Hyperspectral Imaging Using Flexible Endoscopy for Laryngeal Cancer Detection
Abstract
:1. Introduction
2. Instrumentation and Patients
3. Methods
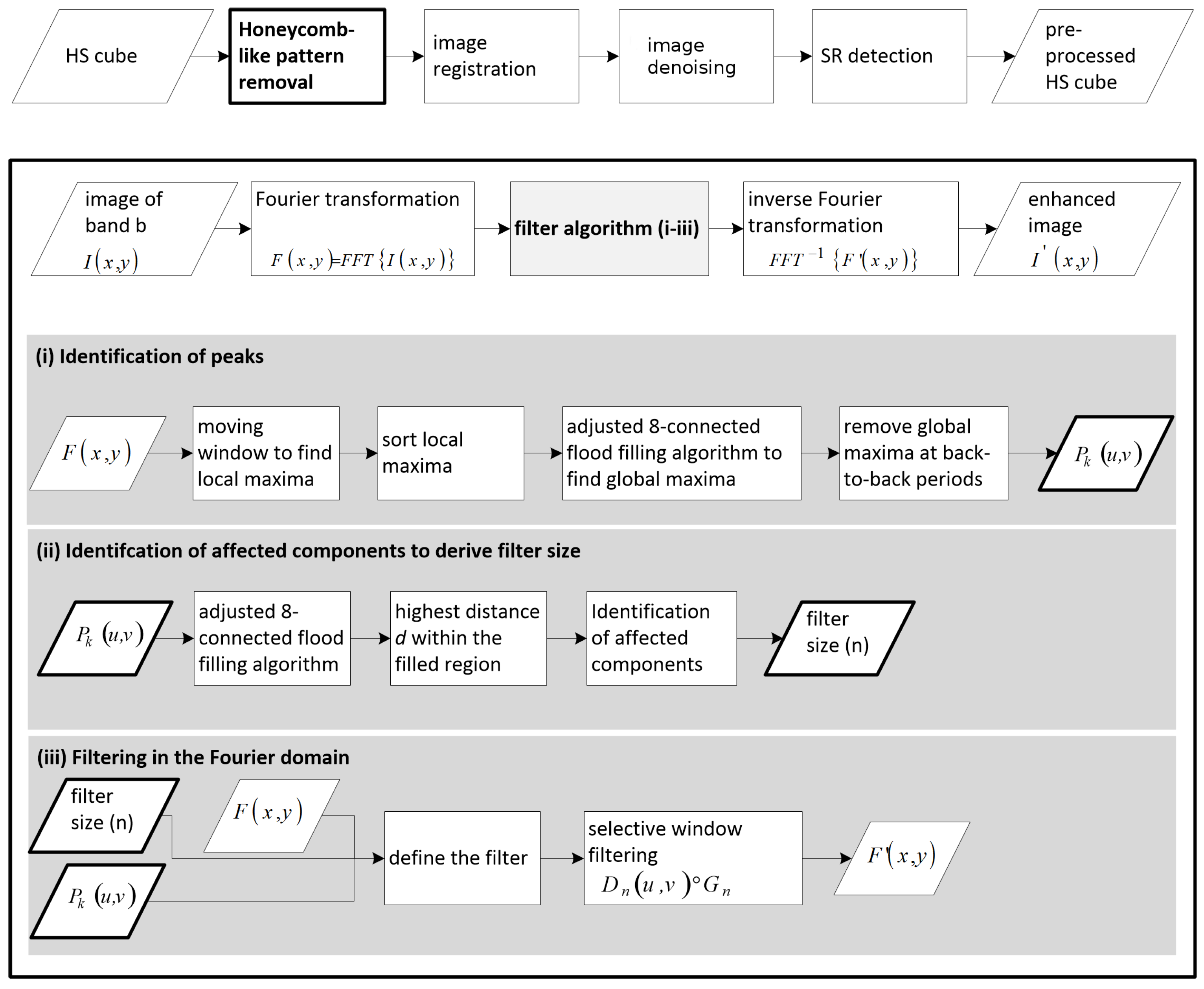
3.1. Removal of Honeycomb-Like Pattern in the Fourier Domain
3.1.1. Identification of Peaks
3.1.2. Identification of Affected Components to Derive Filter Size
3.1.3. Filtering in the Fourier Domain
3.2. Further Pre-Processing and Hyperspectral Classification
3.2.1. Application of the Image Pre-Processor
3.2.2. Illumination
3.2.3. Hyperspectral Classification
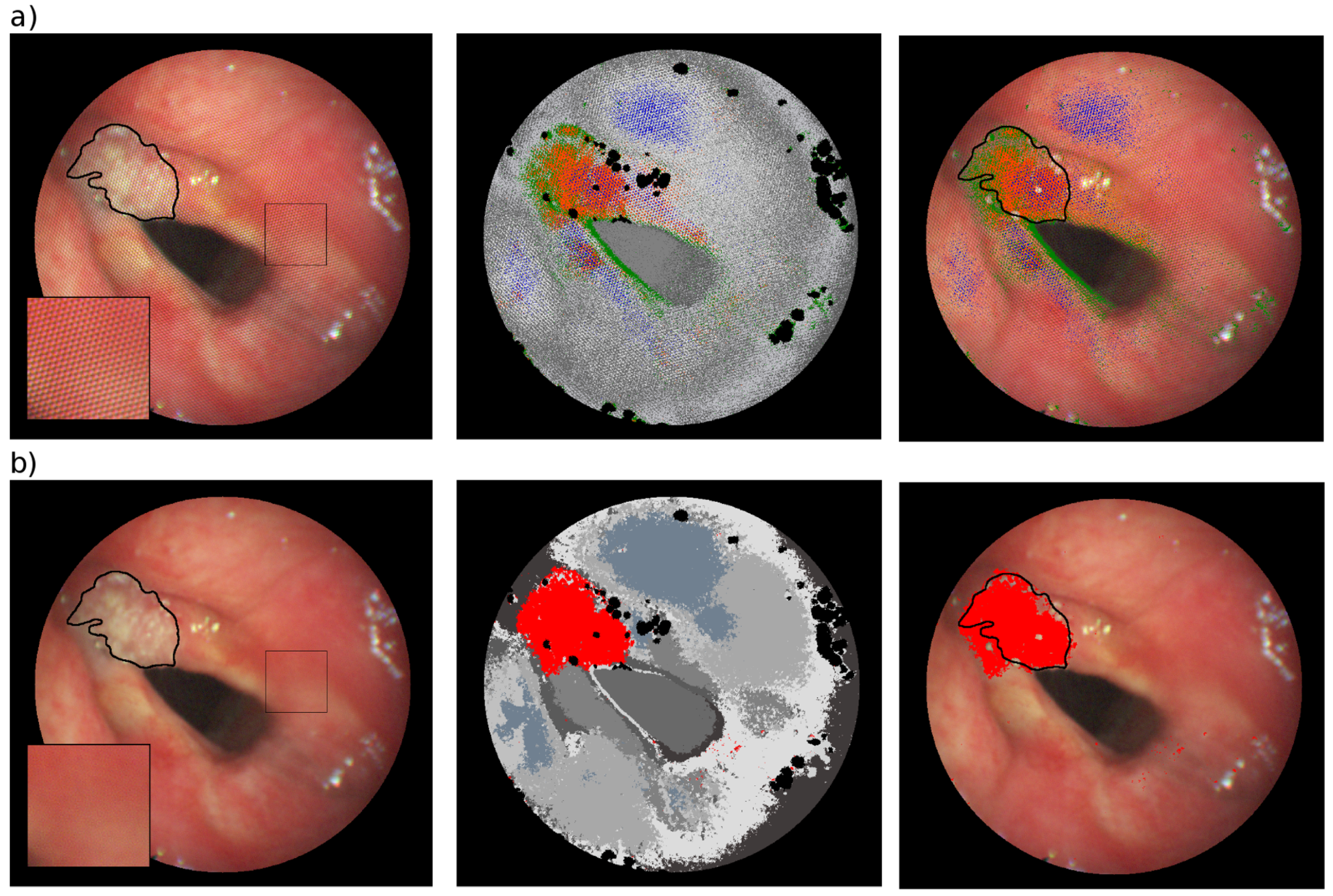
4. Results and Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DC | direct current |
| EM | expectation-maximization |
| FD | Fourier domain |
| FFT | fast Fourier transform |
| GMM | Gaussian mixture model |
| HS | hyperspectral |
| HSI | hyperspectral imaging |
| NBI | narrow band imaging |
| NRI | normalized ratio index |
| RGB | Red Green Blue |
| SD | spatial domain |
| SR | specular reflection |
| USAF | United States Air Force |
References
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef]
- Watanabe, A.; Taniguchi, M.; Tsujie, H.; Hosokawa, M.; Fujita, M.; Sasaki, S. The value of narrow band imaging for early detection of laryngeal cancer. Eur. Arch. Otorhinolaryngol. 2009, 2, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Gono, K.; Yamazaki, K.; Doguchi, N.; Nonami, T.; Obi, T.; Yamaguchi, M.; Ohyama, N.; Machida, H.; Sano, Y.; Yoshida, S.; et al. Endoscopic observation of tissue by narrowband illumination. Opt. Rev. 2003, 10, 211–215. [Google Scholar] [CrossRef]
- Calin, M.A.; Parasca, S.V.; Savastru, R.; Manea, D. Characterization of burns using hyperspectral imaging technique—A preliminary study. Burns 2015, 41, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Meyerle, J.; Burlina, P. Non-invasive estimation of skin thickness from hyperspectral imaging and validation using echography. Comput. Biol. Med. 2015, 57, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Chiba, T.; Nakamizo, A.; Kumashiro, R.; Murata, M.; Akahoshi, T.; Tomikawa, M.; Kikkawa, Y.; Yoshimoto, K.; Mizoguchi, M.; et al. Intraoperative visualization of cerebral oxygenation using hyperspectral image data: A two-dimensional mapping method. Int. J. CARS 2014, 9, 1059–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yudovsky, D.; Nouvong, A.; Schomacker, K.; Pilon, L. Monitoring temporal development and healing of diabetic foot ulceration using hyperspectral imaging. J. Biophotonics 2011, 4, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Thies, B.; Gerstner, A.O.H. Hyperspectral hybrid method classification for detecting altered mucosa of the human larynx. Int. J. Health Geogr. 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Gerstner, A.O.H.; Laffers, W.; Bootz, F.; Fakas, D.L.; Martin, R.; Bendix, J.; Thies, B. Hyperspectral imaging of mucosal surfaces in patients. J. Biophotonics 2012, 5, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Akbari, H.; Uto, K.; Kosugi, Y.; Kojima, K.; Tanaka, N. Cancer detection using infrared hyperspectral imaging. Cancer Sci. 2011, 102, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Kiyotoki, S.; Nishikawa, J.; Okamoto, T.; Hamabe, K.; Saito, M.; Goto, A.; Fujita, Y.; Hamamoto, Y.; Takeuchi, Y.; Satori, S.; et al. New method for detection of gastric cancer by hyperspectral imaging: A pilot study. J. Biomed. Opt. 2013, 18. [Google Scholar] [CrossRef] [PubMed]
- Leitner, R.; Biasio, M.D.; Arnold, T.; Dinh, C.V.; Loog, M.; Duin, R.P.W. Multi-spectral video endoscopy system for the detection of cancerous tissue. Pattern Recognit. Lett. 2013, 34, 85–93. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Li, Q. Tongue tumor detection in medical hyperspectral images. Sensors 2012, 12, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Fei, B. Medical hyperspectral imaging: A review. J. Biomed. Opt. 2014, 19. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.; Bouma, B.; Iftimia, N.; Yelin, R.; Tearney, G. Spectrally-modulated full-field optical coherence microscopy for ultrahigh-resolution endoscopic imaging. Opt. Commun. 2006, 14, 8675–8684. [Google Scholar] [CrossRef]
- Winter, C.; Rupp, S.; Elter, M.; Münzenmayer, C.; Gerhäuser, H.; Wittenberg, T. Automatic adaptive enhancement for images obtained with fiberscopic endoscopes. IEEE Trans. Biomed. Eng. 2006, 53, 2035–2046. [Google Scholar] [CrossRef]
- Lee, C.Y.; Han, J.H. Integrated spatio-spectral method for efficiently suppressing honeycomb pattern artifact in imaging fiber bundle microscopy. Opt. Commun. 2013, 306, 67–73. [Google Scholar] [CrossRef]
- Aizenberg, I.; Butakoff, C. A windowed Gaussian notch filter for quasi-periodic noise removal. Image Vis. Comput. 2008, 26, 1347–1353. [Google Scholar] [CrossRef]
- Regeling, B.; Laffers, W.; Gerstner, A.O.H.; Westermann, S.; Müller, N.A.; Schmidt, K.; Bendix, J.; Thies, B. Development of an image pre-processor for operational hyperspectral laryngeal cancer detection. J. Biophotonics 2015. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Woods, R. Digital Image Processing; Prentice Hall: Upper Saddle River, NJ, USA, 2008. [Google Scholar]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Burger, W.; Burger, M.J. Digitale Bildverarbeitung: Eine Algorithmische Einführung Mit Java, 3rd ed.; Springer Vieweg: Berlin, Germany, 2015. [Google Scholar]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Dempster, A.P.; Laird, N.M.; Rubin, D.B. Maximum Likelihood from Incomplete Data via the EM Algorithm. J. R. Stat. Soc. Ser. B 1977, 39, 1–38. [Google Scholar]
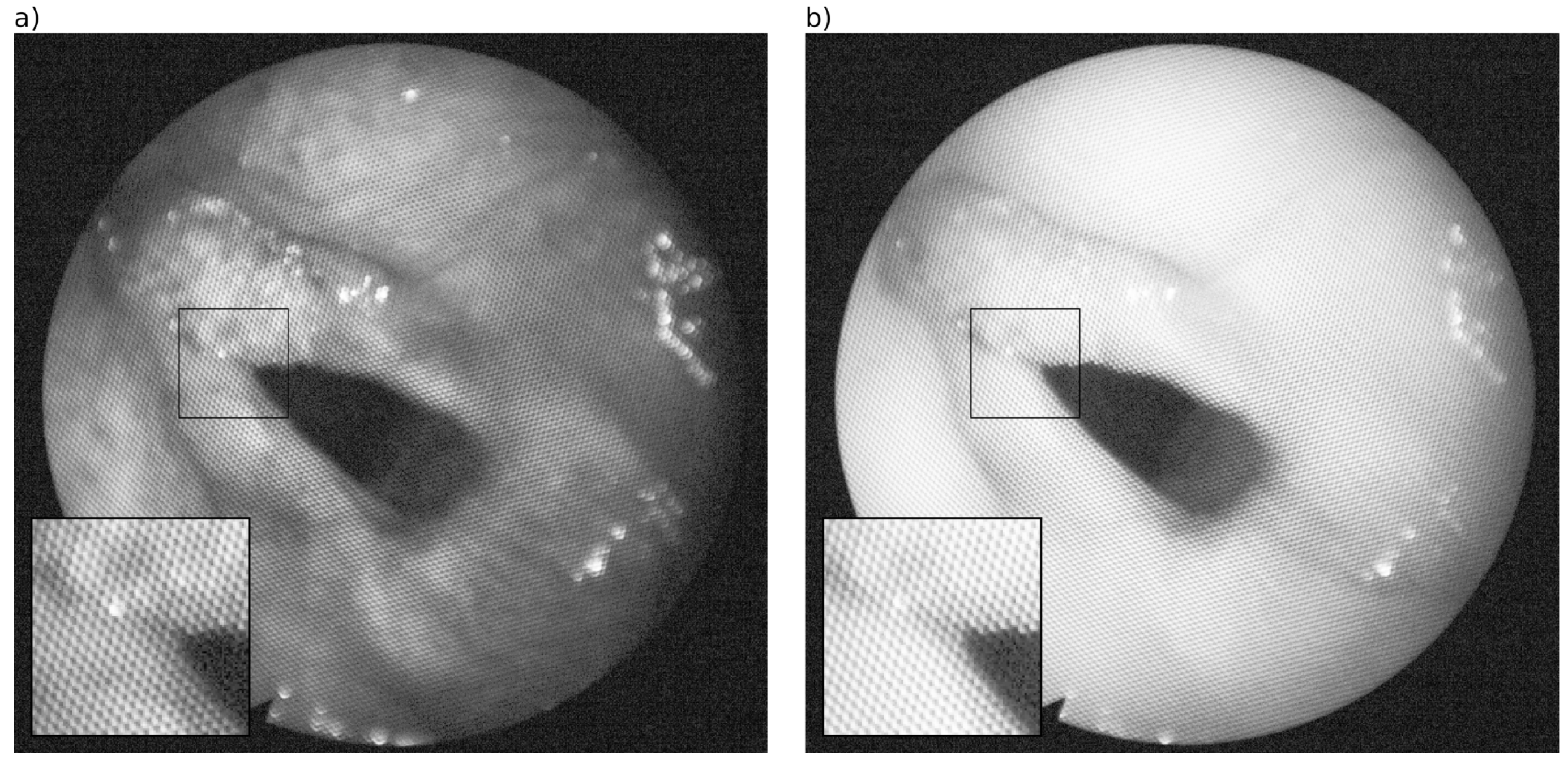

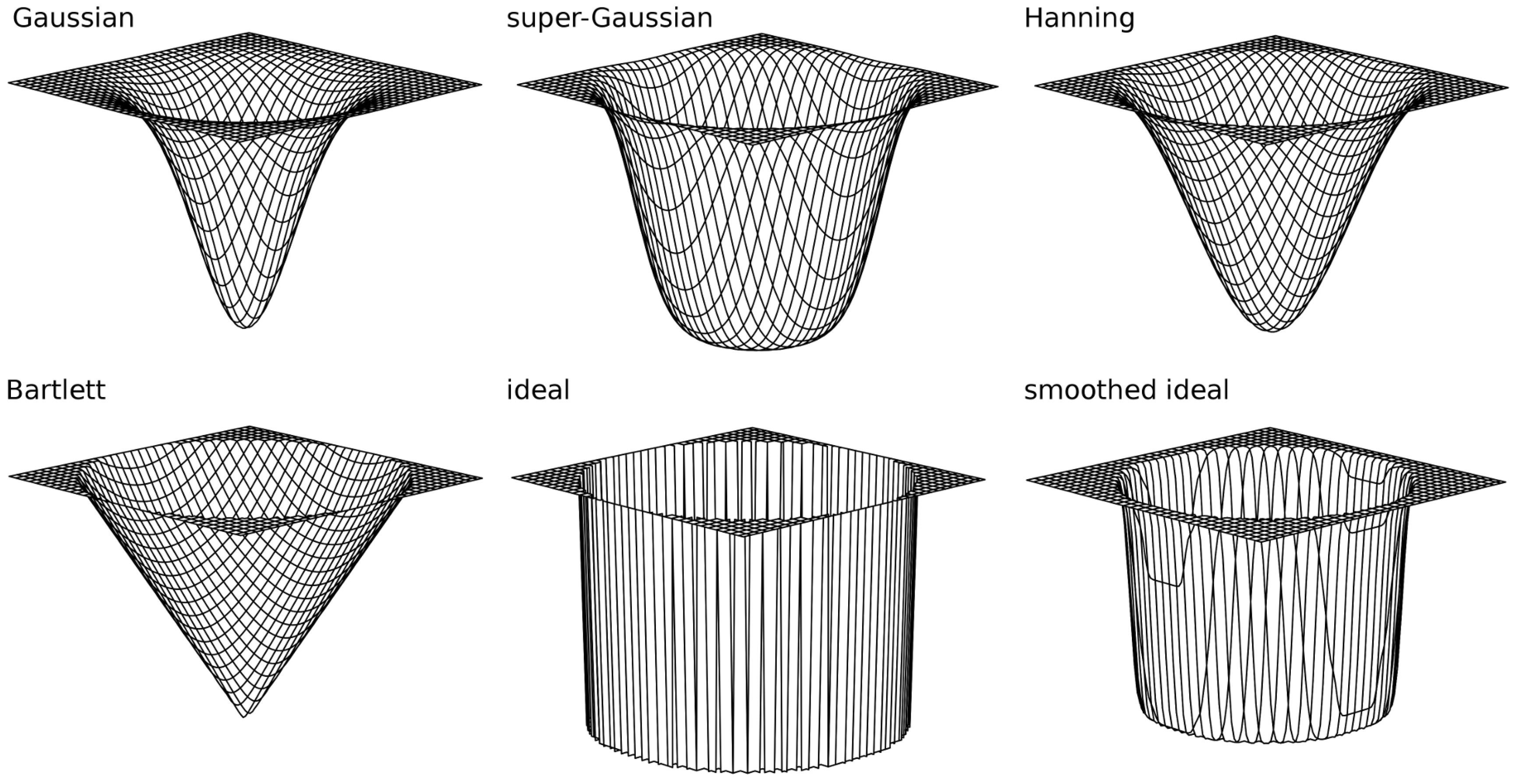



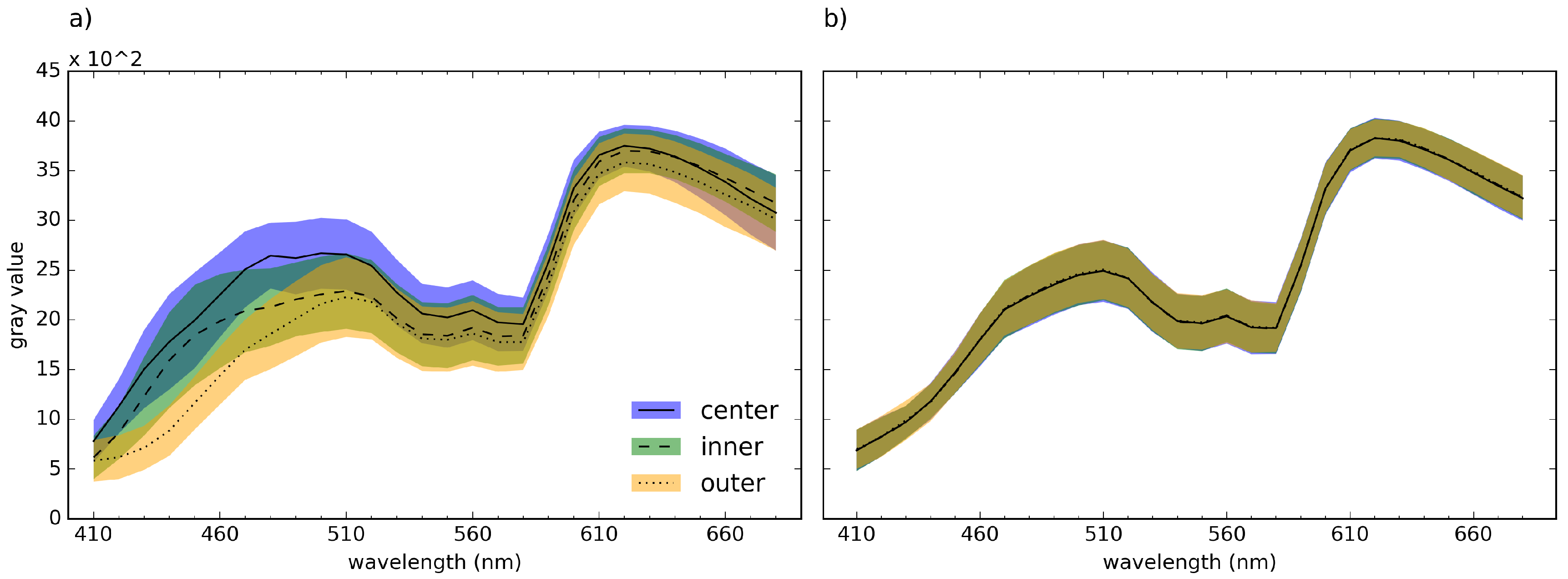

| s | r | q () | q () | |
|---|---|---|---|---|
| Our Method | ||||
| Gaussian | 0.820 (0.054) | 0.638 (0.066) | 0.729 (0.030) | 0.784 (0.039) |
| Super-Gaussian | 0.858 (0.056) | 0.600 (0.044) | 0.729 (0.028) | 0.806 (0.042) |
| Hanning | 0.851 (0.059) | 0.572 (0.105) | 0.711 (0.067) | 0.796 (0.057) |
| Bartlett | 0.827 (0.053) | 0.617 (0.059) | 0.722 (0.028) | 0.785 (0.038) |
| Ideal | 0.851 (0.060) | 0.572 (0.105) | 0.711 (0.067) | 0.796 (0.057) |
| Smoothed ideal | 0.856 (0.056) | 0.586 (0.071) | 0.721 (0.044) | 0.802 (0.046) |
| Star-shaped | 0.795 (0.044) | 0.622 (0.065) | 0.709 (0.024) | 0.760 (0.028) |
| SD-Gaussian | ||||
| kernel size: 3 × 3 | 0.692 (0.006) | 0.672 (0.036) | 0.682 (0.018) | 0.688 (0.008) |
| kernel size: 17 × 17 | 0.936 (0.025) | 0.408 (0.041) | 0.672 (0.019) | 0.831 (0.018) |
| Unfiltered | 0.000 (0.000) | 0.736 (0.030) | 0.368 (0.015) | 0.147 (0.006) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regeling, B.; Thies, B.; Gerstner, A.O.H.; Westermann, S.; Müller, N.A.; Bendix, J.; Laffers, W. Hyperspectral Imaging Using Flexible Endoscopy for Laryngeal Cancer Detection. Sensors 2016, 16, 1288. https://doi.org/10.3390/s16081288
Regeling B, Thies B, Gerstner AOH, Westermann S, Müller NA, Bendix J, Laffers W. Hyperspectral Imaging Using Flexible Endoscopy for Laryngeal Cancer Detection. Sensors. 2016; 16(8):1288. https://doi.org/10.3390/s16081288
Chicago/Turabian StyleRegeling, Bianca, Boris Thies, Andreas O. H. Gerstner, Stephan Westermann, Nina A. Müller, Jörg Bendix, and Wiebke Laffers. 2016. "Hyperspectral Imaging Using Flexible Endoscopy for Laryngeal Cancer Detection" Sensors 16, no. 8: 1288. https://doi.org/10.3390/s16081288






