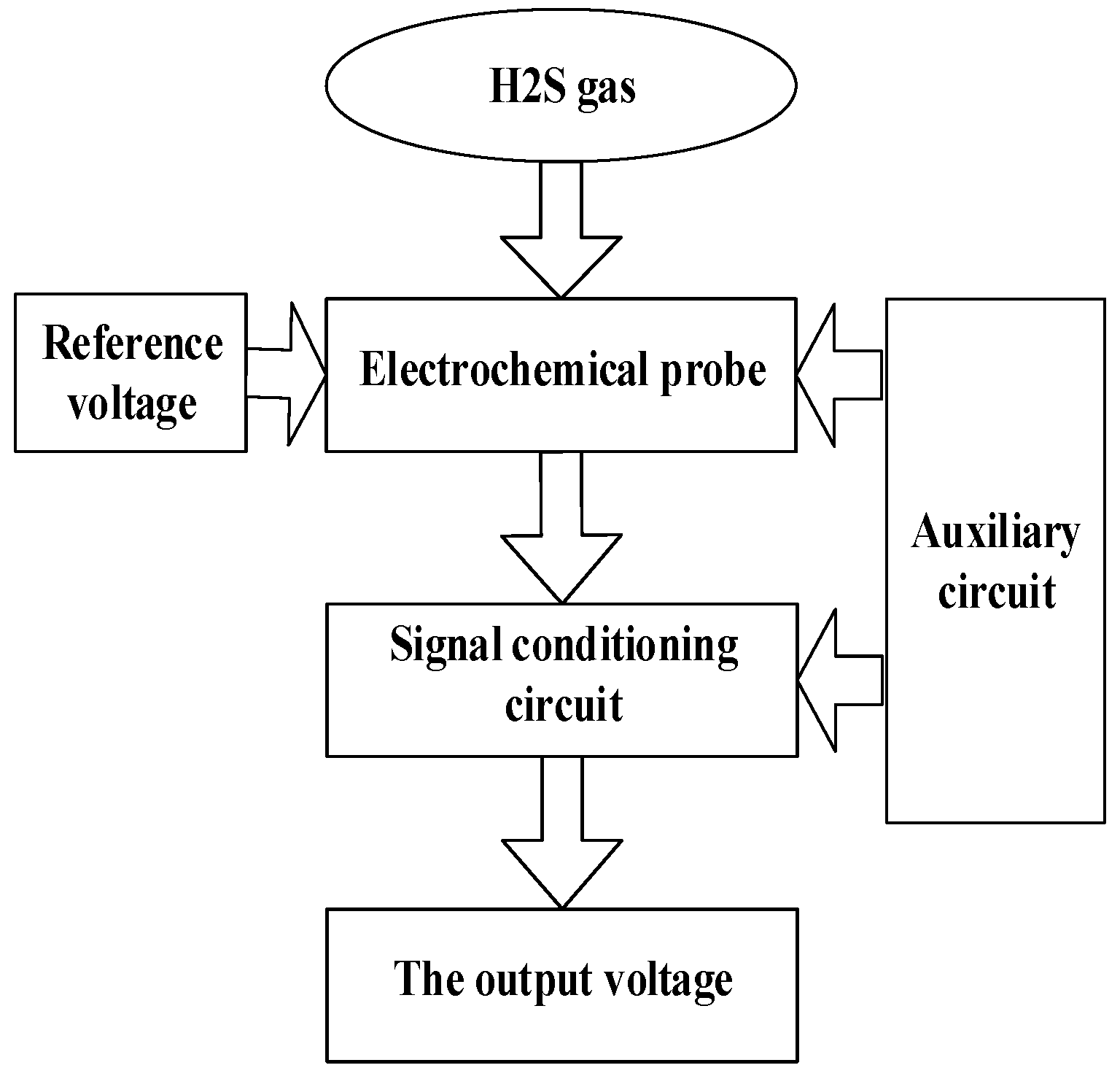
2.3. Sensor Set-up
- (1)
- Three-electrode sensor: It can directly contact hydrogen sulfide gas and convert the hydrogen sulfide gas concentration to a proportional current.
- (2)
- Bias circuit: It can provide a stable voltage for the sensors to control the electrochemical reaction.
- (3)
- Signal-conditioning circuit: It can convert the electrical signal, which is proportional to the gas concentration, into a voltage signal and amplify the voltage signal, which is easily measured by the microcontroller.
- (4)
- Auxiliary circuit: It includes the power supply circuits and peripheral circuits.
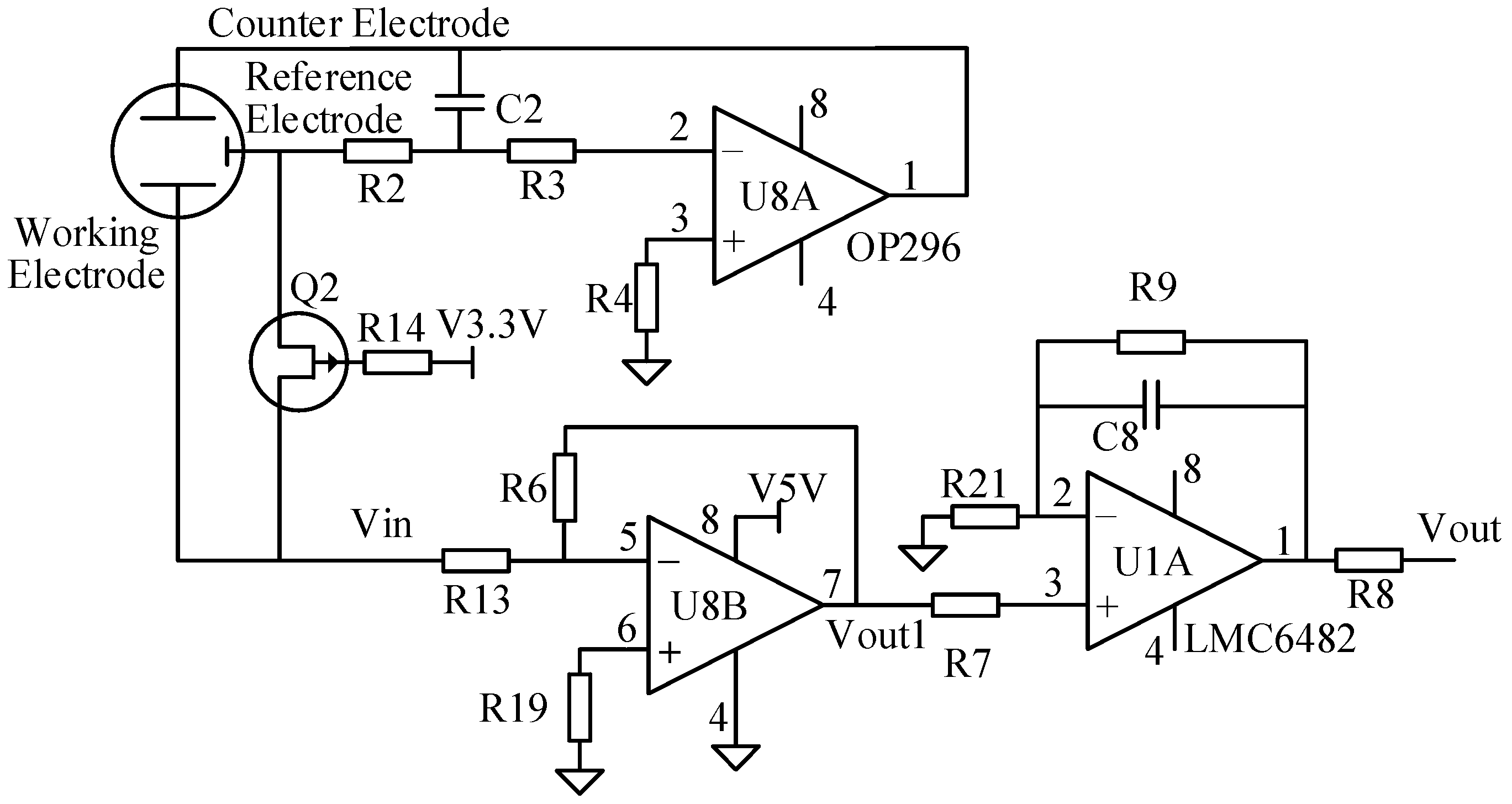
2.3.1. Signal-Conditioning Modules
2.3.2. Constant Voltage Control Module for the Reference Electrode
3. Results
3.1. Calibration of H2S Gas Sensor
3.2. Analysis of the Sensor Performance
3.2.1. Accuracy Analysis
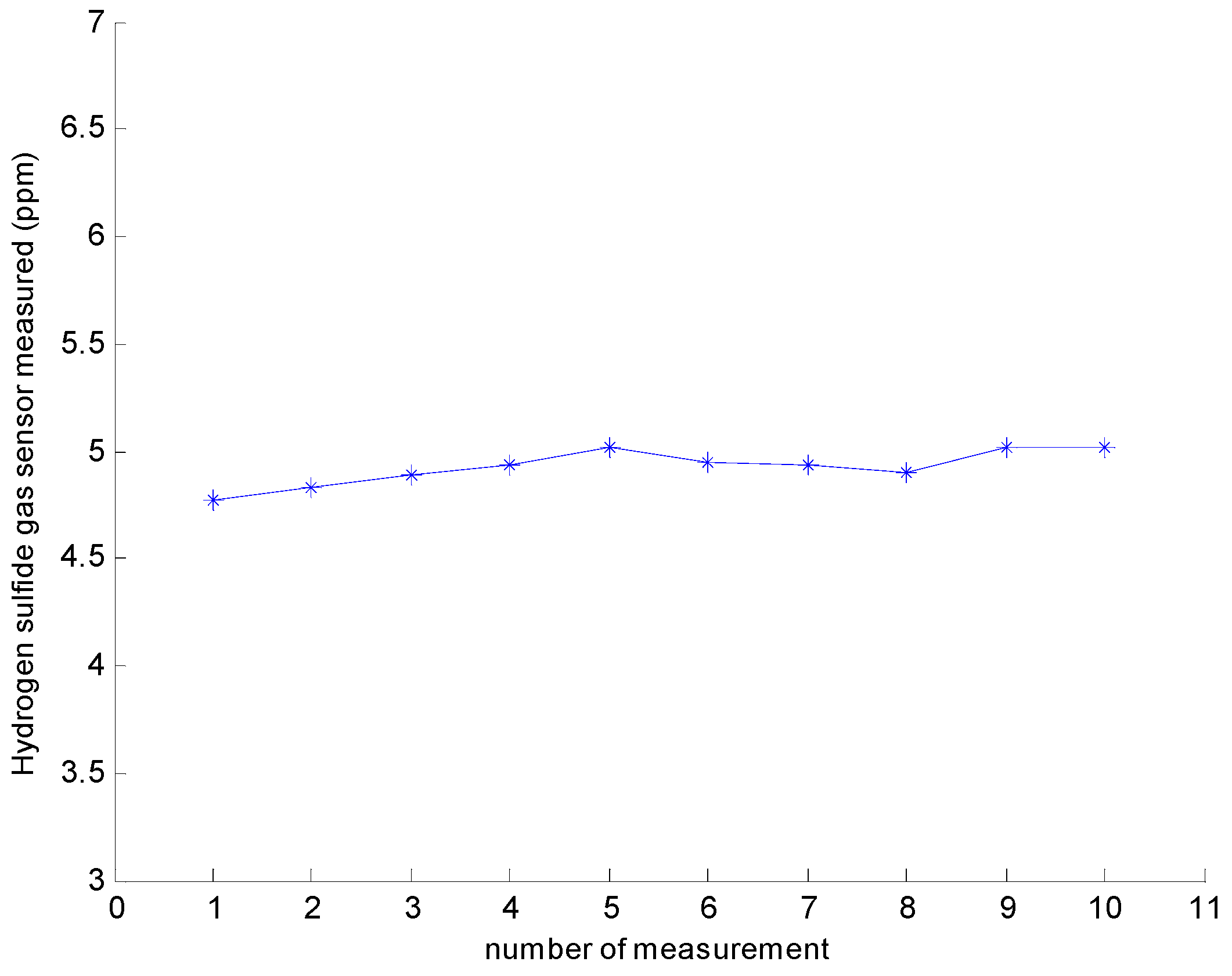
3.2.2. Repeatability
3.2.3. Stability
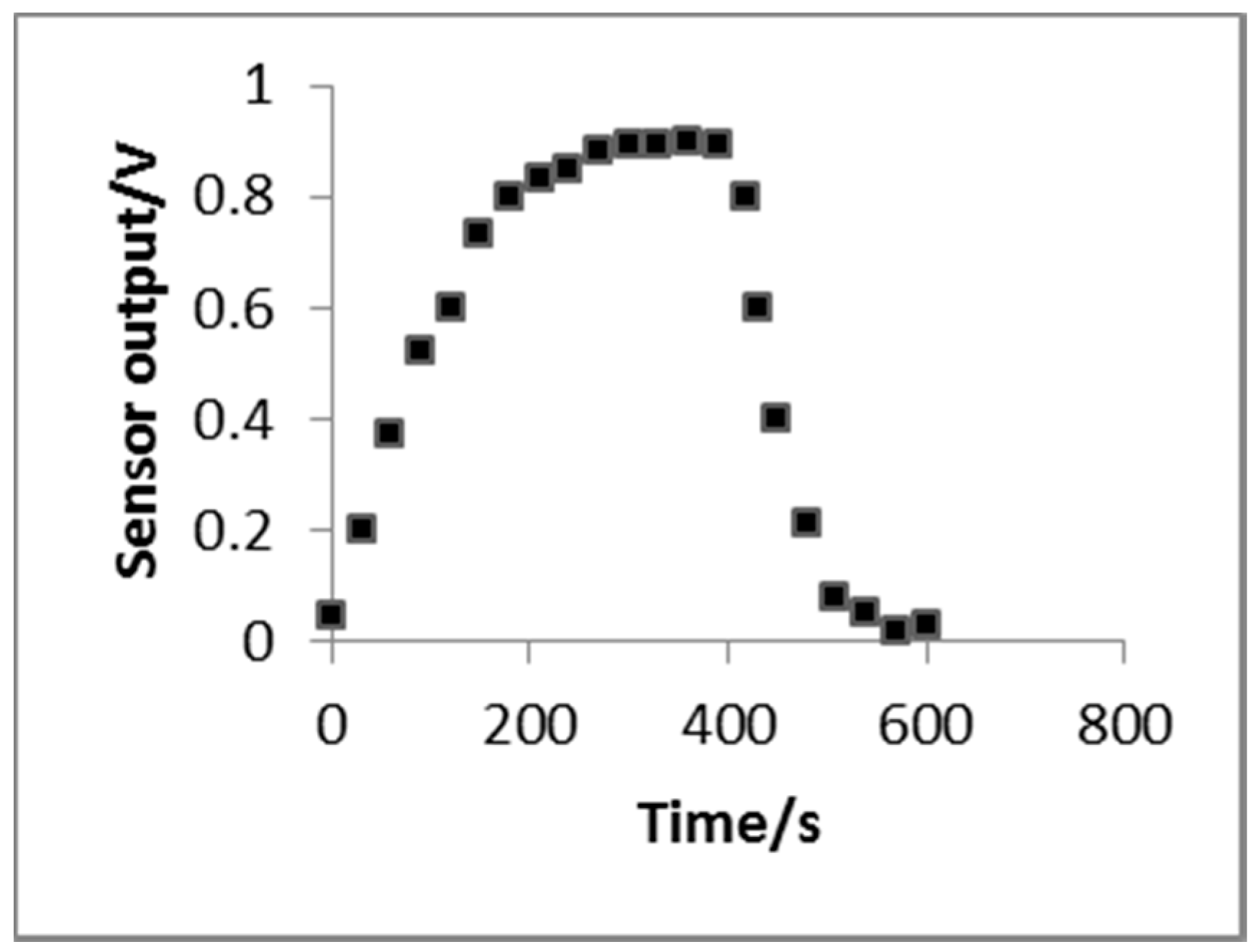
3.2.4. Response Time-to-Recovery
3.3. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lemaire, G.; Franzluebbers, A.; de Faccio Carvalho, P.C.; Dedieu, B. Integrated crop-livestock systems: Strategies to achieve synergy between agricultural production and environmental quality. Agric. Ecosyst. Environ. 2014, 190, 4–8. [Google Scholar] [CrossRef]
- Ni, J.Q.; Heber, A.J.; Darr, M.J.; Lim, T.T.; Diehl, C.A.; Bogan, B.W. Air quality monitoring and on-site computer system for livestock and poultry environment studies. Trans. ASABE 2009, 52, 937–947. [Google Scholar]
- Wang, Y.; Huang, M.; Meng, Q.; Wang, Y. Effects of atmospheric hydrogen sulfide concentration on growth and meat quality in broiler chickens. Poult. Sci. 2011, 90, 2409–2414. [Google Scholar] [CrossRef] [PubMed]
- Hoff, S.J.; Harmon, J.D.; Bundy, D.S.; Zelle, B.C. Source and receptor ammonia and hydrogen sulfide concentrations in communities with and without swine emission sources. Appl. Eng. Agric. 2009, 25, 975–986. [Google Scholar] [CrossRef]
- Pandey, S.K.; Kim, K.; Tang, K. A review of sensor-based methods for monitoring hydrogen sulfide. TrAC Trends Anal. Chem. 2012, 32, 87–99. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Dong, D.; Zheng, W.; Zhao, X. A review of contact sensors used for monitoring malodorous gas in animal facilities. Adv. Mater. Res. 2013, 629, 655–661. [Google Scholar] [CrossRef]
- Fang, G.J.; Liu, Z.L.; Liu, C.Q.; Yao, K.L. Room temperature H2S sensing properties and mechanism of CeO2-SnO2 sol-gel thin films. Sens. Actuator B Chem. 2000, 66, 46–48. [Google Scholar] [CrossRef]
- Shewale, P.S.; Patil, V.B.; Shin, S.W.; Kim, J.H.; Uplane, M.D. H2S gas sensing properties of nanocrystalline Cu-doped ZnO thin films prepared by advanced spray pyrolysis. Sens. Actuator B Chem. 2013, 186, 226–234. [Google Scholar] [CrossRef]
- Chaudhari, G.N.; Alvi, M.; Wankhade, H.G.; Bodade, A.B.; Manorama, S.V. Nanocrystalline chemically modified CdIn2O4 thick films for H2S gas sensor. Thin Solid Films 2012, 520, 4057–4062. [Google Scholar] [CrossRef]
- Li, C.; Zhu, D.; Wang, X.; Xu, J.; Wang, B. H2S gas sensor based on nanocrystalline ZnO synthesized by electrochemical-deposition. Rare Met. Mater. Eng. 2006, 353, 197–199. [Google Scholar]
- Asad, M.; Sheikhi, M.H. Highly sensitive wireless H2S gas sensors at room temperature based on CuO-SWCNT hybrid nanomaterials. Sens. Actuator B Chem. 2016, 231, 474–483. [Google Scholar] [CrossRef]
- Ayesh, A.I.; Abu-Hani, A.F.S.; Mahmoud, S.T.; Haik, Y. Selective H2S sensor based on CuO nanoparticles embedded in organic membranes. Sens. Actuator B Chem. 2016, 231, 593–600. [Google Scholar] [CrossRef]
- Liu, J.H.; Huang, X.J.; Ye, G.; Liu, W.; Jiao, Z.; Chao, W.L.; Zhou, Z.B.; Yu, Z.L. H2S detection sensing characteristic of CuO/SnO2 sensor. Sensors 2003, 3, 110–118. [Google Scholar] [CrossRef]
- Tamaki, J.; Maekawa, T.; Miura, N.; Yamazoe, N. CuO-SnO2 element for highly sensitive and selective detection of H2S. Sens. Actuator B Chem. 1992, 9, 197–203. [Google Scholar] [CrossRef]
- Kneer, J.; Knobelspies, S.; Bierer, B.; Woellenstein, J.; Palzer, S. New method to selectively determine hydrogen sulfide concentrations using CuO layers. Sens. Actuator B Chem. 2016, 222, 625–631. [Google Scholar] [CrossRef]
- Kong, X.H.; Li, Y.D. High sensitivity of CuO modified SnO2 nanoribbons to H2S at room temperature. Sens. Actuator B Chem. 2005, 105, 449–453. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, D.; Zheng, W.; Wang, J. Optical methods for monitoring harmful gas in animal facilities. Opt. Eng. 2014, 53, 061602. [Google Scholar] [CrossRef]
- Choi, M.; Hawkins, P. Development of an optical hydrogen sulphide sensor. Sens. Actuator B Chem. 2003, 90, 211–215. [Google Scholar] [CrossRef]
- Gauglitz, G. Direct optical sensors: Principles and selected applications. Anal. Bioanal. Chem. 2005, 381, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Park, C.O.; Fergus, J.W.; Miura, N.; Park, J.; Choi, A. Solid-state electrochemical gas sensors. Ionics 2009, 15, 261–284. [Google Scholar] [CrossRef]
- Kroll, A.V.; Smorchkov, V.I. Electrochemical solid-state micro-sensor for hydrogen determination. Sens. Actuator B Chem. 1996, 34, 462–465. [Google Scholar] [CrossRef]
- Vandecruys, F.; Brauns, E.; Engelen, W.; de Schutter, F.; Vangrunderbeek, J. A H2S-sensor for on-line measurements in a coal gasification system. Solid State Ion. 1998, 112, 95–102. [Google Scholar] [CrossRef]
- Wang, Y.R.; Yan, H.Q.; Wang, E. Solid polymer electrolyte-based hydrogen sulfide sensor. Sens. Actuator B Chem. 2002, 87, 115–121. [Google Scholar] [CrossRef]
- Hu, W.; Du, Y. Design of an amperometric hydrogen salfide gas sensor. Anal. Instrum. 1997, 1, 18–20. [Google Scholar]
- Fu, C. Development and Application of the Henhouse Environment Temperature and Humidity Monitoring System. Master’s Thesis, Agricultural University of Hebei, Baoding, China, 2013. [Google Scholar]
- Fu, T. Sensing properties and mechanism of gas sensor for H2S and NO2 based on [Cu5(bipyO2)6Cl8]Cl2. Sens. Actuator B Chem. 2007, 123, 1113–1119. [Google Scholar] [CrossRef]
- Jain, G.H.; Patil, L.A. CuO-doped BSST thick film resistors for ppb level H2S gas sensing at room temperature. Sens. Actuator B Chem. 2007, 123, 246–253. [Google Scholar] [CrossRef]
- Tang, D.L.; Wang, Y.; Guo, F.; Zhao, D. Optical H2S gas sensor based on spectrum-absorption. Chin. J. Sens. Actuators 2005, 23, 458–460. [Google Scholar]
- Fard, H.D.; Khatami, S.; Izadi, N.; Koohsorkhi, J.; Rashidi, A. Design and fabrication of hydrogen sulfide (H2S) gas sensor using PtSi/Porous n-Si Schottky Diode. Sens. Mater. 2013, 25, 297–308. [Google Scholar]
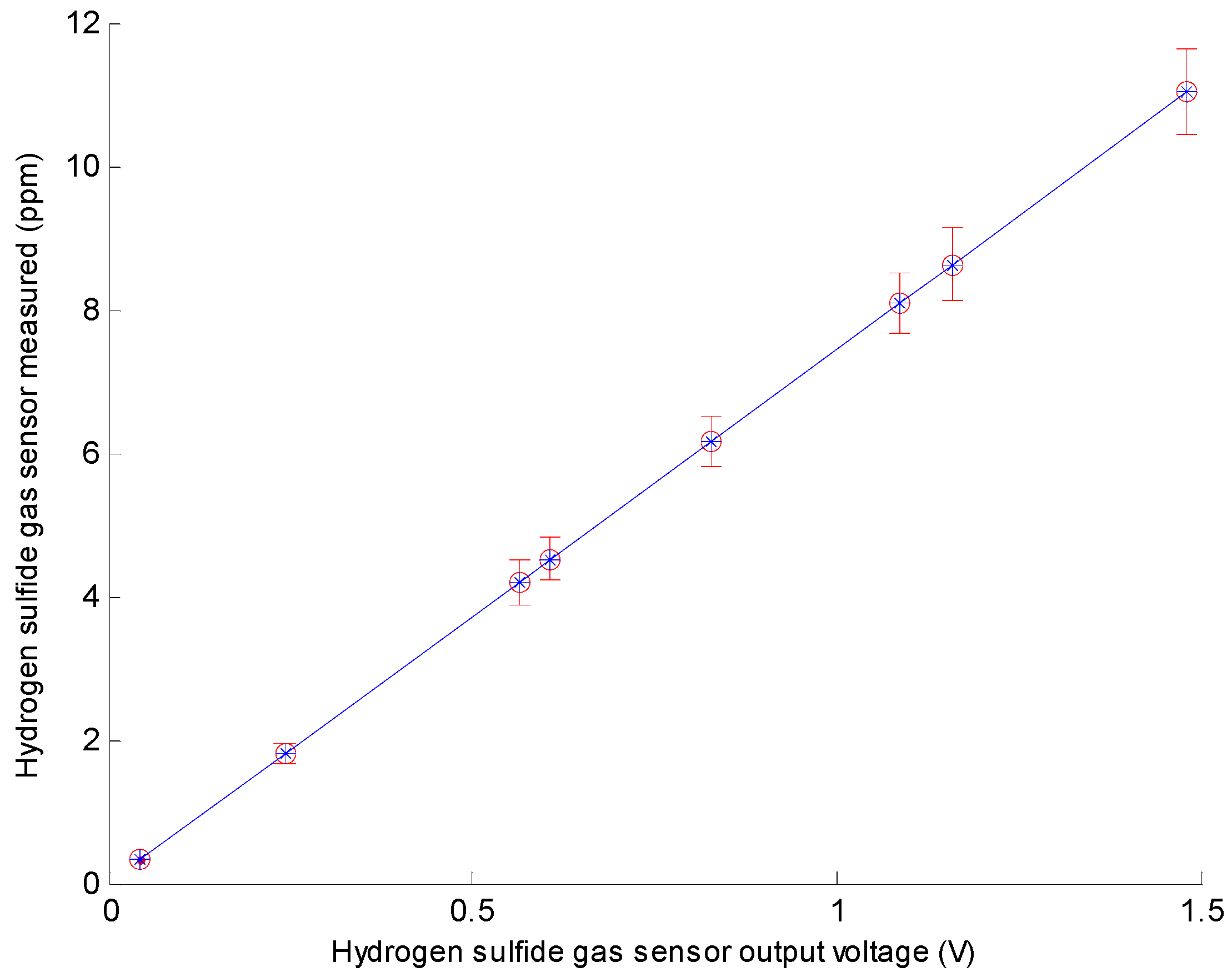
| Hydrogen Sulfide Gas Samples | Setting Concentration (ppm) | Hydrogen Sulfide Gas Sensor Output Voltage (V) | Hydrogen Sulfide Gas Sensor Measured (ppm) |
|---|---|---|---|
| 1 | 0 | 0.044 | 0.327 |
| 2 | 2 | 0.242 | 1.800 |
| 3 | 4 | 0.565 | 4.204 |
| 4 | 5 | 0.607 | 4.516 |
| 5 | 6 | 0.827 | 6.153 |
| 6 | 8 | 1.087 | 8.088 |
| 7 | 9 | 1.159 | 8.624 |
| 8 | 11 | 1.482 | 11.027 |
| Sample | Result (ppm) | The Average (ppm) | Absolute Error (ppm) | Relative Tolerance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||||
| 3 | 2.669 | 2.721 | 2.863 | 2.932 | 2.993 | 2.921 | 2.901 | 2.899 | 2.862 | 0.078 | 2.72% |
| 5 | 4.771 | 4.825 | 4.892 | 4.931 | 5.012 | 4.952 | 4.938 | 4.901 | 4.903 | 0.054 | 1.10% |
| 7 | 6.665 | 6.891 | 6.937 | 6.991 | 7.002 | 7.159 | 7.013 | 7.059 | 6.965 | 0.083 | 1.19% |
| Sample | Measurement Result (ppm) | Average Value | Fractional Error | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||
| 5.00 | 4.86 | 4.79 | 4.89 | 4.93 | 4.89 | 4.91 | 5.09 | 4.98 | 5.12 | 5.10 | 4.96 | 2.68% |
| 6.00 | 6.12 | 6.09 | 6.16 | 6.11 | 6.13 | 6.01 | 5.90 | 5.92 | 6.06 | 6.10 | 6.06 | 1.17% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, L.; He, M.; Yu, H.; Li, D. An H2S Sensor Based on Electrochemistry for Chicken Coops. Sensors 2016, 16, 1398. https://doi.org/10.3390/s16091398
Zeng L, He M, Yu H, Li D. An H2S Sensor Based on Electrochemistry for Chicken Coops. Sensors. 2016; 16(9):1398. https://doi.org/10.3390/s16091398
Chicago/Turabian StyleZeng, Lihua, Mei He, Huihui Yu, and Daoliang Li. 2016. "An H2S Sensor Based on Electrochemistry for Chicken Coops" Sensors 16, no. 9: 1398. https://doi.org/10.3390/s16091398






