1. Introduction
Lung diseases are some of the most common medical conditions [
1]. Many people suffer from lung diseases stemming from smoking, infections, and genetics. The lungs are the primary organs expanding and relaxing thousands of times each day to bring in oxygen and push out carbon dioxide. As one inhales, the breath travels through the mouth or nose to the windpipe and lungs, and further moves through smaller tubes to deliver oxygen throughout the organ. A number of lung diseases adversely affect lung function by affecting the airways. These include asthma, chronic obstructive pulmonary disease (COPD), chronic bronchitis, emphysema, acute bronchitis, and cystic fibrosis [
2].
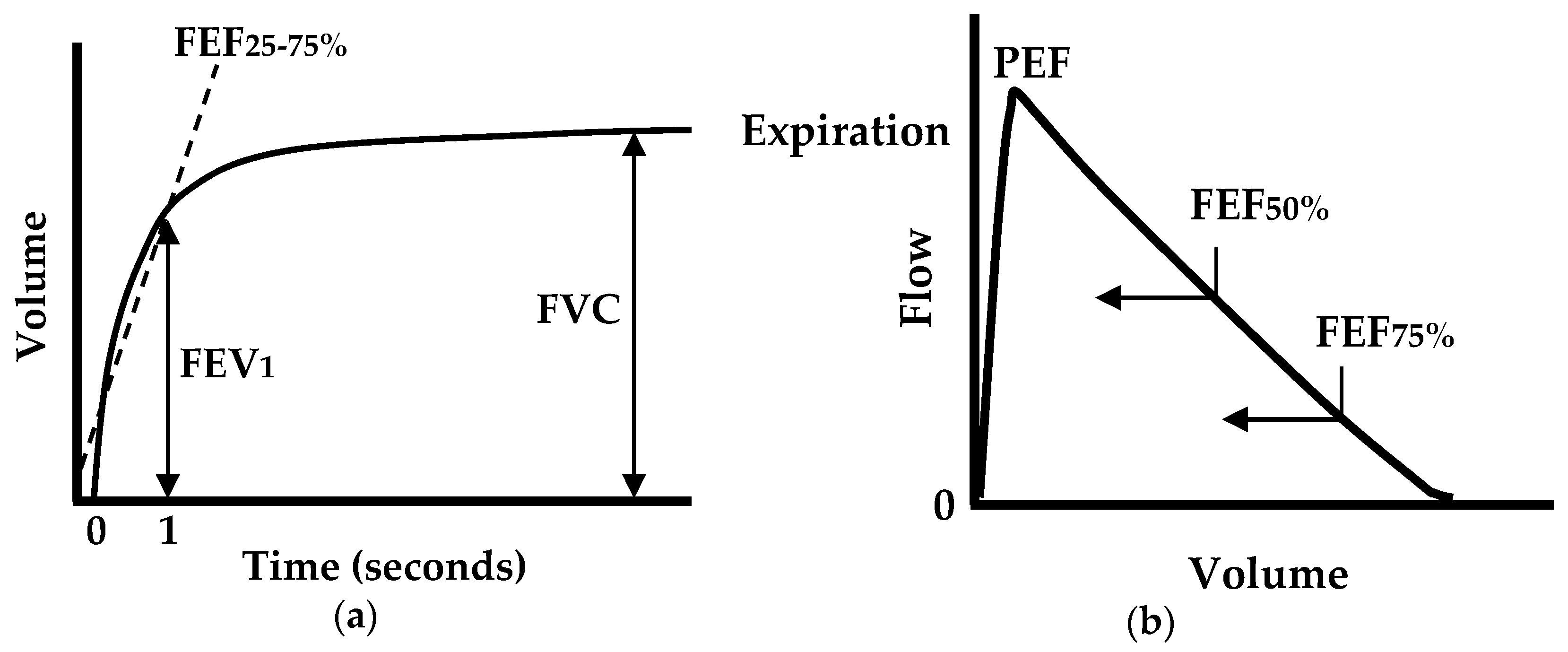
For lung function evaluation, pulmonary function tests (PFTs) have found widespread clinical use. PFTs can help diagnose asthma, allergies, chronic bronchitis, respiratory infections, lung fibrosis, bronchiectasis, and COPD by evaluating how well the lungs take in and release air. For basic PFTs, a spirometer is the main piece of equipment, and an important clinical diagnostic device that should be used by all primary care and most specialist physicians [
3]. Indeed, it was reported that 66% of primary care offices owned and used their own spirometer at the primary contact point. This low amount should reflect, in part, the need for alternatives to the spirometer [
4,
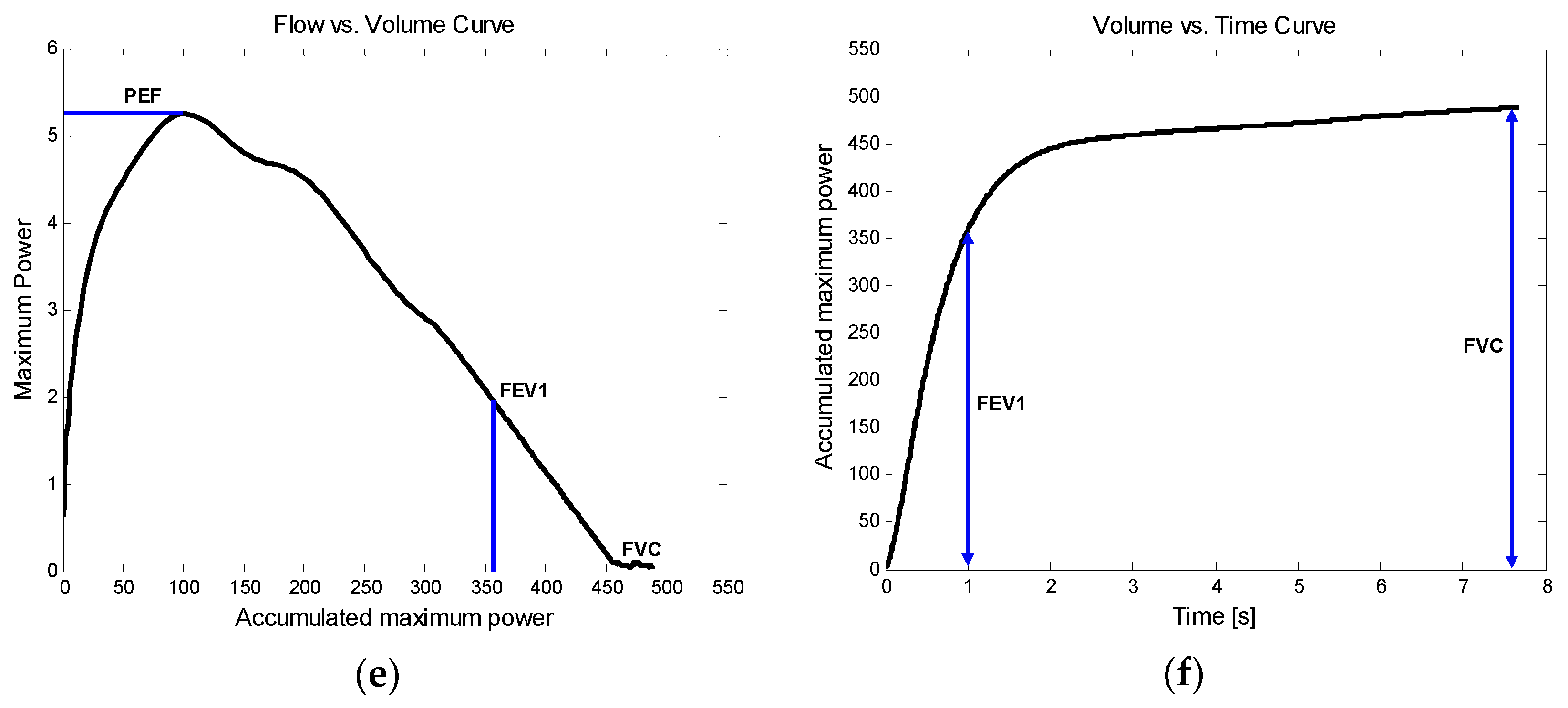
5]. A standard spirometer measures forced expiratory flow and displays a volume-time curve and a flow–volume loop (or curve), as shown in
Figure 1. From the measurements, the following parameters are quantized: the forced vital capacity (FVC); the forced expiratory volume in one second (FEV
1); the FEV
1/FVC ratio; the peak expiratory flow (PEF); the forced expiratory flow at 25%, 50%, or 75% of FVC (FEF
25, FEF
50, and FEF
75); and the forced expiratory flow between 25% and 75% (FEF
25%–75%) [
6,
7,
8]. All of these parameters are clinically important in assessing lung function.
Among these parameters, the most common clinical measures are FVC, FEV
1, FEV
1/FVC, and PEF, as they are used to quantify the degree of airflow limitation in chronic lung diseases, such as asthma, COPD, and cystic fibrosis. The FEV
1/FVC ratio has been widely accepted, especially for assessing COPD, which obstructs the main airway. In 1983, the European Community for Coal and Steel (ECCS) initially defined airway obstruction with an FEV
1/FVC ratio below the lower fifth percentile of a large healthy reference group, which is the statistically-defined lower limit of normal (LLN) [
9]. The same definition was followed by the American Thoracic Society (ATS) in 1991 [
10], the European Respiratory Society (ERS) in 1993 [
11], and the National Lung Health Education Program (NLHEP) in 2000 [
12]. Depending on the organization and year, the definition has occasionally been modified by using a fixed value instead of the LLN. In 1987, ATS defined airway obstruction with an FEV
1/FVC ratio lower than the fixed value of 0.75 [
13], followed by the British Thoracic Society (BTS) with 0.70 in 1997 [
14], the National Institute for Health and Clinical Excellence (NICE) with 0.75 in 2004 [
15], the ATS and ERS with 0.70 in 2004 [
16], and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) with 0.75 in 2007 [
15]. In this way, the FEV
1/FVC ratio has been a widely accepted standard for assessing COPD, as summarized in
Table 1 [
15].
Home spirometry may provide the convenient way for patients who live great distances from their clinics and research facilities. It also allows patients to monitor more frequently for detecting changes in lung function, which results in in earlier treatment of exacerbations, more rapid recovery, reduced healthcare cost, and improved outcomes [
17,
18]. Even though there are portable spirometers available, they are expensive, which limits the popularity to many people. Since the smartphone is popular and capable for users to measure forced exhalation with its built-in microphone, it can be a cheap home spirometer available to smartphone users. In addition, since smartphone technology has the capacity for users to communicate with physicians (e.g., using cloud technology), smartphone-based home spirometry has the potential in providing early diagnosis of lung disease for those who may have breathing problems on a day-to-day basis [
19,
20,
21,
22,
23]. Recently, a smartphone-based approach that measures lung function using the built-in microphone has been introduced [
24]. In their pilot study, the researchers demonstrated the extraction of the FVC, FEV
1, PEF, and FEV
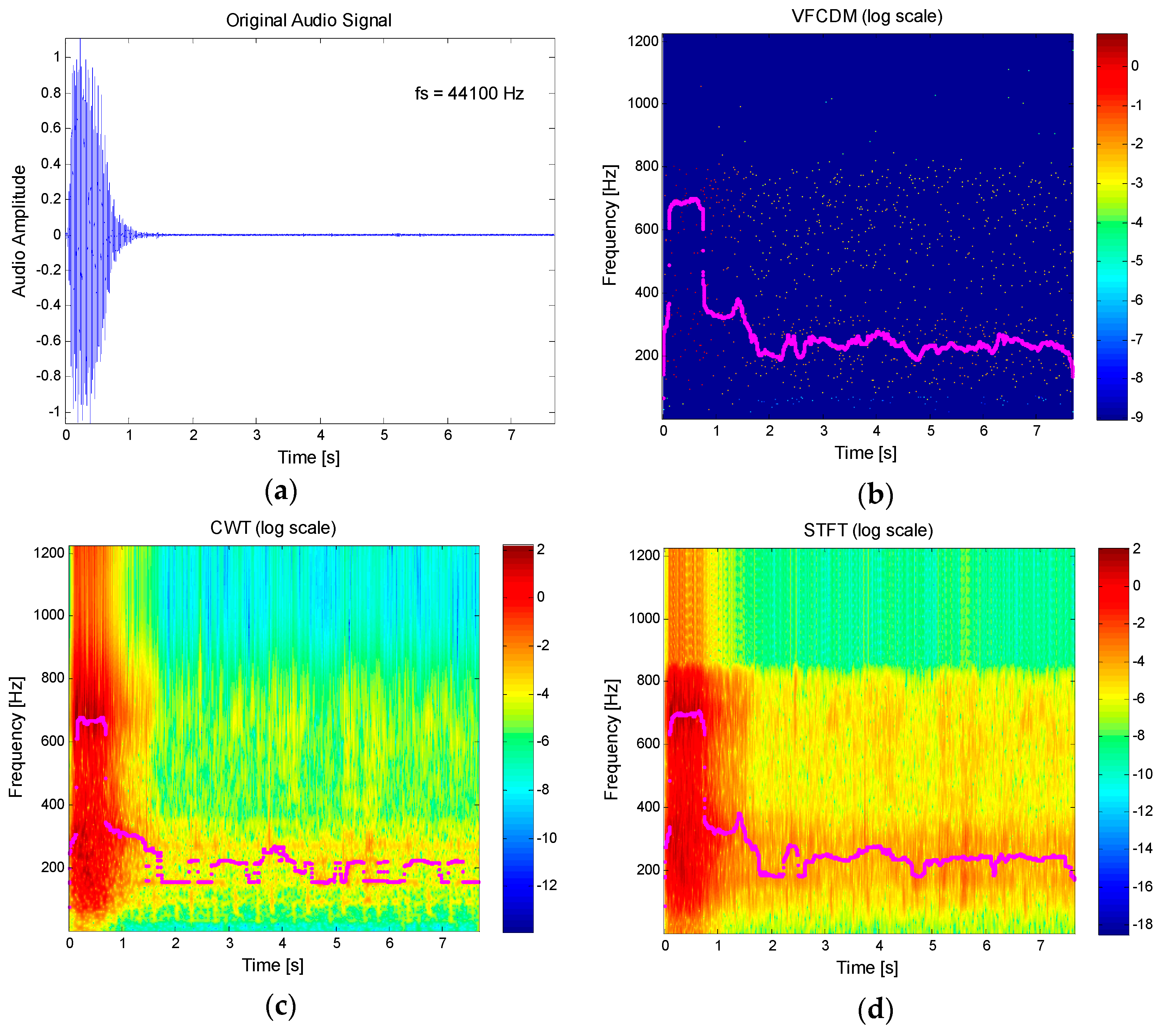
1/FVC parameters. However, the approach used the spectrogram of the signal based on the short-time Fourier transform (STFT) with 30 ms frames. The STFT has a limitation in its time-frequency resolution capability, which is due to the uncertainty principle. The Heisenberg-Gabor inequality states that the time–bandwidth product of a signal is the lower bound by some constant. This means that a signal cannot be simultaneously narrow in time and frequency domains. These limitations in resolution were one of the reasons for the invention of wavelet theory. Recently, the variable frequency complex demodulation method (VFCDM) has shown a higher resolution than any other time-frequency spectrum methods, such as the smoothed pseudo Wigner-Ville (SPWV), short-time Fourier transform (STFT), and the wavelet transform (WT) methods [
25,
26,
27,
28].
In this paper, VFCDM-based lung function parameter estimation is presented by using an audio signal recorded from a smartphone built-in microphone. The estimation method of the FEV1/FVC ratio is first presented, and the accuracy of the VFCDM is then compared with that of CWT and STFT. The main contribution is to introduce the smartphone built-in microphone-based FEV1/FVC ratio estimation by using the high-resolution time-frequency spectrum, VFCDM, which accurately detects the main time-varying resonance frequency. To evaluate our proposed method, we compared the estimated FEV1/FVC ratio with the parameters clinically obtained from PFTs. In addition, we evaluated the other parameter estimation of FVC, FEV1, and PEF.
4. Discussion
We presented a smartphone-based lung function test using a high-resolution time-frequency spectrum from a smartphone built-in microphone. Even though the time-frequency spectrum is not a sole component for the result accuracy, it is the key algorithm to increase the accuracy. In [
24], the audio from a phone was buffered into 30 ms frames and each frame was windowed to quantify the magnitude spectrogram of the signal. On the other hand, we down-sampled to 2450 Hz and visualized the time-frequency spectrum with the time interval of 1/2450 s, which is equivalent to 0.41 ms, approximately. Hence, our method has approximately 73 times higher time resolution than the previous work. More specifically, to estimate the FEV
1/FVC ratio, VFCDM provided lower absolute errors than CWT and STFT by 6.52 and 6.67, respectively. In addition, VFCDM provided lower RMSEs than CWT and STFT by 5.94 and 6.62, respectively. The results suggest that the VFCDM approach provided higher accuracy of the FEV
1/FVC ratio than CWT and STFT due to high resolution of the time-frequency spectrum. However, we also found that only the FEV
1/FVC ratio can be accurately estimated using the smartphone built-in microphone since the ratio as a relative value can be obtained directly without the estimates of FVC and FEV
1. These two factors, as well as PEF, are subjective and dependent on the subject’s familiarization with the test and performance of the forced exhalation. More specifically, each individual may exhale with slightly different angles toward a built-in microphone even with the add-on mouthpiece. In addition, each individual may bite the mouthpiece deeply or shallowly. Each individual may also move or shake the smartphone during the forced exhalation test. Those factors affect the time-frequency spectrum, and may result in inaccurate estimation of FVC, FEV
1, and PEF. Healthy subjects #2, #9, and #11 embodied such cases. Regarding FEV
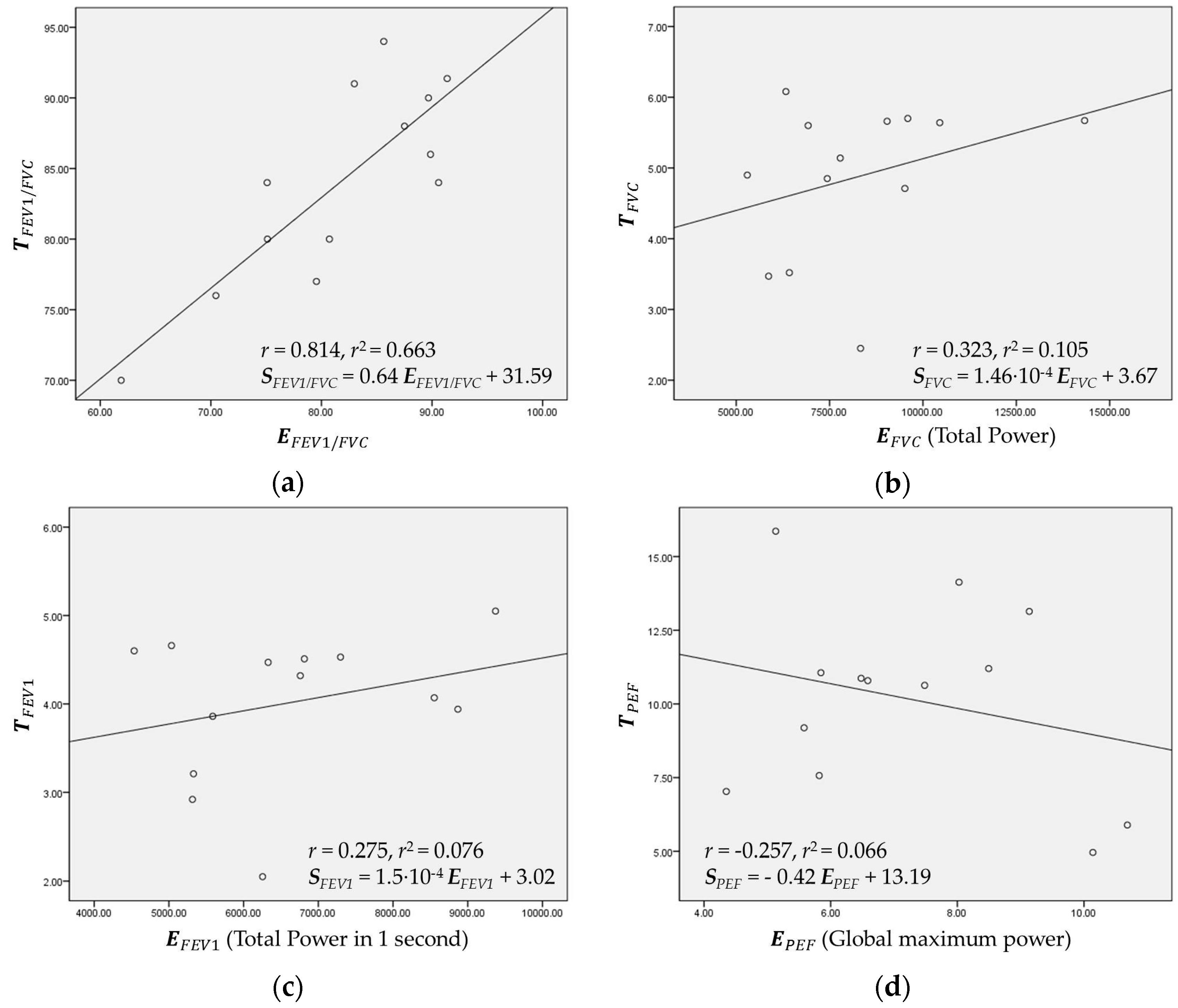
1/FVC, if the three subjects #2, #9, and #11 were excluded, the estimation results from the ten other healthy subjects provided very low absolute errors and RMSEs of 3.573 and 4.760, respectively. Regarding FVC, FEV
1, and PEF, the correlation
r value could increase to 0.763, 0.928, and 0.823, respectively. Thus, the limited and cautious force of exhalation toward the built-in microphone is necessary to increase the estimation accuracy. Furthermore, the future research needs to be toward operation without the add-on adapter. Furthermore, the additional condition needs to be considered: each individual should keep the distance constant between a lip and a microphone. Then, a user may have a trouble keeping the fixed distance between the lip and microphone before the test every time.
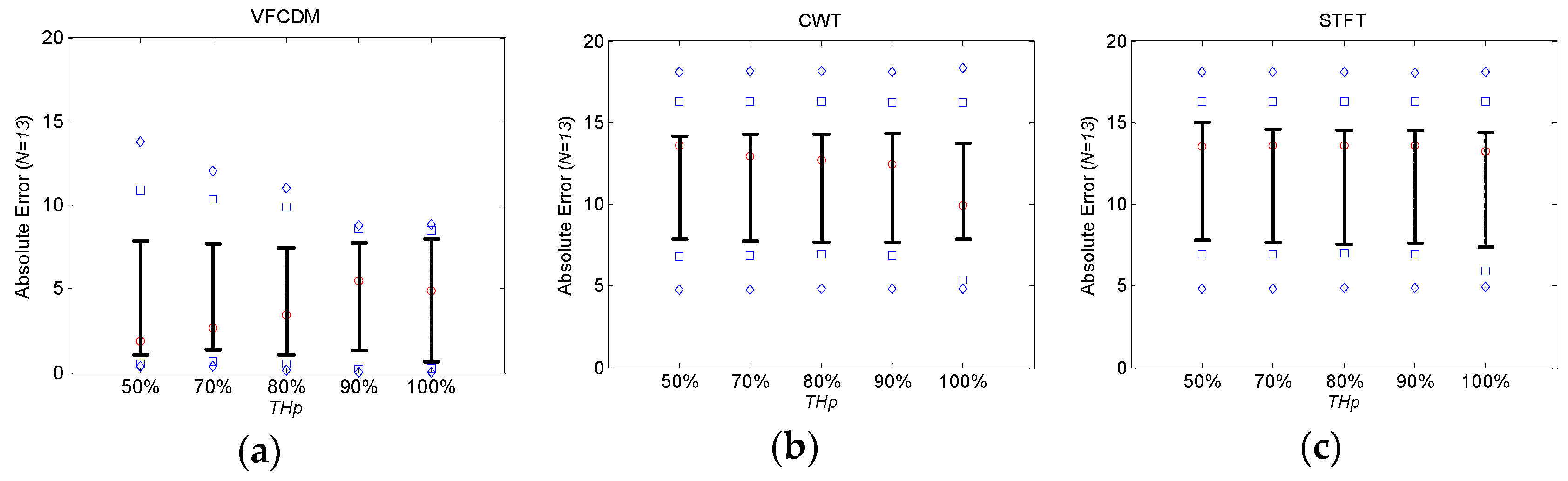
We also found that AE and RMSE of FEV
1/FVC ratios from COPD patients were relatively high with 10.30% ± 10.59% and 14.48%, respectively, even with VFCDM. These high errors were mainly from the artifact sound caused by the narrow windpipe, especially for COPD patients. Most COPD patients have a narrow windpipe, which causes artifact sound even in routine life. In the case of forced exhalation, the artifact sound is more dominating and the real forced exhalation sound is severely affected by the artifacts. Then, the time-varying main frequency cannot be accurately detected, and eventually the instantaneous frequency with maximum power at each sample cannot appropriately represent the volume of the PFT test. More specifically, in the portion of the sound without the artifacts, our proposed method with VFCDM provides higher accuracy than CWT and STFT. In the portion of the sound with the artifacts, there is no difference among VFCDM, CWT, and STFT. This reflects that the results from healthy subjects have higher accuracy than those from COPD patients. In addition, this reflects that the results with VFCDM have higher accuracy than CWT and STFT for both healthy subjects and COPD patients. Thus, the dispersion trend according to
THp is observed only in healthy subjects as shown in
Figure 4 and
Figure 5. More research regarding artifact sound detection and filtering should be directed to be clinically available in the future.
In our study, among the healthy subjects, seven subjects were aged between 20 and 29 (group A), and the other six subjects were aged between 30 and 39 (group B). For groups A and B, RMSE values were 6.13 and 4.76, and AE values were 4.96 ± 3.89 and 3.93 ± 2.93. However, due to a small number of subjects, the effect of age is not clear from the results. Similarly, for male (N = 10) and female (N = 3) groups from healthy subjects, RMSE values were 4.61 and 7.90, and AE values were 3.48 ± 3.18 and 7.84 ± 1.17. From COPD patients, RMSE values were 15.17 and 11.92, and AE values were 10.65 ± 11.38 and 9.14 ± 99.36, respectively. Similarly with the gender effect, due to a small number of subjects and patients, the effect of gender is not clear from the results. Thus, further studies need to rigorously validate the FEV1/FVC ratio and revise the regression model for FVC, FEV1, and PEF by considering a larger number of subjects with age and gender matching.