Test-Retest Reliability of an Automated Infrared-Assisted Trunk Accelerometer-Based Gait Analysis System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocol
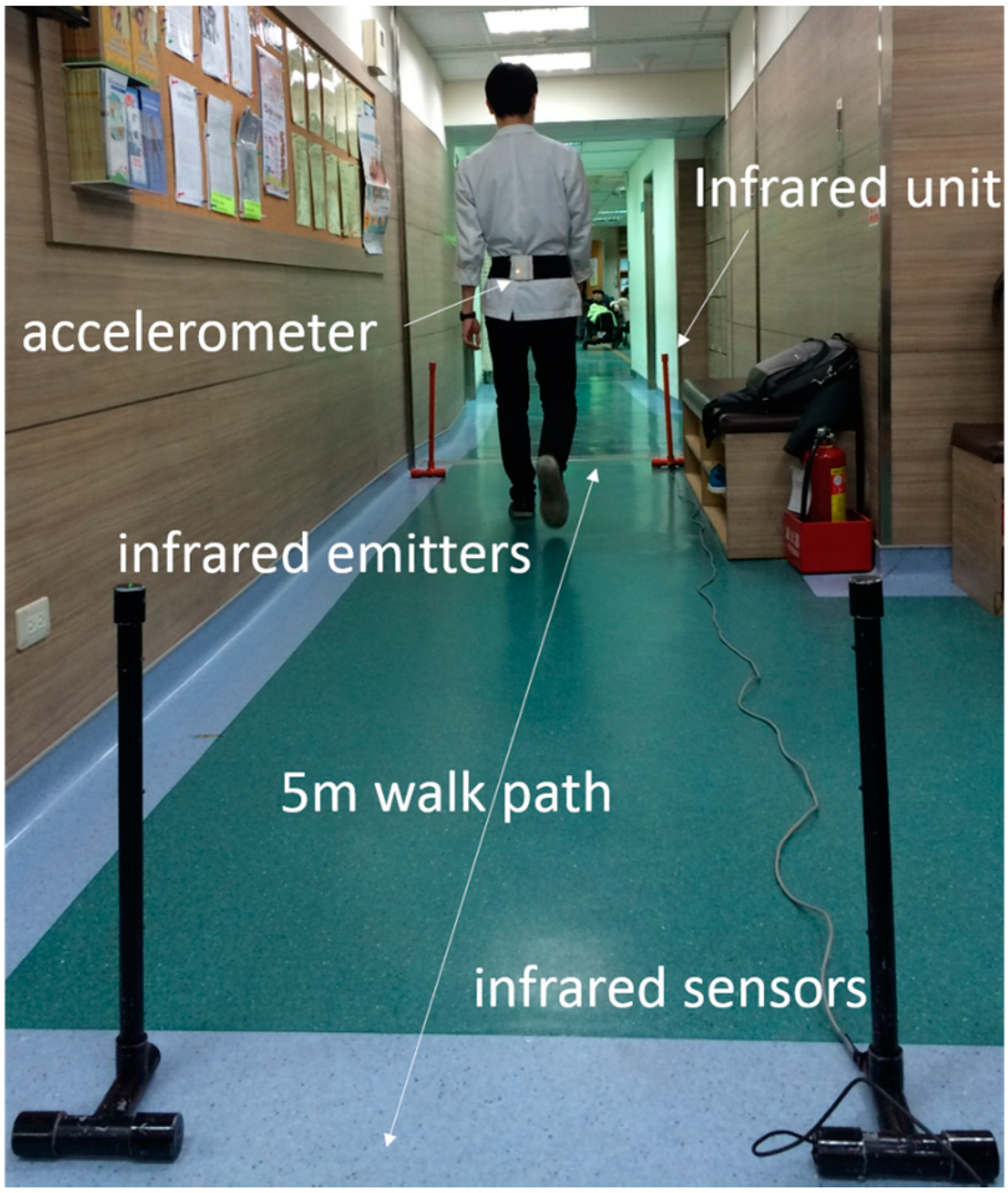
2.3. Equipment
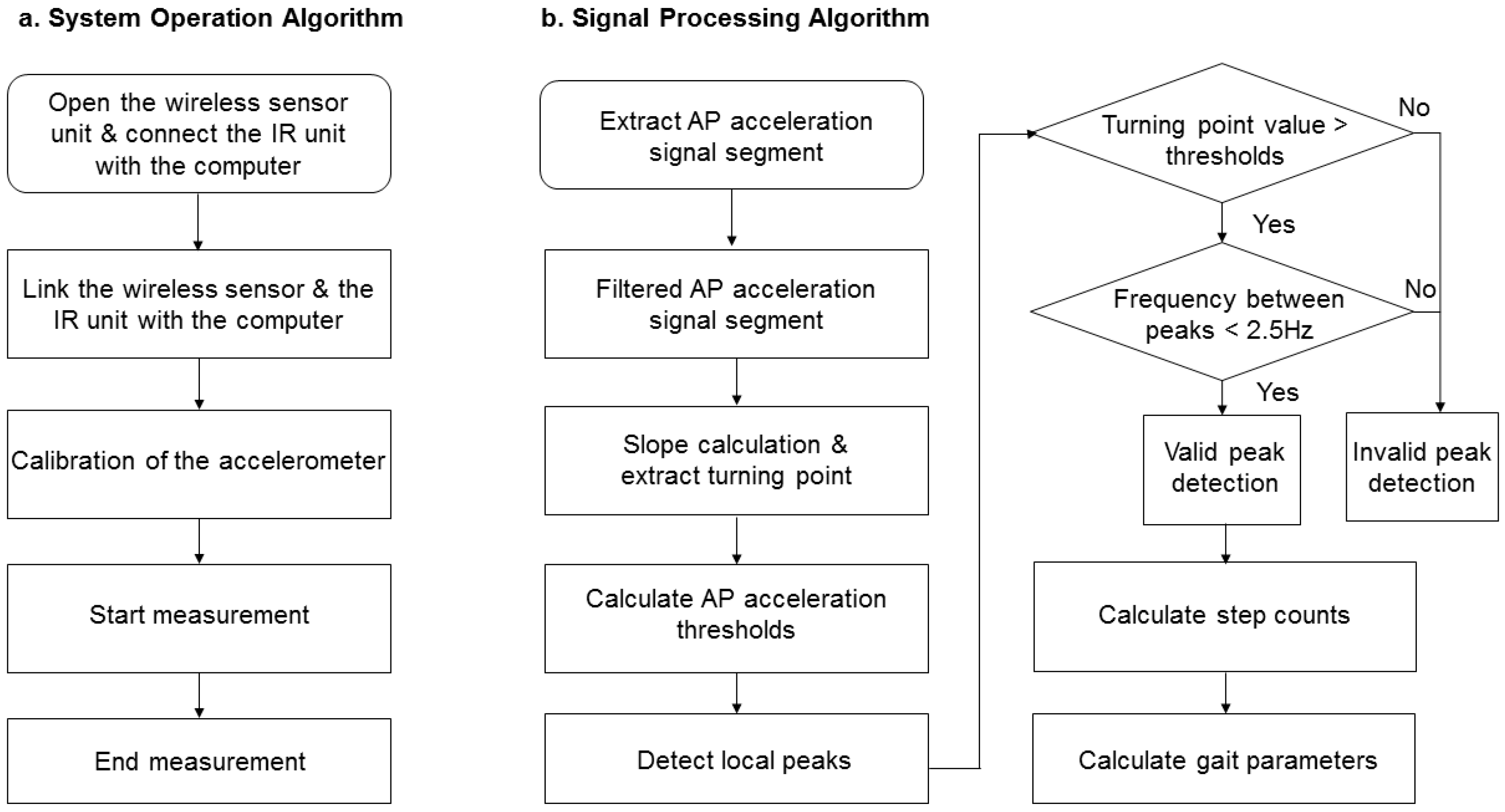
2.4. Gait Characteristics
2.5. Statistical Analysis
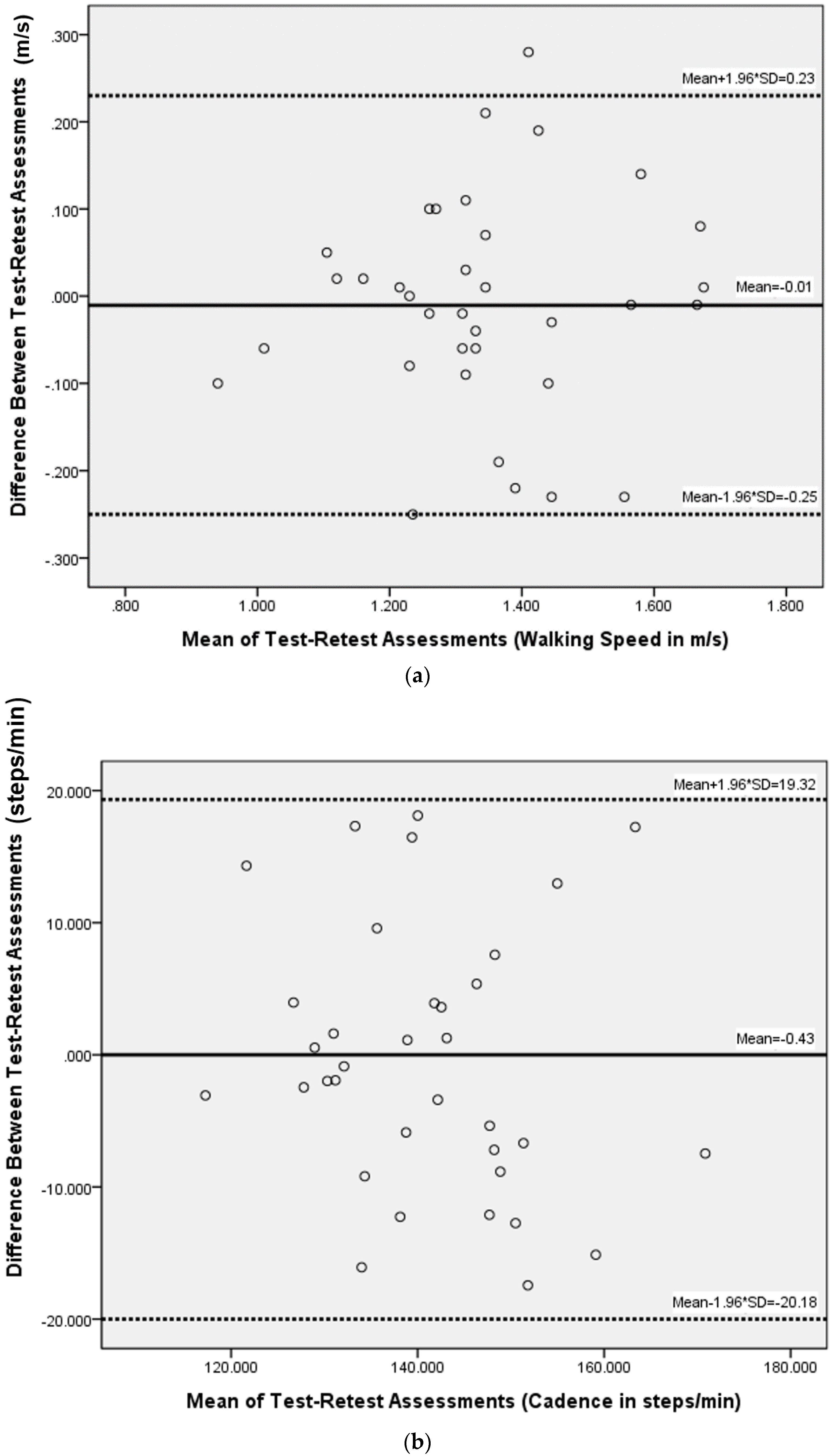
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| AP | anterior-posterior |
| AGA | accelerometer-based gait analysis |
| ICC | intraclass correlation coefficient |
| IR | infrared |
| ML | medial-lateral |
| RMS | root mean square |
| RMSR | root mean square ratio |
| SDD | smallest detectable difference |
| SEM | standard error of measurement |
References
- Mahlknecht, P.; Kiechl, S.; Bloem, B.R.; Willeit, J.; Scherfler, C.; Gasperi, A.; Rungger, G.; Poewe, W.; Seppi, K. Prevalence and burden of gait disorders in elderly men and women aged 60–97 years: A population-based study. PLoS ONE 2013, 8, e69627. [Google Scholar] [CrossRef] [PubMed]
- Stolze, H.; Klebe, S.; Baecker, C.; Zechlin, C.; Friege, L.; Pohle, S.; Deuschl, G. Prevalence of gait disorders in hospitalized neurological patients. Mov. Disord. 2005, 20, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Tinetti, M.E.; Baker, D.I.; McAvay, G.; Claus, E.B.; Garrett, P.; Gottschalk, M.; Koch, M.L.; Trainor, K.; Horwitz, R.I. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N. Engl. J. Med. 1994, 331, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Snijders, A.H.; van de Warrenburg, B.P.; Giladi, N.; Bloem, B.R. Neurological gait disorders in elderly people: Clinical approach and classification. Lancet Neurol. 2007, 6, 63–74. [Google Scholar] [CrossRef]
- Sudarsky, L. Gait disorders: Prevalence, morbidity, and etiology. Adv. Neurol. 2001, 87, 111–117. [Google Scholar] [PubMed]
- Nevitt, M.C.; Cummings, S.R. Type of fall and risk of hip and wrist fractures: The study of osteoporotic fractures. The study of osteoporotic fractures research group. J. Am. Geriatr. Soc. 1993, 41, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait speed and survival in older adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Baker, R. Gait analysis methods in rehabilitation. J. Neuroeng. Rehabil. 2006, 3. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, M.; Lund, H.; Moe-Nilssen, R.; Bliddal, H.; Danneskiod-Samsoe, B. Test-retest reliability of trunk accelerometric gait analysis. Gait Posture 2004, 19, 288–297. [Google Scholar] [CrossRef]
- Howell, D.; Osternig, L.; Chou, L.S. Monitoring recovery of gait balance control following concussion using an accelerometer. J. Biomech. 2015, 48, 3364–3368. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, A.; Yoshida, K.; Genno, H.; Murata, A.; Matsuzawa, S.; Nakamura, K.; Nakamura, A.; Ikeda, S. Clinical assessment of standing and gait in ataxic patients using a triaxial accelerometer. Cerebellum Ataxias 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Senden, R.; Grimm, B.; Heyligers, I.C.; Savelberg, H.H.; Meijer, K. Acceleration-based gait test for healthy subjects: Reliability and reference data. Gait Posture 2009, 30, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Moe-Nilssen, R.; Helbostad, J.L. Estimation of gait cycle characteristics by trunk accelerometry. J. Biomech. 2004, 37, 121–126. [Google Scholar] [CrossRef]
- Zijlstra, W.; Hof, A.L. Assessment of spatio-temporal gait parameters from trunk accelerations during human walking. Gait Posture 2003, 18, 1–10. [Google Scholar] [CrossRef]
- Ben Mansour, K.; Rezzoug, N.; Gorce, P. Analysis of several methods and inertial sensors locations to assess gait parameters in able-bodied subjects. Gait Posture 2015, 42, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.C.; Lopez, A.M.; Rodriguez-Uria, J.; Alvarez, D.; Alvarez, J.C. Real-time gait event detection for normal subjects from lower trunk accelerations. Gait Posture 2010, 31, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Maffiuletti, N.A.; Gorelick, M.; Kramers-de Quervain, I.; Bizzini, M.; Munzinger, J.P.; Tomasetti, S.; Stacoff, A. Concurrent validity and intrasession reliability of the ideea accelerometry system for the quantification of spatiotemporal gait parameters. Gait Posture 2008, 27, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Sekine, M.; Tamura, T.; Yoshida, M.; Suda, Y.; Kimura, Y.; Miyoshi, H.; Kijima, Y.; Higashi, Y.; Fujimoto, T. A gait abnormality measure based on root mean square of trunk acceleration. J. Neuroeng. Rehabil. 2013, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.C.; Bussmann, J.; Janssen, W.; Horemans, H.; Chastin, S.; Heijenbrok, M.; Stam, H. Accelerometric assessment of different dimensions of natural walking during the first year after stroke: Recovery of amount, distribution, quality and speed of walking. J. Rehabil. Med. 2015, 47, 714–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicchetti, D.V.; Sparrow, S.A. Developing criteria for establishing interrater reliability of specific items: Applications to assessment of adaptive behavior. Am. J. Ment. Defic. 1981, 86, 127–137. [Google Scholar] [PubMed]
- Haley, S.M.; Fragala-Pinkham, M.A. Interpreting change scores of tests and measures used in physical therapy. Phys. Ther. 2006, 86, 735–743. [Google Scholar] [PubMed]
- Schreuders, T.A.; Roebroeck, M.E.; Goumans, J.; van Nieuwenhuijzen, J.F.; Stijnen, T.H.; Stam, H.J. Measurement error in grip and pinch force measurements in patients with hand injuries. Phys. Ther. 2003, 83, 806–815. [Google Scholar] [PubMed]
- Lu, W.S.; Wang, C.H.; Lin, J.H.; Sheu, C.F.; Hsieh, C.L. The minimal detectable change of the simplified stroke rehabilitation assessment of movement measure. J. Rehabil. Med. 2008, 40, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.L.; Hsieh, C.L.; Wu, R.M.; Tai, C.H.; Lin, C.H.; Lu, W.S. Minimal detectable change of the timed “up & go” test and the dynamic gait index in people with parkinson disease. Phys. Ther. 2011, 91, 114–121. [Google Scholar] [PubMed]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Tura, A.; Raggi, M.; Rocchi, L.; Cutti, A.G.; Chiari, L. Gait symmetry and regularity in transfemoral amputees assessed by trunk accelerations. J. Neuroeng. Rehabil. 2010, 7. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.; Allard, P.; Prince, F.; Labelle, H. Symmetry and limb dominance in able-bodied gait: A review. Gait Posture 2000, 12, 34–45. [Google Scholar] [CrossRef]
- Kuan, T.S.; Tsou, J.Y.; Su, F.C. Hemiplegic gait of stroke patients: The effect of using a cane. Arch. Phys. Med. Rehabil. 1999, 80, 777–784. [Google Scholar] [CrossRef]
- Nilsson, J.; Thorstensson, A. Ground reaction forces at different speeds of human walking and running. Acta Physiol. Scand. 1989, 136, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Demonceau, M.; Donneau, A.F.; Croisier, J.L.; Skawiniak, E.; Boutaayamou, M.; Maquet, D.; Garraux, G. Contribution of a trunk accelerometer system to the characterization of gait in patients with mild-to-moderate parkinson’s disease. IEEE J. Biomed. Health Inform. 2015, 19, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Rispens, S.M.; Pijnappels, M.; van Schooten, K.S.; Beek, P.J.; Daffertshofer, A.; van Dieen, J.H. Consistency of gait characteristics as determined from acceleration data collected at different trunk locations. Gait Posture 2014, 40, 187–192. [Google Scholar] [CrossRef] [PubMed]




| Mean (SD) | Min. | Max. | |
|---|---|---|---|
| Age (years) | 40.0 (15.2) | 23 | 79 |
| Height (cm) | 161.5 (7.9) | 150 | 183 |
| Weight (kg) | 61.6 (13.9) | 43 | 95 |
| Occasion 1, Mean (SD) | Occasion 2, Mean (SD) | Paired t Test | ICC (95% Confidence Interval) | SEM | SDD | SDD, % | ||
|---|---|---|---|---|---|---|---|---|
| Velocity (m/s) | 1.34 (0.19) | 1.35 (0.18) | 0.62 | 0.87 (0.74–0.93) | 0.065 | 0.180 | 13.4 | |
| Step length (cm) | 56.83 (5.95) | 57.22 (5.67) | 0.62 | 0.81 (0.63–0.91) | 2.516 | 6.974 | 12.2 | |
| Cadence (step/min) | 140.87 (11.85) | 141.30 (13.40) | 0.80 | 0.81 (0.63–0.91) | 5.475 | 15.176 | 10.8 | |
| AP | acceleration RMS (g) | 0.14 (0.41) | 0.13 (0.43) | 0.09 | 0.74 (0.48–0.87) | 0.022 | 0.061 | 44.5 |
| Symmetry | 81.20 (8.61) | 83.30 (7.38) | 0.15 | 0.63 (0.27–0.81) | 4.884 | 13.538 | 16.5 | |
| Stride regularity | 0.58 (0.09) | 0.60 (0.10) | 0.32 | 0.83 (0.67–0.92) | 0.040 | 0.111 | 18.8 | |
| Step regularity | 0.72 (0.08) | 0.72 (0.09) | 0.82 | 0.79 (0.59–0.89) | 0.038 | 0.105 | 14.7 | |
| ML | acceleration RMS (g) | 0.13 (0.05) | 0.14 (0.05) | 0.89 | 0.83 (0.66–0.91) | 0.020 | 0.055 | 41.1 |
| acceleration RMSR | 0.48 (0.11) | 0.48 (0.10) | 0.80 | 0.81 (0.62–0.90) | 0.045 | 0.125 | 26.0 | |
| Symmetry | 81.46 (11.43) | 79.49 (13.76) | 0.46 | 0.38 (−0.23–0.69) | 9.918 | 27.491 | 34.2 | |
| Stride regularity | 0.35 (0.09) | 0.36 (0.09) | 0.75 | 0.72 (0.45–0.86) | 0.049 | 0.136 | 38.2 | |
| Step regularity | 0.44 (0.12) | 0.46 (0.12) | 0.30 | 0.64 (0.28–0.82) | 0.070 | 0.194 | 43.2 | |
| V | acceleration RMS(g) | 0.19 (0.04) | 0.20 (0.05) | 0.10 | 0.88 (0.76–0.94) | 0.015 | 0.042 | 21.4 |
| Symmetry | 84.24 (7.15) | 81.76 (8.58) | 0.09 | 0.62 (0.25–0.81) | 4.894 | 13.565 | 16.3 | |
| Stride regularity | 0.56 (0.09) | 0.55 (0.11) | 0.36 | 0.76 (0.52–0.88) | 0.049 | 0.136 | 24.4 | |
| Step regularity | 0.67 (0.08) | 0.67 (0.11) | 0.85 | 0.55 (0.11–0.77) | 0.062 | 0.172 | 25.7 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-Y.; Tsai, Y.-S.; Yau, C.-S.; Shie, H.-H.; Wu, C.-M. Test-Retest Reliability of an Automated Infrared-Assisted Trunk Accelerometer-Based Gait Analysis System. Sensors 2016, 16, 1156. https://doi.org/10.3390/s16081156
Hsu C-Y, Tsai Y-S, Yau C-S, Shie H-H, Wu C-M. Test-Retest Reliability of an Automated Infrared-Assisted Trunk Accelerometer-Based Gait Analysis System. Sensors. 2016; 16(8):1156. https://doi.org/10.3390/s16081156
Chicago/Turabian StyleHsu, Chia-Yu, Yuh-Show Tsai, Cheng-Shiang Yau, Hung-Hai Shie, and Chu-Ming Wu. 2016. "Test-Retest Reliability of an Automated Infrared-Assisted Trunk Accelerometer-Based Gait Analysis System" Sensors 16, no. 8: 1156. https://doi.org/10.3390/s16081156





