Ultrasonic Sensing of Plant Water Needs for Agriculture
Abstract
:1. Introduction
2. Description of a NC-RUS System for Plant Leaves and Main Design Parameters and Specifications
- Sensors. A couple of wideband and high sensitivity air-coupled ultrasonic transducers (transmitter: Tx and receiver: Rx).
- Electronics. A pulser/receiver to excite Tx and to filter, amplify and digitize the electrical signal in Rx. If an analog pulser/receiver is used, then a digital oscilloscope or a similar device is required to digitize the received signal.
- PC and software. Including: (i) software to control the electronics and display the results, includes a graphical user interface (GUI) and (ii) the software to solve the inverse problem and extract leaf parameters from the measured resonance.
- Structural elements. Including: (i) a system to hold sensors in the right position, (ii) a sample holder that allows the right location of the leaf in-between the ultrasonic sensors and (iii) any system to isolate the measurements from the influence of environmental conditions.
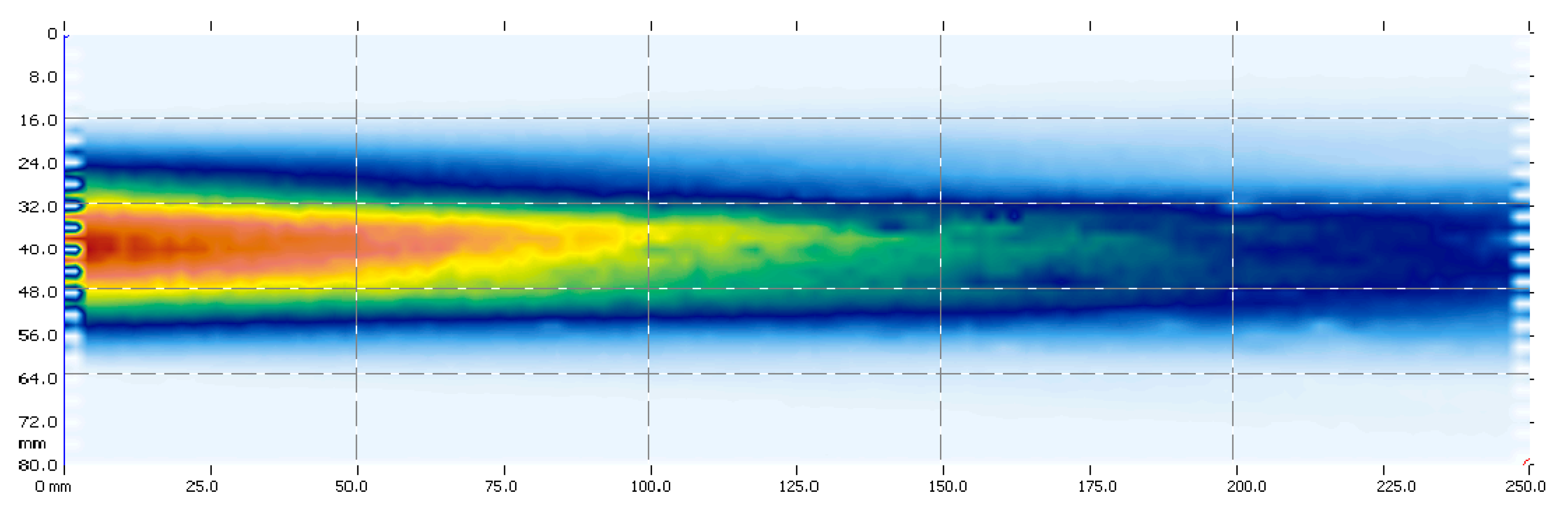
2.1. Size of the Measurement Area and Geometry of the Ultrasonic Field (Beam)
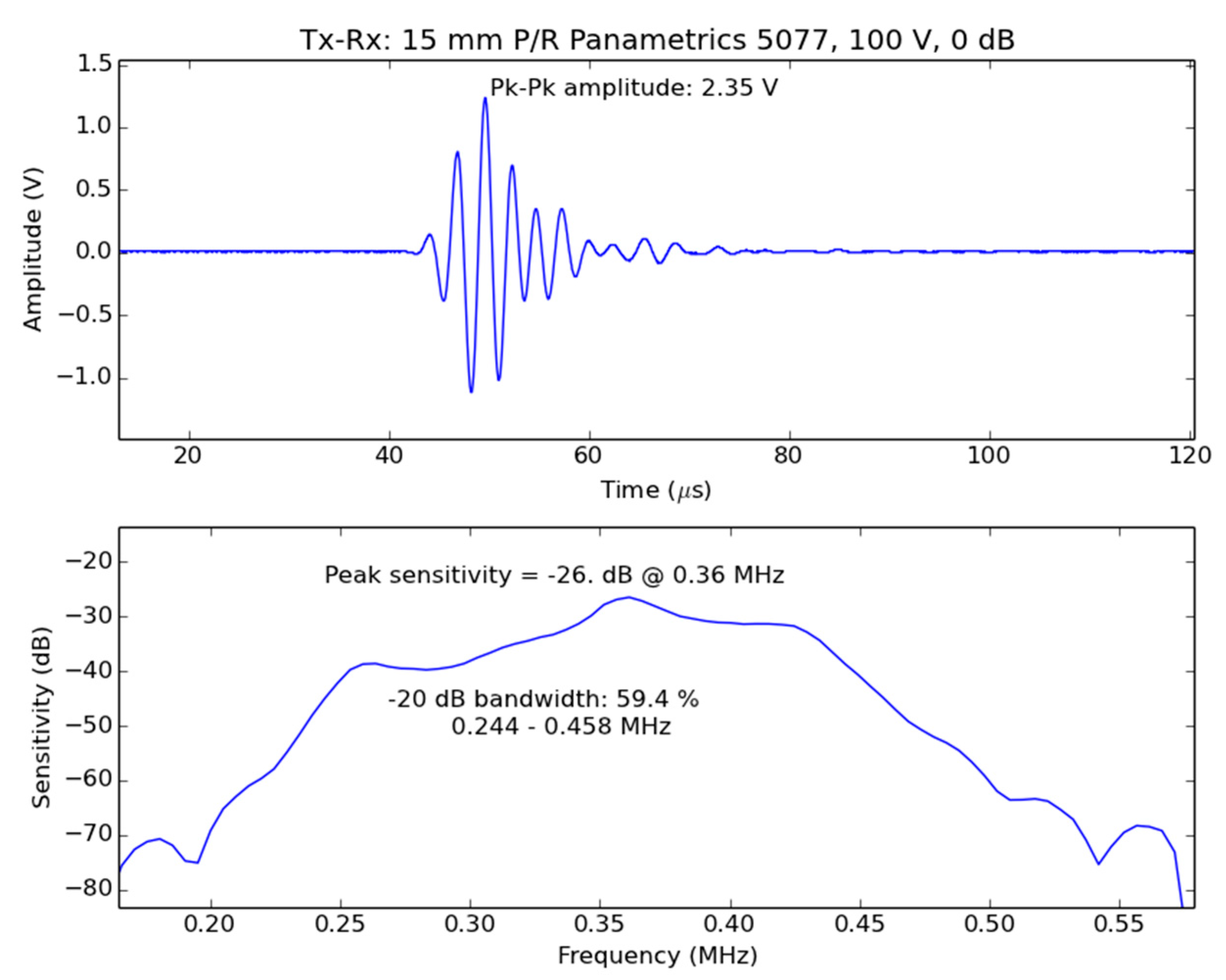
2.2. Centre Frequency and Frequency Bandwidth
2.3. Dynamic Range and SNR
2.4. Separation between the Sensors and the Leaf
2.5. Time of Measurement
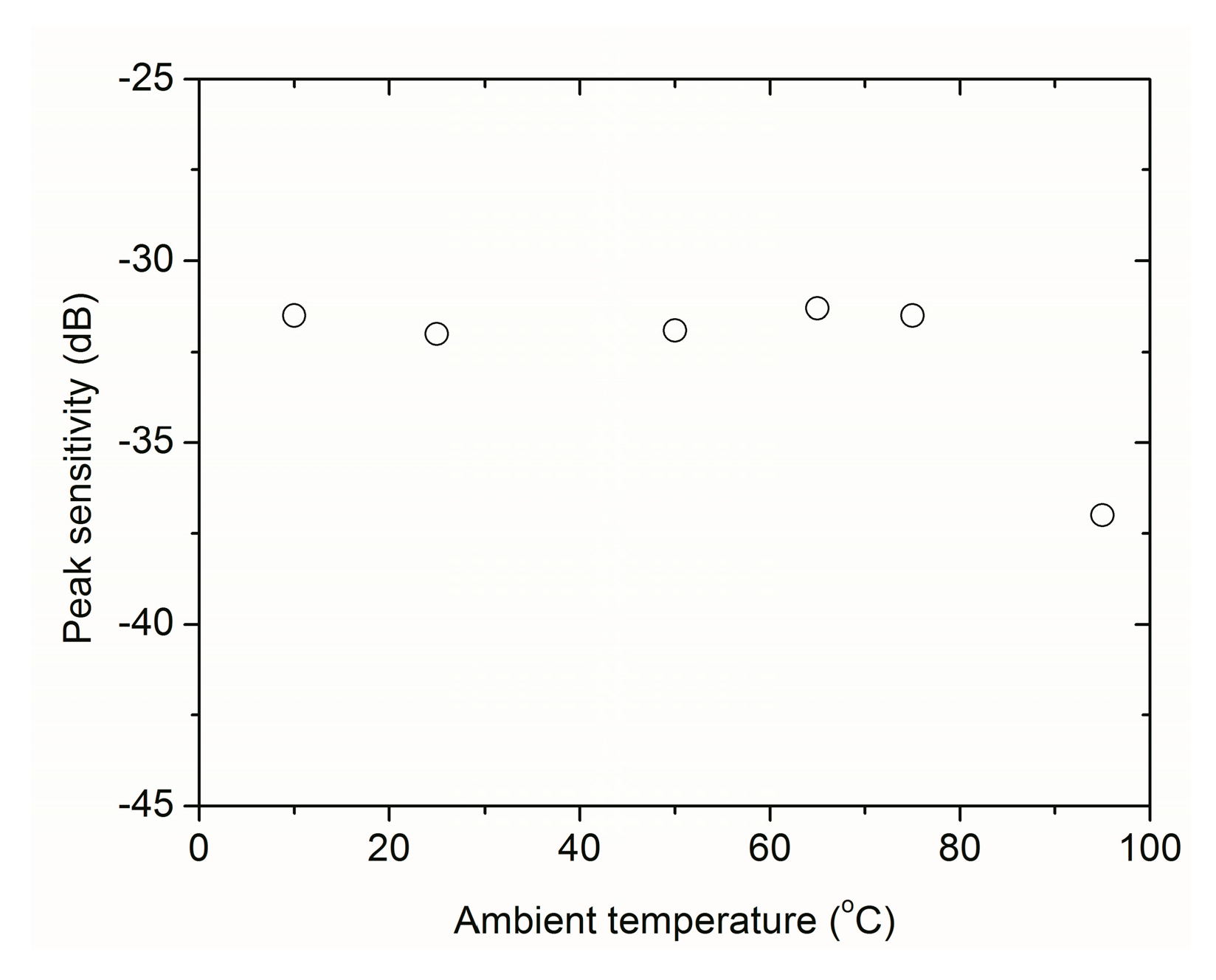
2.6. Portability and Robustness
3. Proposed NC-RUS System for Plant Leaves: General Solution
3.1. Sensors
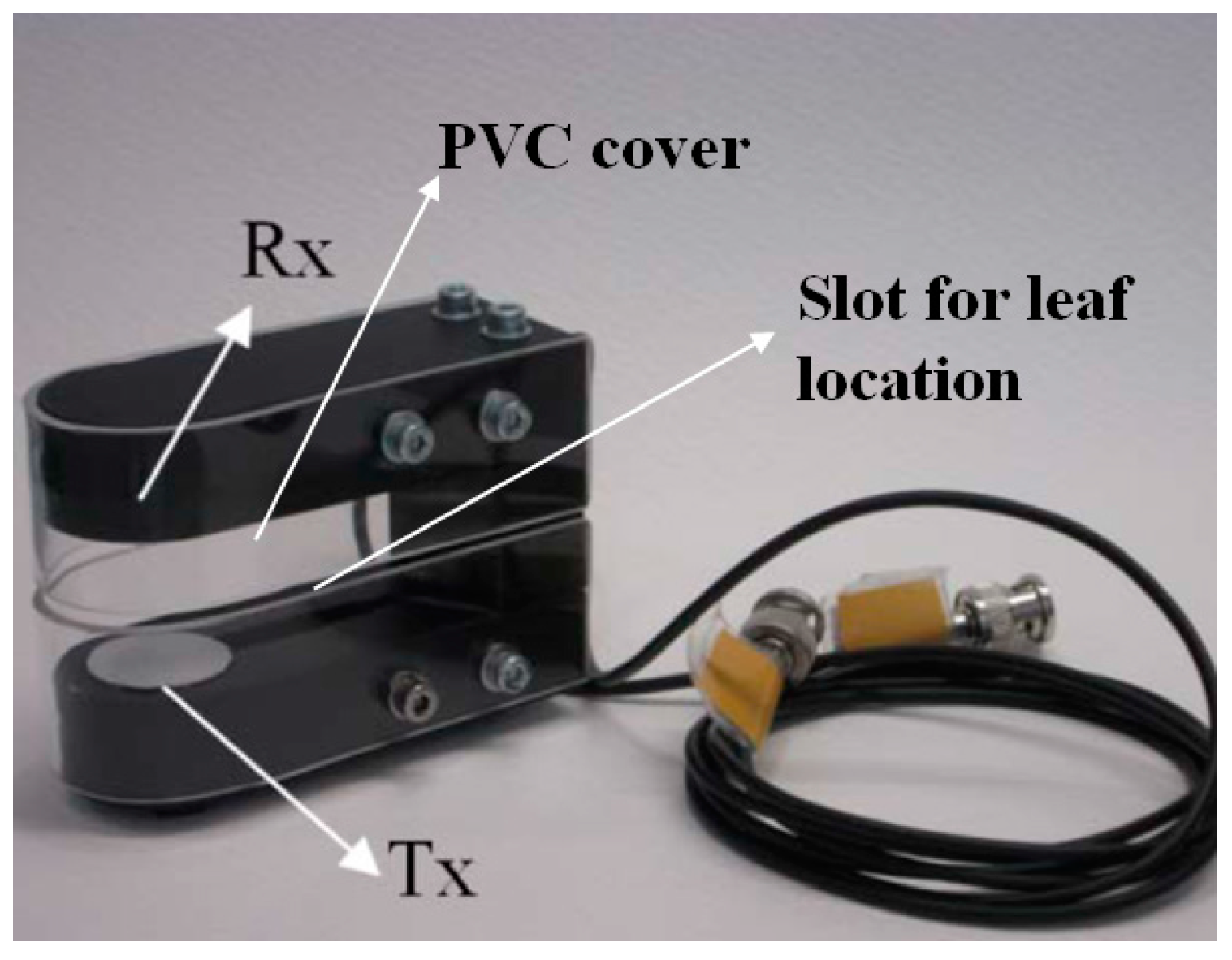
3.2. Mechanical Holder for Sensors and Leaf
3.3. Electronics
3.4. Control Software (GUI)
3.5. Data Processing
3.6. System Integration
4. Specifications of the NC-RUS System for Coffea Arabica and Vitis Vinifera Leaves: Sensors and Structural Elements
4.1. Specific Design of Sensors for Vitis Vinifera Leaves
4.2. Specific Design of Sensors for Coffea Arabica Leaves
5. Examples of Application
5.1. Vitis Vinifera
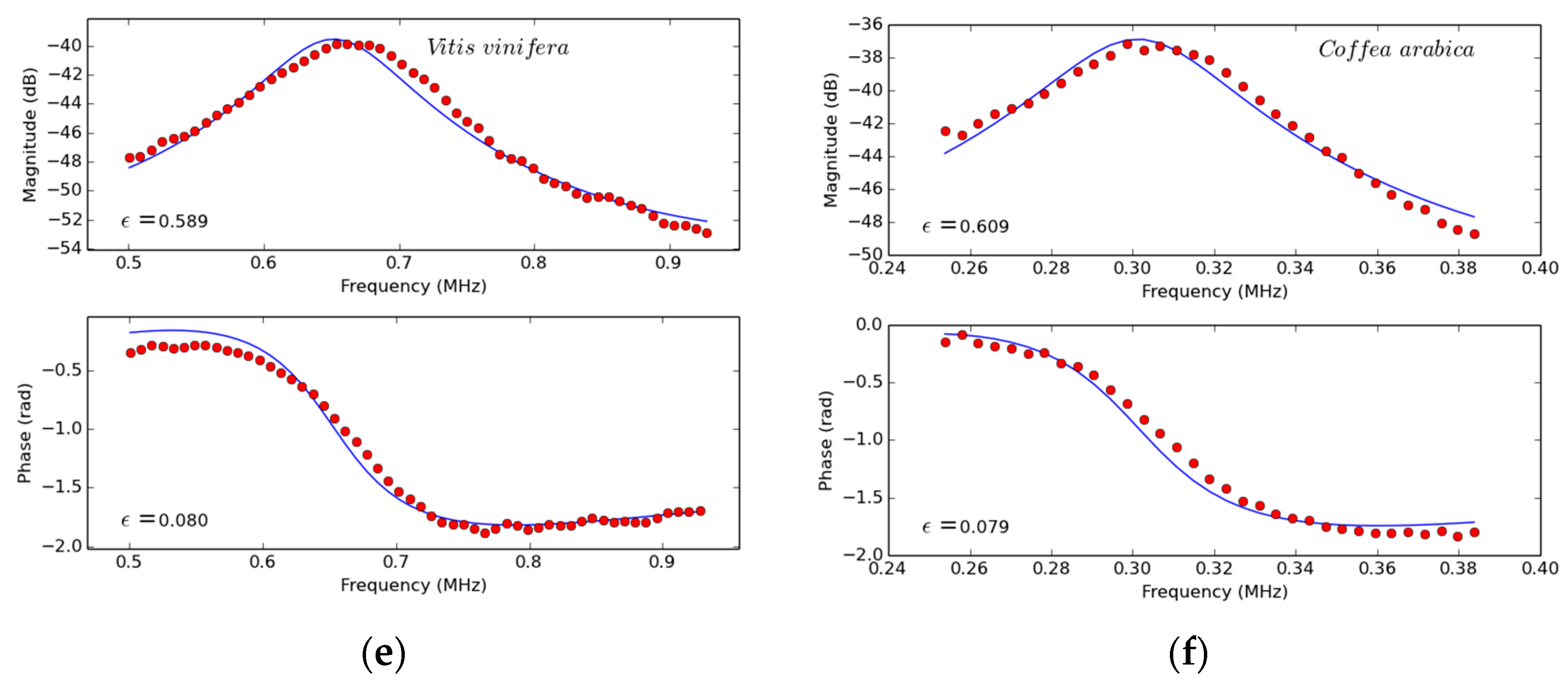
5.1.1. Extraction of Leaf Parameters from the Measured Spectra of the First Thickness Resonance
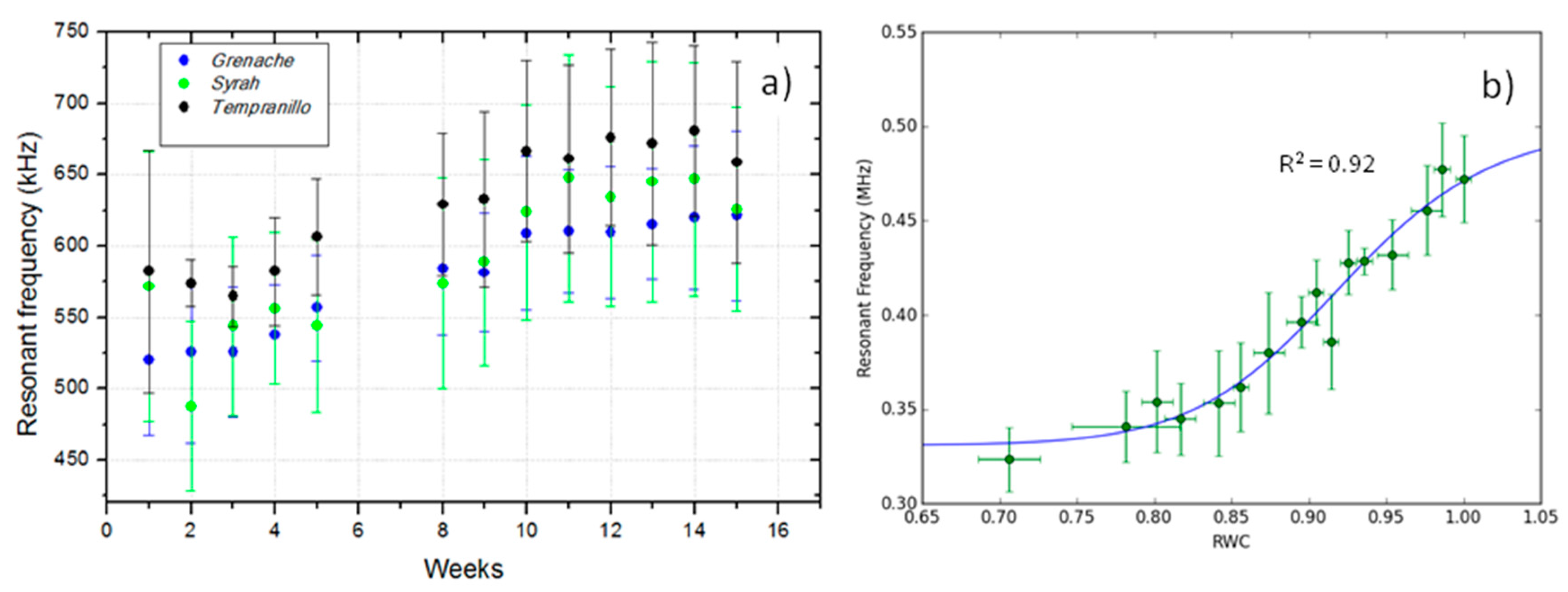
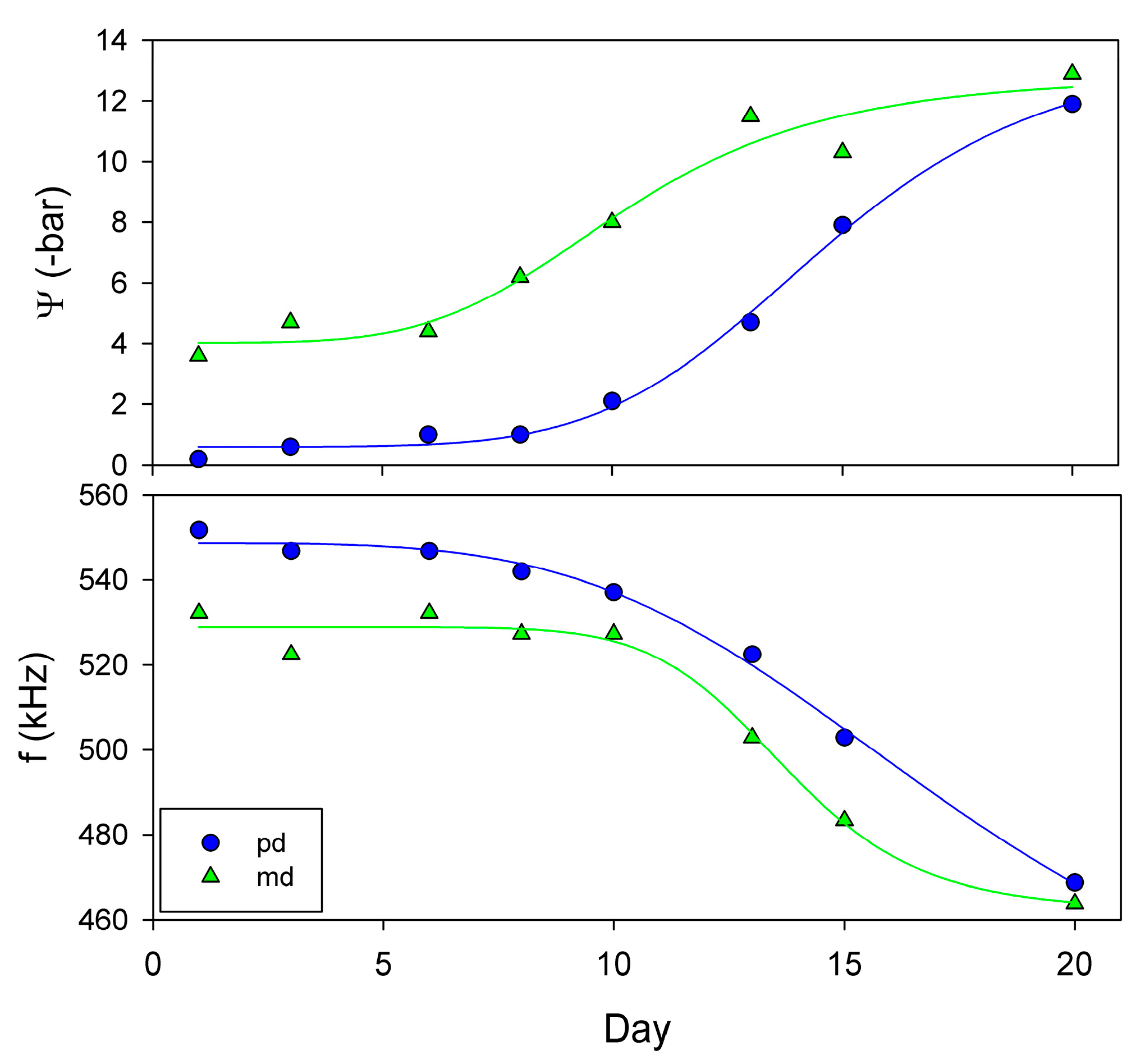
5.1.2. Use of NC-RUS to Monitor Drought Stress in Vitis vinifera
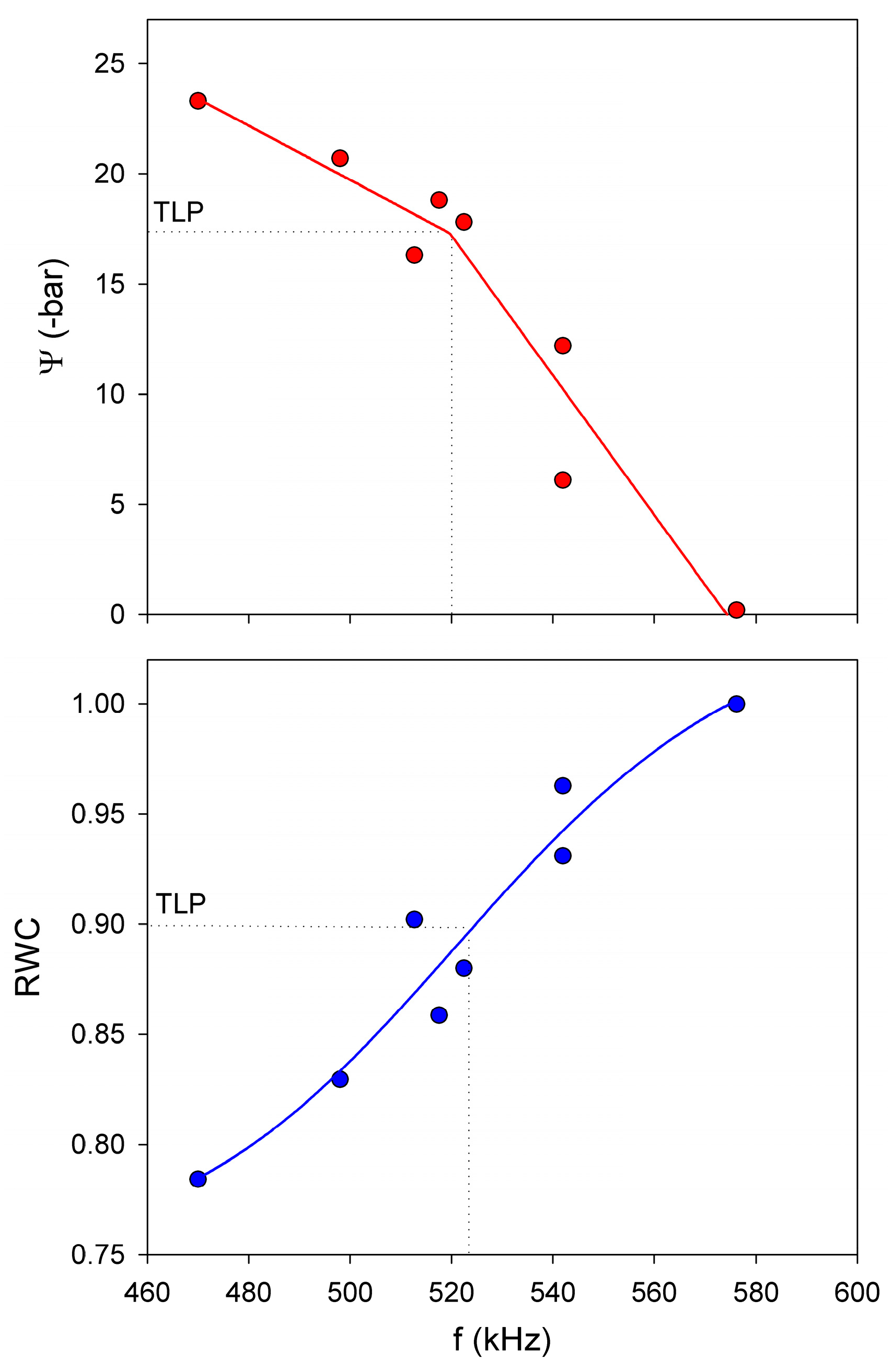
5.1.3. Use of NC-RUS to Monitor the Dehydratation Process of Vitis vinifera Leaves: Relationship between Resonant Frequency, Water Potential and Relative Water Content
5.2. Coffea Arabica
5.2.1. Extraction of Leaf Parameters from the Measured Spectra of the First Thickness Resonance
5.2.2. Extraction of Leaf Parameters from the Measured Spectra of the First Thickness Resonance
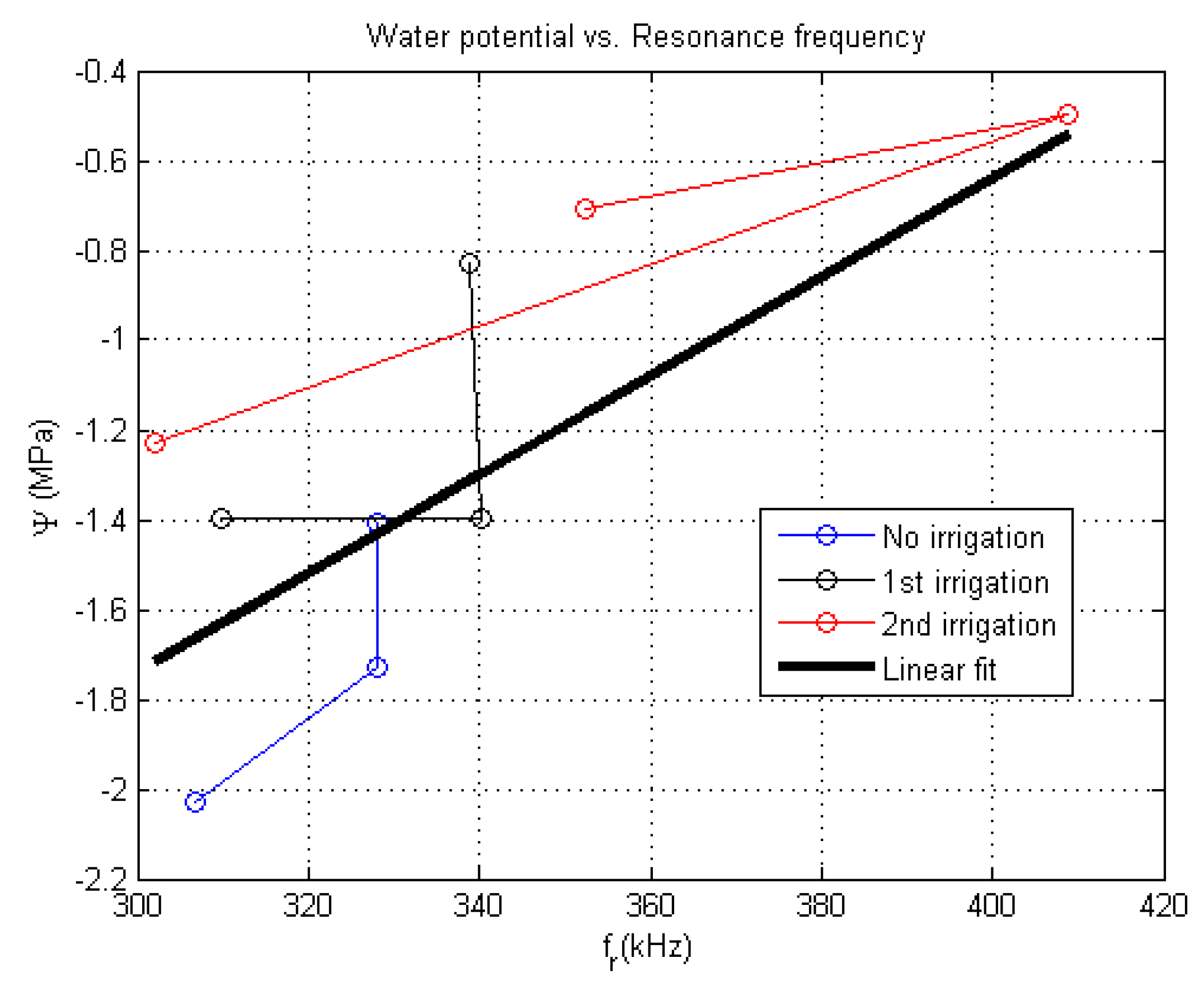
5.2.3. Relationship between Ultrasonic Transmission Spectra and the Water Potential
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pimentel, D.; Berger, B.; Filiberto, D.; Newton, M.; Wolfe, B.; Karabinakis, E.; Clark, S.; Poon, E.; Abbett, E.; Nandagopal, S. Water resources: Agricultural and environmental issues. Bioscience 2004, 54, 909–918. [Google Scholar]
- Taher Kahil, M.; Albiac, J.; Dinar, A.; Calvo, E.; Esteban, E.; Avella, L.; Garcia-Molla, M. Improving the performance of water policies: Evidence from drought in spain. Water 2016, 8, 34. [Google Scholar] [CrossRef]
- European Environment Agency. Water for Agriculture. Available online: http://www.webcitation.org/6fVy4dzts (accessed on 23 Feburary 2016).
- Chaves, M.M.; Santos, T.P.; Souza, C.R.; Ortuño, M.F.; Rodrigues, M.L.; Lopes, C.M.; Maroco, J.P.; Pereira, J.S. Deficit irrigation in grapevine improves water-use efficiency while controlling vigour and production quality. Ann. Appl. Biol. 2007, 150, 237–252. [Google Scholar] [CrossRef]
- Flexas, J.; Galmés, J.; Gallé, A.; Gulías, J.; Pou, A.; Ribas-Carbo, M.; Tomás, M.; Medrano, H. Improvement of water use efficiency in grapevines. Aust. J. Grape. Wine. Res. 2010, 16, 106–121. [Google Scholar] [CrossRef]
- Medrano, H.; Escalona, J.M.; Cifre, J.; Bota, J.; Flexas, J. A ten-year study on the physiology of two Spanish grapevine cultivars under field conditions: Effects of water availability from leaf photosynthesis to grape yield and quality. Funct. Plant. Biol. 2003, 30, 607–619. [Google Scholar] [CrossRef]
- Keller, M.; Smithyman, R.P.; Mills, L.J. Interactive effects of deficit irrigation and crop load on Cabernet Sauvignon in an arid climate. Am. J. Enol. Vitic. 2008, 59, 221–234. [Google Scholar]
- Pou, A.; Gulias, J.; Moreno, M.; Tomas, M.; Medrano, H.; Cifre, J. Cover cropping in Vitis vinifera L. cv. Manto Negro vineyards under Mediterranean conditions: Effects on plant vigour, yield and grape quality. J. Int. Sci. Vigne Vin. 2011, 45, 223–234. [Google Scholar]
- Dinero Magazine. Available online: http://www.webcitation.org/6hmmJBuhP (accessed on 26 March 2016).
- Ramírez Builes, V.H.; Jaramillo Robledo, A.; Arcila Pulgarín, J. Rangos adecuados de lluvia para el cultivo de café en Colombia. Avances técnicos Cenicafe, Federación Nacional de Cafeteros de Colombia 2010, 395, 1–8. [Google Scholar]
- Marin, F.R.; Angelocci, L.R.; Righi, E.Z.; Sentelhas, P.C. Evapotranspiration and Irrigation Requirements of a Coffee Plantation in Southern Brazil. Exp. Agric. 2005, 41, 187–197. [Google Scholar] [CrossRef]
- Gleice A. de, A.; Scalco, M.S.; Guimarães, R.J.; Colombo, A.; Dominghetti, A.W.; de Matos, N.M.S. Drip Irrigation in Coffee Crop under Different Planting Densities: Growth and Yield in Southeastern Brazil. Revista Brasileira de Engenharia Agrícola E Ambiental 2014, 18, 1116–1123. [Google Scholar]
- Lopes Serra, E.; Scalco, M.S.; Guimaraes, R.J.; Colombo, A.; Ramalho, A.; Mesquita, C.H. Production Functions of Irrigated Coffee under Different Planting Densities. Coffee Sci. Lavras 2013, 8, 149–157. [Google Scholar]
- Chemura, A.; Mahoya, C.; Kutywayo, D.; Chidoko, P.; Services, S. The Growth Response of Coffee Plants to Organic Manure, Inorganic Fertilizers and Integrated Soil Fertility Management under Different Irrigation. Int. J. Recycl. Org. Waste Agric. 2014, 3, 1–9. [Google Scholar] [CrossRef]
- Barrs, H.D. Determination of water deficits in plant tissues. In Water Deficits and Plant Growth; Kozlowski, T.T., Ed.; Academic Press: New York, NY, USA, 1968; Volume 1, pp. 235–368. [Google Scholar]
- Slavík, B. Methods of Studying Plant Water Relations; Springer: Berlin, Germany, 1974. [Google Scholar]
- Jones, H.G. Monitoring plant and soil water status: Established and novel methods revisited and their relevance to studies of drought tolerance. J. Exp. Bot. 2007, 58, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Koide, R.T.; Robichaux, R.H.; Morse, S.R.; Smith, C.M. Plant water status, hydraulic resistance and capacitance. In Plant Physiological Ecology: Field Methods and Instrumentation; Pearcy, R.W., Ehleringer, J., Mooney, H.A., Rundel, P.W., Eds.; Chapman and Hall: London, UK, 1989; pp. 161–183. [Google Scholar]
- Savage, M.J.; Wiebe, H.H.; Cass, A. Effect of cuticular abrasion on thermocouple psychrometric in situ measurement of leaf water potential. J. Exp. Bot. 1984, 35, 36–42. [Google Scholar] [CrossRef]
- Mc Burney, T. The relationship between leaf thickness and plant water potential. J. Exp. Bot. 1992, 43, 327–335. [Google Scholar] [CrossRef]
- Lintilhac, P.M.; Wei, C.; Tanguay, J.J.; Outwater, J.O. Ball tonometry: A rapid, non-destructive method for measuring cell turgor pressure in thin-walled plant cells. J. Plant Growth Regul. 2000, 19, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Grant, O.M.; Tronina, L.; Chaves, M.M. Exploring thermal imaging variables for the detection of stress responses in grapevine under different irrigation regimes. J. Exp. Bot. 2006, 58, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Peguero-Pina, J.J.; Morales, F.; Flexas, J.; Gil-Pelegrín, E.; Moya, I. Photochemistry, remotely sensed physiological reflectance index and de-epoxidation state of the xanthophyll cycle in Quercus coccifera under intense drought. Oecologia 2008, 156, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, D.; Reuss, R.; Westhoff Gebner, P.; Bauer, W.; Bamberg Bentrup, F.W.; Zimmermann, U. A novel, noninvasive, online-monitoring, versatile and easy plant-based probe for measuring leaf water status. J. Exp. Bot. 2008, 59, 3157–3167. [Google Scholar] [CrossRef] [PubMed]
- Migliori, A.; Darling, T.W.; Baiardo, J.P.; Freibert, F. Resonant ultrasound spectroscopy. Exp. Methods Phys. Sci. 2001, 39, 189–220. [Google Scholar]
- Migliori, A.; Sarrao, J.L.; Visscher, W.M.; Bell, T.M.; Lei, M.; Fisk, Z.; Leisure, R.G. Resonant ultrasound spectroscopic techniques for measurement of the elastic moduli of solids. Physica B 1993, 183, 1–24. [Google Scholar] [CrossRef]
- Migliori, A.; Darling, T.W. Resonant ultrasound spectroscopy for materials studies and non-destructive testing. Ultrasonics 1996, 34, 473–476. [Google Scholar] [CrossRef]
- Leisure, R.G.; Willis, F.A. Resonant Ultrasound Spectroscopy. J. Phys. Condens. Matter 1997, 9, 6001–6029. [Google Scholar] [CrossRef]
- Hutchins, D.A.; Wright, W.M.D. Ultrasonic measurements in polymeric materials using air-coupled capacitance transducers. J. Acoust. Soc. Am. 1994, 96, 1634–1642. [Google Scholar] [CrossRef]
- Schindel, D.W.; Hutchins, D.A. Through-thickness characterization of solids by wideband air-coupled ultrasound. Ultrasonics 1995, 33, 11–17. [Google Scholar] [CrossRef]
- Álvarez-Arenas, T.E.G.; Montero, F.; Moner-Girona, M.; Rodrı́guez, E.; Roig, A.; Molins, E. Viscoelasticity of silica aerogels at ultrasonic frequencies. Appl. Phys. Lett. 2002, 81, 1198. [Google Scholar] [CrossRef]
- Álvarez-Arenas, T.E.G. Air-coupled ultrasonic spectroscopy for the study of membrane filters. J. Membr. Sci. 2003, 213, 195–207. [Google Scholar] [CrossRef]
- Alvarez-Arenas, T.E.G. Simultaneous determination of the ultrasound velocity and the thickness of solid plates from the analysis of thickness resonances using air-coupled ultrasound. Ultrasonics 2010, 50, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Arenas, T.E.G.; Calás, H.; Cuello, J.E.; Fernández, A.; Muñoz, M. Noncontact ultrasonic spectroscopy applied to the study of polypropylene ferroelectrets. J. Appl. Phys. 2010, 108, 074110. [Google Scholar] [CrossRef]
- Álvarez-Arenas, T.E.G.; Sancho-Knapik, D.; Peguero-Pina, J.J.; Gil-Pelegrín, E. Noncontact and noninvasive study of plant leaves using air-coupled ultrasounds. Appl. Phys. Lett. 2009, 95, 193702. [Google Scholar] [CrossRef]
- Sancho-Knapik, D.; Álvarez-Arenas, T.E.G.; Peguero Pina, J.J.; Gil-Pelegrín, E. Air-coupled broadband ultrasonic spectroscopy as a new non-invasive and non-contact method for the determination of leaf water status. J. Exp. Bot. 2010, 61, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Knapik, D.; Álvarez-Arenas, T.E.G.; Peguero-Pina, J.J.; Fernandez, V.; Gil-Pelegrín, E. Relationship between ultrasonic properties and structural changes in the mesophyll during leaf dehydration. J. Exp. Bot. 2011, 62, 3637–3645. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Knapik, D.; Calás, H.; Peguero-Pina, J.J.; Ramos-Fernández, A.; Gil-Pelegrín, E.; Álvarez-Arenas, T.E.G. Air-coupled ultrasonic resonance spectroscopy for the study of the relationship between plant leaves elasticity and their water content. IEEE Trans. Ultrason. Ferroelect. Freq. Control 2012, 59, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Knapik, D.; Peguero-Pina, J.J.; Medrano, H.; Fariñas, M.D.; Álvarez-Arenas, T.E.G.; Gil-Pelegrín, E. Ultrasonic spectroscopy allows a rapid determination of the relative water content at the turgor loss point. A comparison with pressure-volume curves in 13 woody species. Tree Physiol. 2013, 33, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Fariñas, M.D.; Sancho-Knapik, D.; Peguero-Pina, J.J.; Gil-Pelegrín, E.; Gómez Alvarez-Arenas, T. Ultrasonic monitoring of plants response to environmental stimuli. Ultrasound. Med. Biol. 2014, 40, 2183–2194. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Arenas, T.E.G. Acoustic impedance matching of piezoelectric transducers to the air. IEEE Trans. Ultrason. Ferroelec. Freq. Control 2004, 51, 624–633. [Google Scholar] [CrossRef]
- Álvarez-Arenas, T.E.G. Air-coupled ultrasonic transducers. In Ultrasound in Food Processing: Recent Advances; Villamiel, M., García-Pérez, J.V., Montilla, M., Cárcel, A., Benedito, J., Eds.; Wiley: New York, NY, USA, 2016. [Google Scholar]
- Álvarez-Arenas, T.E.G. Solution of Inverse Problems in PyData Madrid, 8–10 April 2016. Available online: https://github.com/TomasGAA/Pydata_Madrid2016_Solution_Inverse_problem (accessed on 12 July 2016).
- US-BIOMAT. Available online: https://us-biomat.com/resources/code-2/ or https://github.com/usbiomat/ultrasonic-thickness-resonance (accessed on 12 July 2016).
- Perez-Martin, A.; Flexas, J.; Ribas-Carbo, M.; Bota, J.; Tomas, M.; Infante, J.M. Interactive effects of soil water deficit and air vapour pressure deficit on mesophyll conductance to CO2 in Vitis vinifera and Olea europaea. J. Exp. Botany 2009, 60, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Corcuera, L.; Camarero, J.J.; Gil-Pelegrín, E. Functional groups in Quercus species derived from the analysis of pressure-volume curves. Trees 2002, 16, 465–472. [Google Scholar] [CrossRef]
- Sancho-Knapik, D.; Peguero-Pina, J.J.; Medrano, H.; Fariñas, M.D.; Álvarez-Arenas, T.E.G.; Gil-Pelegrín, E. The reflectivity in the S-Band and the broadband ultrasonic spectroscopy as new tools for the study of water relations in Vitis vinifera L. Physiol. Plant. 2013, 148, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, M.K.; Scoffoni, C.; Sack, L. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: A global meta-analysis. Ecol. Lett. 2012, 15, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Pompelli, M.F.; Cabrini, E.C.; Claudjane, M.; Leite, J. Leaf anatomy, ultrastructure and plasticity of Coffea arabica L. in response to light and nitrogen Resumo. Biotemas 2012, 25, 13–28. [Google Scholar] [CrossRef]







| Design Parameter | Elements of the NC-RUS System Affected | Goal |
|---|---|---|
| 1. Size of the measurement area | Sensors | Smaller than leaf half width |
| 2. Geometry of the ultrasonic field (beam) | Sensors | Plane wave incident on the leaf surface |
| 3. Centre frequency | Sensors and electronics | 6 dB band of the thickness resonance band of the leaves to be studied (and expected range of variation) must be included within the frequency band of the NC-RUS system |
| 4. Frequency band | Sensors and electronics | |
| 5. Dynamic range and SNR | Sensors and electronics | Large enough to cope with expected losses for the leaves of the species of interest. |
| 6. Separation between sensors and leaf | Sensors holder | To avoid any overlap of the through transmitted signal with reverberations in the air cavities between transducers and samples. |
| 7. Time of measurement. | PC and software analysis | Fast enough to permit processing measurements right after the acquisition. |
| 8. Portability and robustness | All | To allow for field measurements. Resistance against environmental conditions (wind, moisture, heat, etc.) |
| Species | Diameter of the Beam | Centre Frequency (kHz) | Frequency Band (kHz) | SNR * (dB) |
|---|---|---|---|---|
| Vitis vinifera | <25 mm | 650 | 400–900 | >65 |
| Coffea arabica | <20 mm | 300 | 200–400 | >60 |
| Species | T (μm) | LMA (g/m2) | (MPa) | α (Np/m) |
|---|---|---|---|---|
| Vitis vinifera | 174 | 220 | 68 | 1625 |
| Coffea arabica | 210 | 153 | 12 | 1070 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez Álvarez-Arenas, T.; Gil-Pelegrin, E.; Ealo Cuello, J.; Fariñas, M.D.; Sancho-Knapik, D.; Collazos Burbano, D.A.; Peguero-Pina, J.J. Ultrasonic Sensing of Plant Water Needs for Agriculture. Sensors 2016, 16, 1089. https://doi.org/10.3390/s16071089
Gómez Álvarez-Arenas T, Gil-Pelegrin E, Ealo Cuello J, Fariñas MD, Sancho-Knapik D, Collazos Burbano DA, Peguero-Pina JJ. Ultrasonic Sensing of Plant Water Needs for Agriculture. Sensors. 2016; 16(7):1089. https://doi.org/10.3390/s16071089
Chicago/Turabian StyleGómez Álvarez-Arenas, Tomas, Eustaquio Gil-Pelegrin, Joao Ealo Cuello, Maria Dolores Fariñas, Domingo Sancho-Knapik, David Alejandro Collazos Burbano, and Jose Javier Peguero-Pina. 2016. "Ultrasonic Sensing of Plant Water Needs for Agriculture" Sensors 16, no. 7: 1089. https://doi.org/10.3390/s16071089