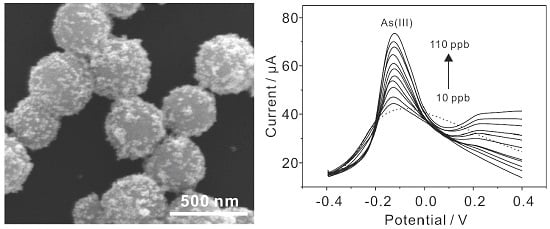
Electrochemical Sensing toward Trace As(III) Based on Mesoporous MnFe2O4/Au Hybrid Nanospheres Modified Glass Carbon Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Apparatus
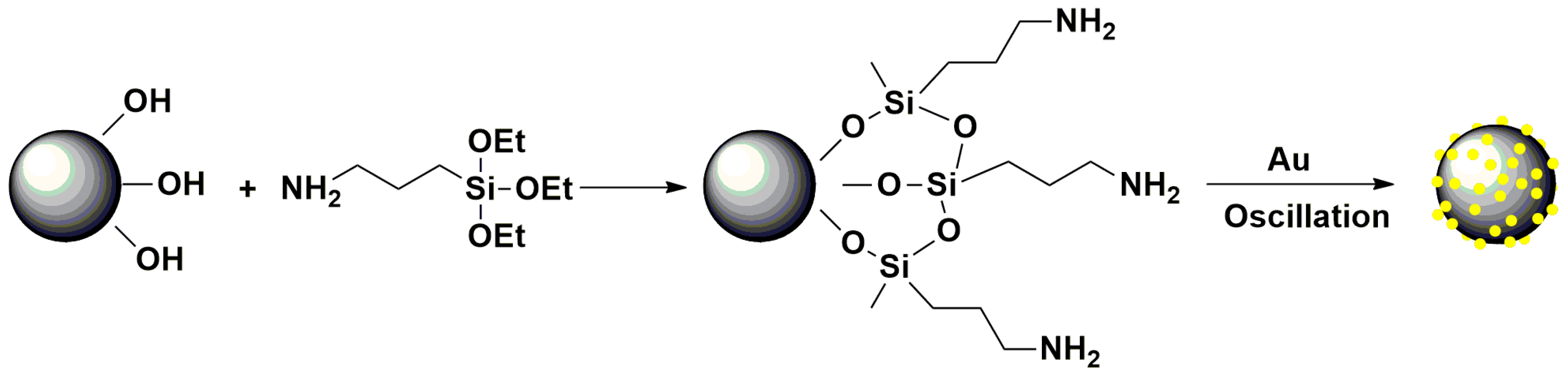
2.3. Preparation of Mesoporous MnFe2O4/Au Hybrid Nanospheres
2.4. Preparation of Mesoporous MnFe2O4/Au Hybrid Nanospheres Modified GCE
2.5. Electrochemical Measurements
3. Results and Discussion
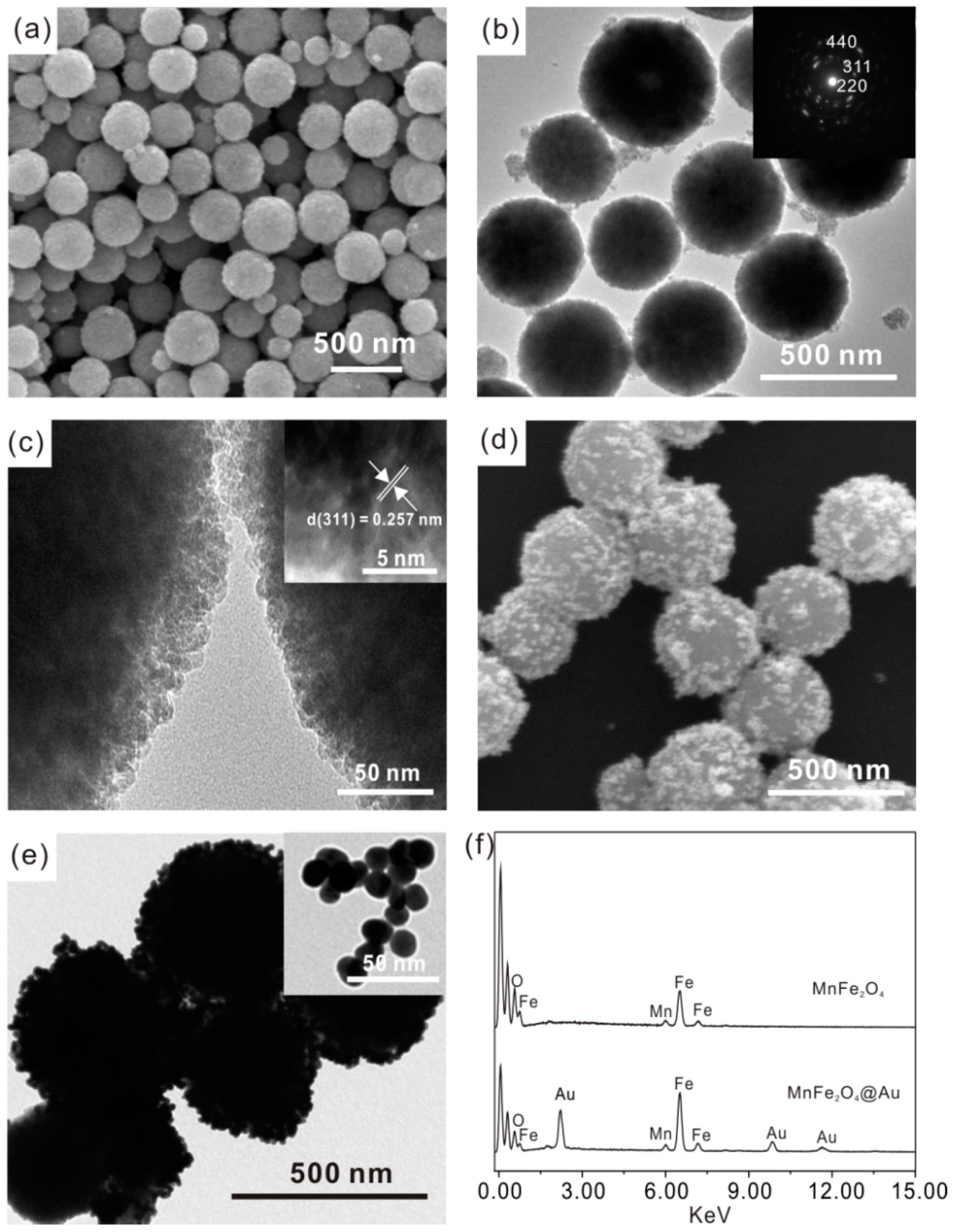
3.1. Characterization of Mesoporous MnFe2O4/Au Hybrid Nanospheres
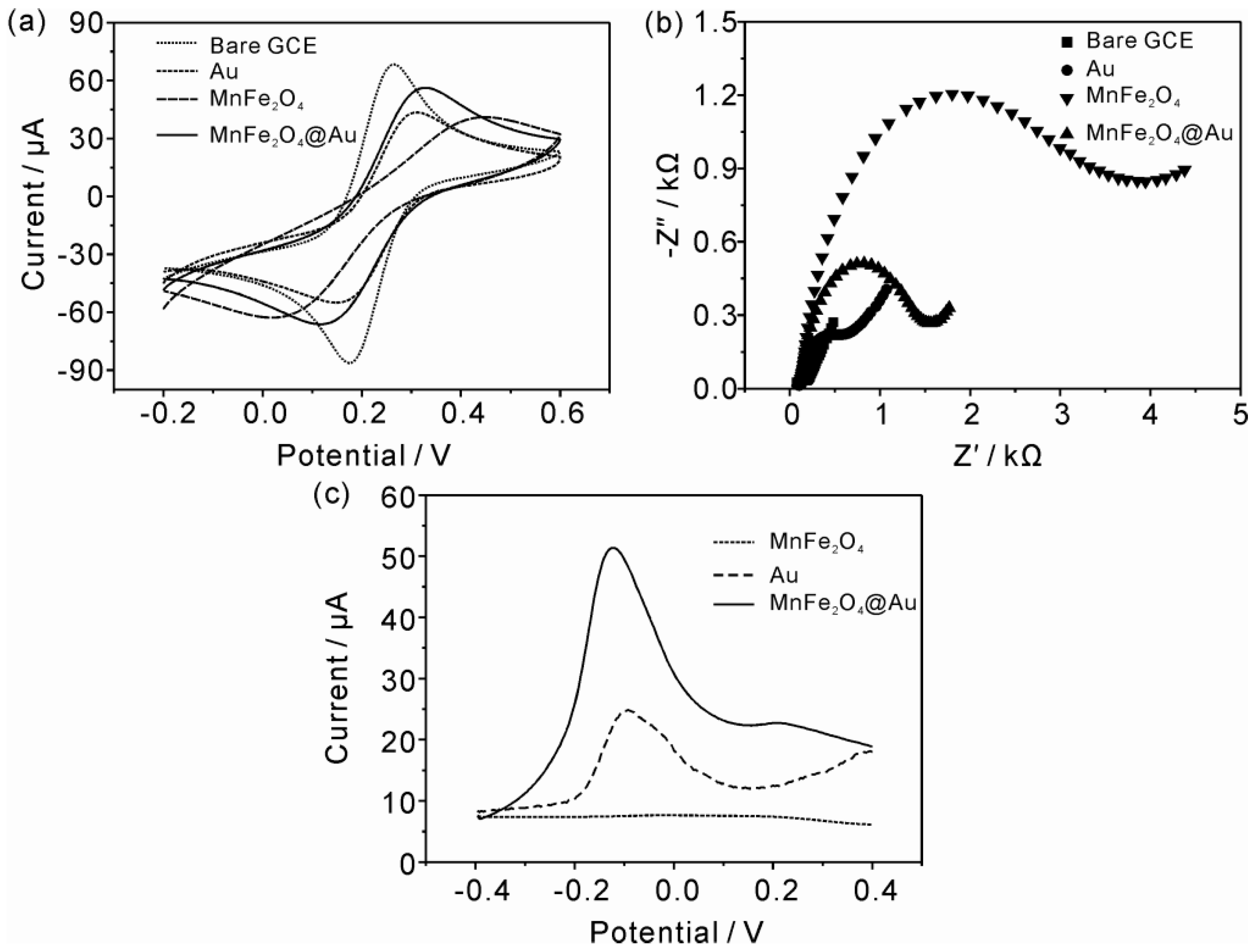
3.2. Electrochemical Characterization of Mesoporous MnFe2O4/Au Hybrid Nanospheres Modified GCE
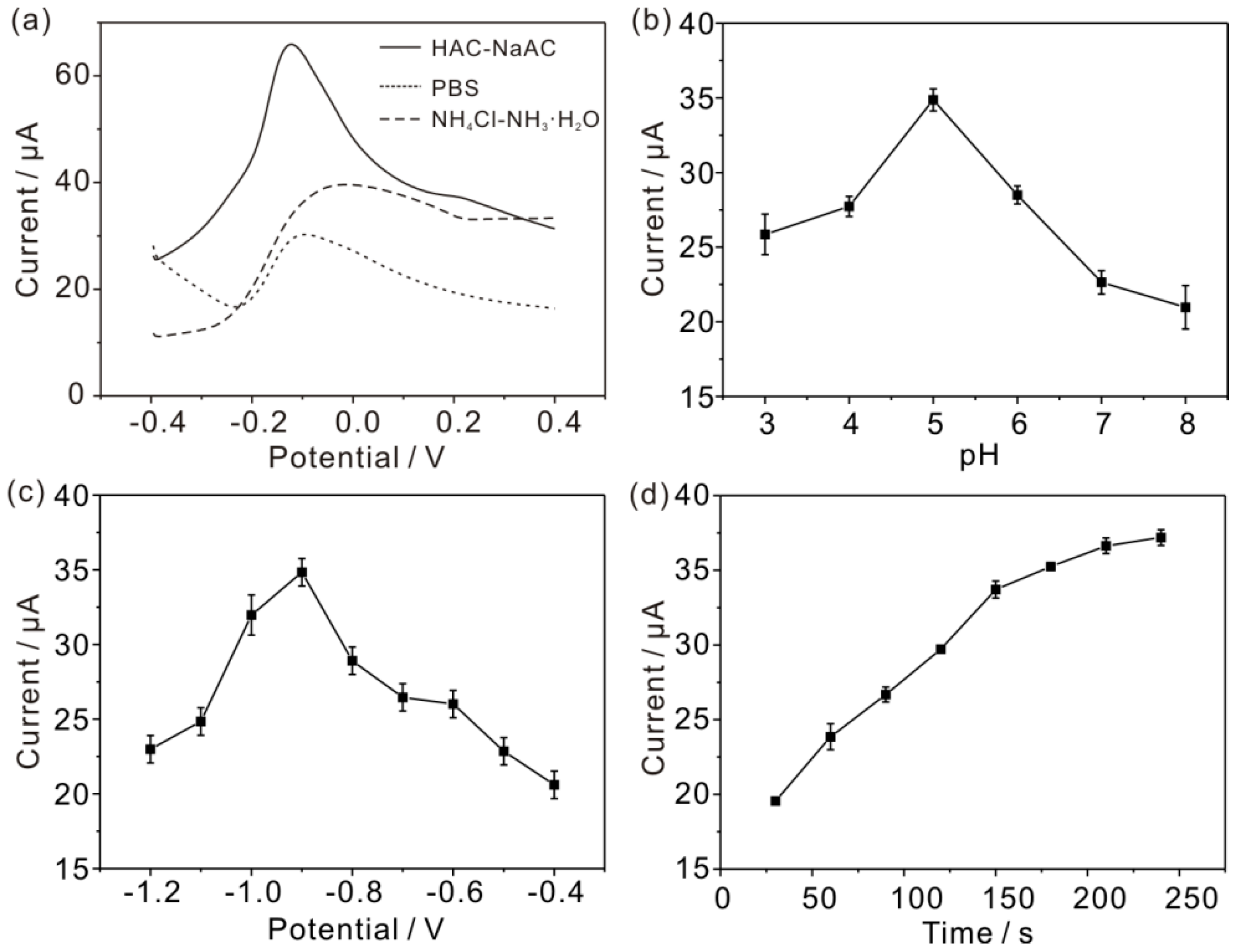
3.3. Optimum Experimental Conditions of Electrochemical Detection of As(III)
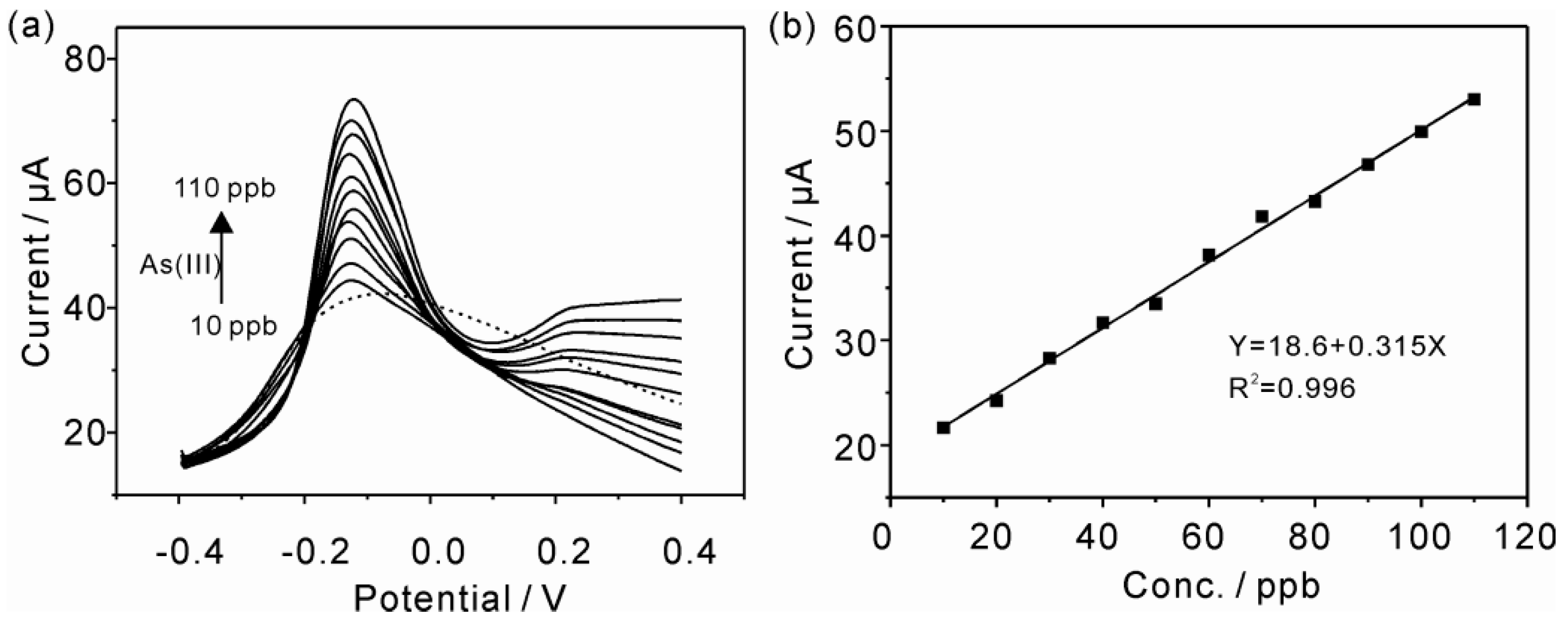
3.4. Electrochemical Detection of As(III) with MnFe2O4/Au Hybrid Nanospheres Modified GCE
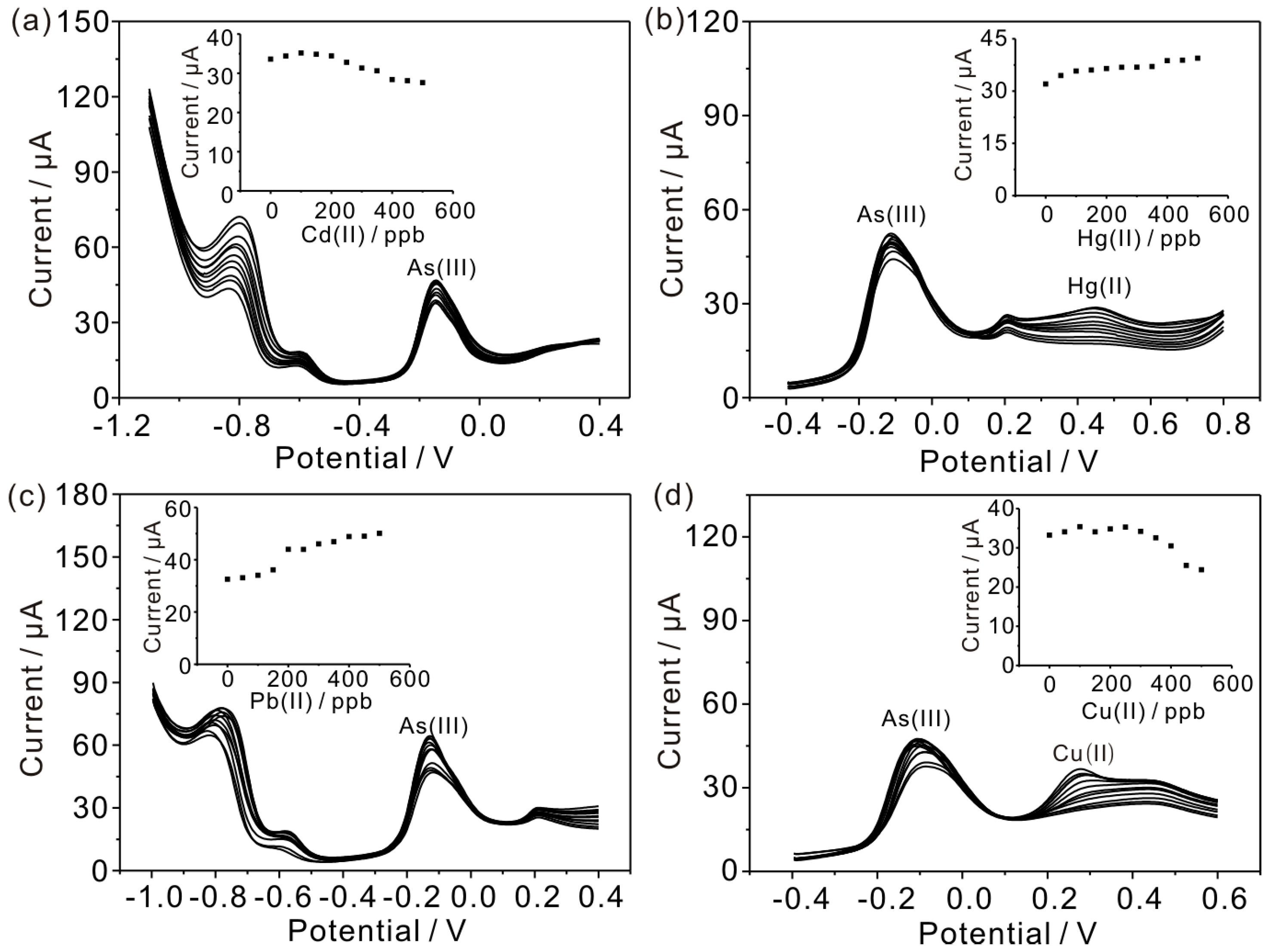
3.5. Interference Measurements
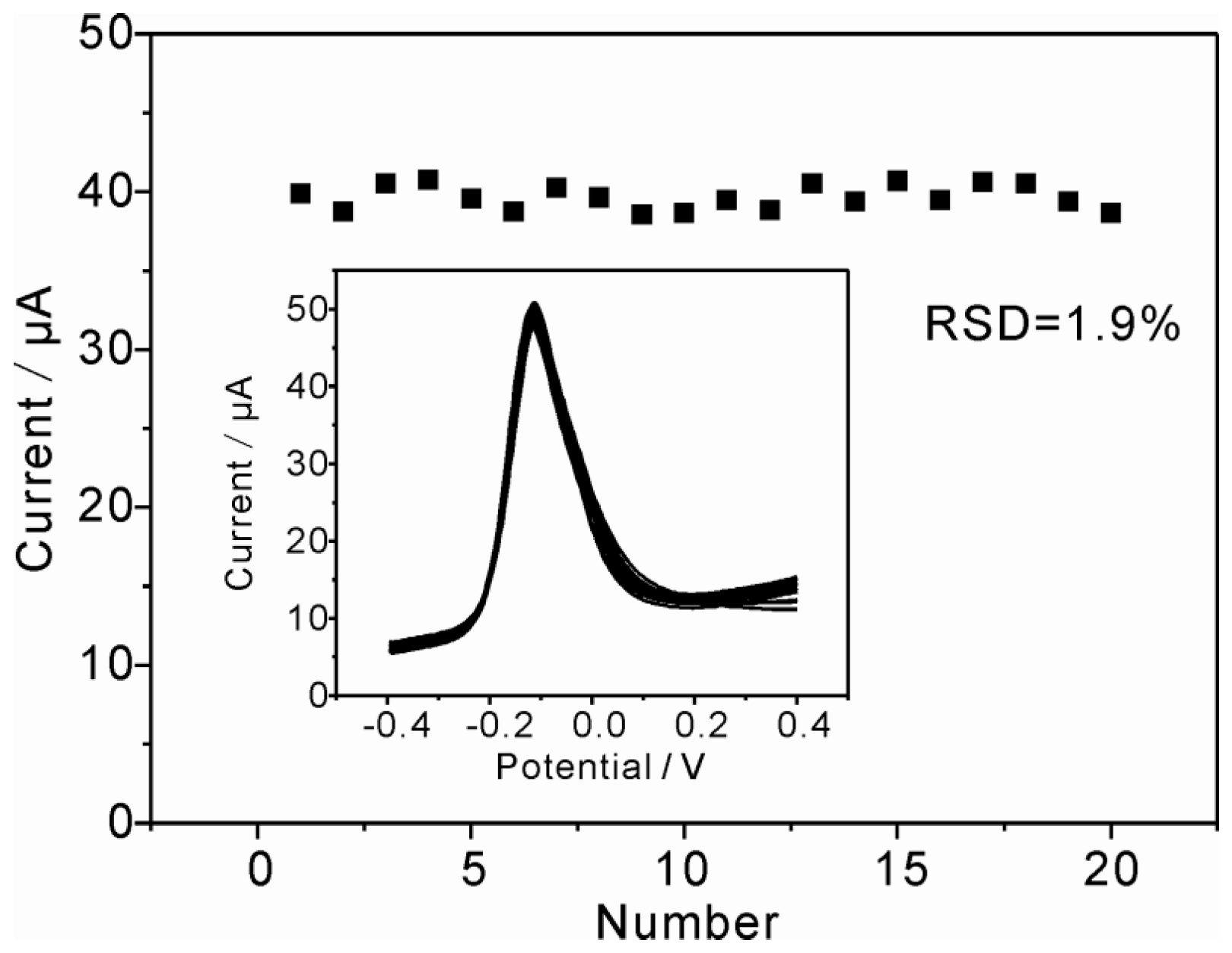
3.6. Reproducibility, Stability and Repeatability
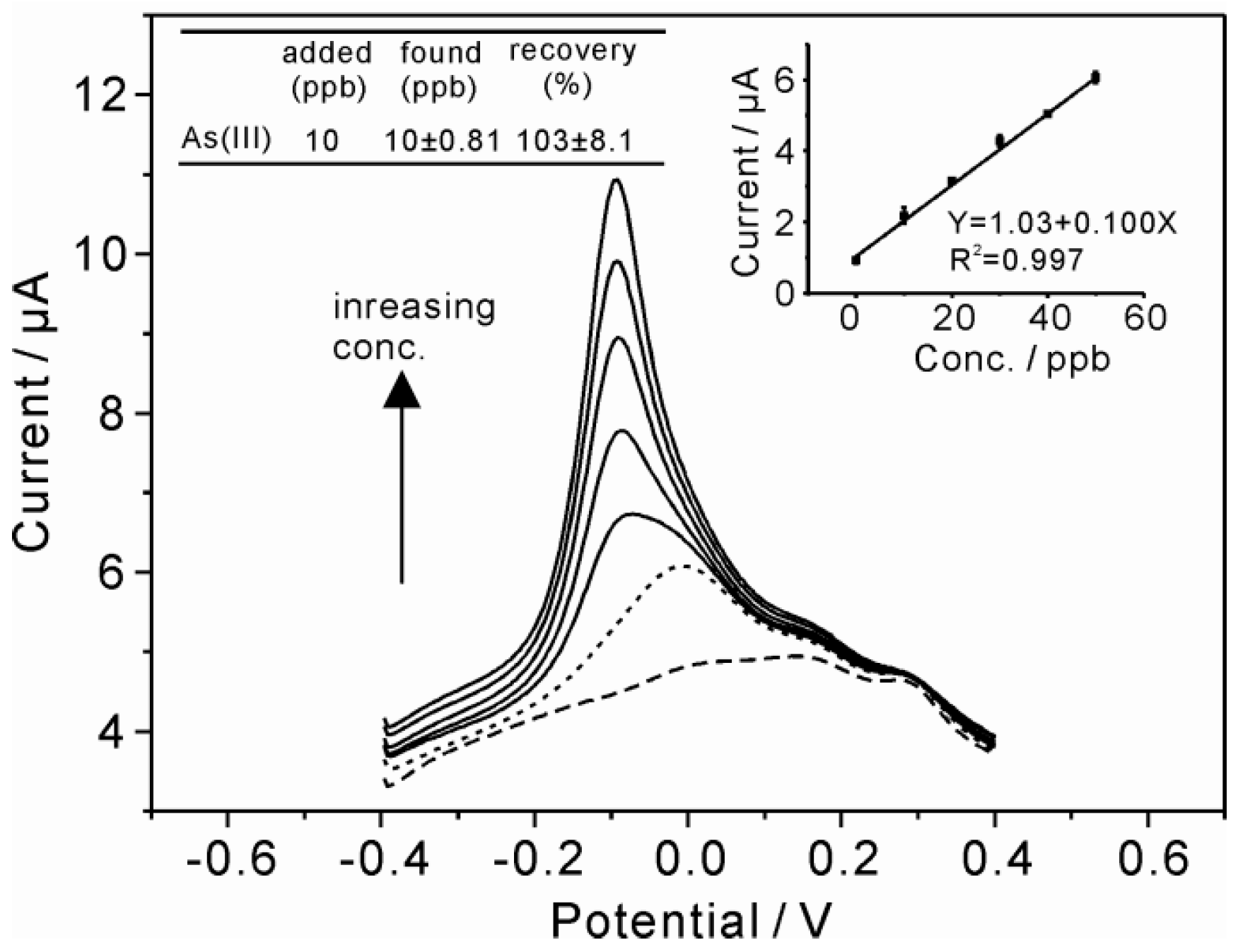
3.7. Real Sample Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Jena, B.K.; Raj, C.R. Gold nanoelectrode ensembles for the simultaneous electrochemical detection of ultratrace arsenic, mercury, and copper. Anal. Chem. 2008, 80, 4836–4844. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Nekrassova, O.; Hyde, M.E.; Compton, R.G. Anodic stripping voltammetry of arsenic(III) using gold nanoparticle-modified electrodes. Anal. Chem. 2004, 76, 5924–5929. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, J.; Jia, X.; Han, Y.; Wang, E. Electrochemical determination of arsenic(III) on mercaptoethylamine modified Au electrode in neutral media. Anal. Chim. Acta 2012, 733, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Mcgaw, E.A.; Swain, G.M. A comparison of boron-doped diamond thin-film and Hg-coated glassy carbon electrodes for anodic stripping voltammetric determination of heavy metal ions in aqueous media. Anal. Chim. Acta 2006, 575, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Wonsawat, W.; Dungchai, W.; Motomizu, S.; Chuanuwatanakul, S.; Chailapakul, O. Highly Sensitive Determination of Cadmium and Lead Using a Low-cost Electrochemical Flow-through Cell Based on a Carbon Paste Electrode. Anal. Sci. 2012, 28, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.G.; Huang, X.J. Voltammetric determination of inorganic arsenic. Trend Anal. Chem. 2014, 60, 25–35. [Google Scholar] [CrossRef]
- Luong, J.H.T.; Lam, E.; Male, K.B. Recent advances in electrochemical detection of arsenic in drinking and ground waters. Anal. Methods 2014, 6, 6157–6169. [Google Scholar] [CrossRef]
- Munoz, E.; Palmero, S. Analysis and speciation of arsenic by stripping potentiometry: A review. Talanta 2005, 65, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.X.; Yin, L.B. Electrochemical performance of heterostructured Au-Pd bimetallic nanoparticles toward As(III) aqueous media. Electrochem. Commun. 2012, 22, 57–60. [Google Scholar] [CrossRef]
- Simm, A.O.; Banks, C.E.; Compton, R.G. Sonically assisted electroanalytical detection of ultratrace arsenic. Anal. Chem. 2004, 76, 5051–5055. [Google Scholar] [CrossRef] [PubMed]
- Kopanica, M.; Novotny, L. Determination of traces of arsenic(III) by anodic stripping voltammetry in solutions, natural waters and biological material. Anal. Chim. Acta 1998, 368, 211–218. [Google Scholar] [CrossRef]
- Majid, E.; Hrapovic, S.; Liu, Y.L.; Male, K.B.; Luong, J.H.T. Electrochemical determination of arsenite using a gold nanoparticle modified glassy carbon electrode and flow analysis. Anal. Chem. 2006, 78, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Wildgoose, G.G.; Salter, C.; Crossley, A.; Compton, R.G. Electroanalysis using macro-, micro-, and nanochemical architectures on electrode surfaces. Bulk surface modification of glassy carbon microspheres with gold nanoparticles and their electrical wiring using carbon nanotubes. Anal. Chem. 2006, 78, 6102–6108. [Google Scholar] [CrossRef] [PubMed]
- Rassaei, L.; Sillanpaeae, M.; French, R.W.; Compton, R.G.; Marken, F. Arsenite determination in phosphate media at electroaggregated gold nanoparticle deposits. Electroanalysis 2008, 20, 1286–1292. [Google Scholar] [CrossRef]
- Zakharova, E.A.; Noskova, G.N.; Kabakaev, A.S.; Rees, N.V.; Compton, R.G. Gold microelectrode ensembles: cheap, reusable and stable electrodes for the determination of arsenic (V) under aerobic conditions. Int. J. Environ. Anal. Chem. 2013, 93, 1105–1115. [Google Scholar] [CrossRef]
- Salimi, A.; Manikhezri, H.; Hallaj, R.; Soltanian, S. Electrochemical detection of trace amount of arsenic(III) at glassy carbon electrode modified with cobalt oxide nanoparticles. Sens. Actuators B Chem. 2008, 129, 246–254. [Google Scholar] [CrossRef]
- Gao, C.; Yu, X.Y.; Xiong, S.Q.; Liu, J.H.; Huang, X.J. Electrochemical detection of arsenic(III) completely free from noble metal: Fe3O4 microspheres-room temperature ionic liquid composite showing better performance than gold. Anal. Chem. 2013, 85, 2673–2680. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Z.G.; Zhao, Z.Q.; Liu, J.H.; Huang, X.J. SnO2 tube-in-tube nanostructures: Cu@C nanocable templated synthesis and their mutual interferences between heavy metal ions revealed by stripping voltammetry. Small 2013, 9, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, C.T.; Mayo, J.T.; Yu, W.W.; Prakash, A.; Falkner, J.C.; Yean, S.; Cong, L.L.; Shipley, H.J.; Kan, A.; Tomson, M.; et al. Low-field magnetic separation of monodisperse Fe3O4 nanocrystals. Science 2006, 314, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Liu, T.; Xue, W.; Wang, X.C. Arsenic(III) oxidation/adsorption behaviors on a new bimetal adsorbent of Mn-oxide-doped Al oxide. Chem. Eng. J. 2012, 192, 343–349. [Google Scholar] [CrossRef]
- Zhang, G.S.; Qu, J.H.; Liu, H.J.; Liu, R.P.; Wu, R.C. Preparation and evaluation of a novel Fe-Mn binary oxide adsorbent for effective arsenite removal. Water Res. 2007, 41, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Maity, A.; Ghosh, U.C. Manganese associated nanoparticles agglomerate of iron(III) oxide: Synthesis, characterization and arsenic(III) sorption behavior with mechanism. J. Hazard. Mater. 2010, 184, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.H.; Wang, L.; Wang, J.; Sheng, G.P.; Liu, J.H.; Yu, H.Q.; Huang, X.J. Superparamagnetic mesoporous ferrite nanocrystal clusters for efficient removal of arsenite from water. Crystengcomm 2013, 15, 7895–7903. [Google Scholar] [CrossRef]
- Zhou, S.F.; Han, X.J.; Fan, H.L.; Zhang, Q.X.; Liu, Y.Q. Electrochemical detection of As(III) through mesoporous MnFe2O4 nanocrystal clusters by square wave stripping voltammetry. Electrochim. Acta 2015, 174, 1160–1166. [Google Scholar] [CrossRef]
- Wu, S.G.; Zhao, Q.P.; Zhou, L.; Zhang, Z.X. Stripping analysis of trace arsenic based on the MnOx/AuNPs composite film modified electrode in alkaline media. Electroanalysis 2014, 26, 1840–1849. [Google Scholar] [CrossRef]
- Cui, H.; Yang, W.Y.; Li, X.J.; Zhao, H.; Yuan, Z.B. An electrochemical sensor based on a magnetic Fe3O4 nanoparticles and gold nanoparticles modified electrode for sensitive determination of trace amounts of arsenic(III). Anal. Methods 2012, 4, 4176–4181. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.; Peng, Q.; Wang, X.; Chen, J.; Li, Y. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. 2005, 117, 2842–2845. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S.; Wang, E. A general route to construct diverse multifunctional Fe3O4/metal hybrid nanostructures. Chemistry 2009, 15, 2416–2424. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dong, S.J.; Wang, E. Constructing carbon-nanotube/metal hybrid nanostructures using homogeneous TiO2 as a spacer. Small 2008, 4, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, Z.K.; Deng, Y.H.; Zou, Y.; Li, C.Y.; Guo, X.H.; Xiong, L.Q.; Gao, Y.; Li, F.Y.; Zhao, D.Y. Highly water-dispersible biocompatible magnetite particles with low cytotoxicity stabilized by citrate groups. Angew. Chem. Int. Ed. 2009, 48, 5875–5879. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Wei, H.H.; Zhang, L.Z. Efficient removal of heavy metal ions with biopolymer template synthesized mesoporous titania beads of hundreds of micrometers size. Environ. Sci. Technol. 2012, 46, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Chen, J.S.; Lou, X.W. Highly efficient removal of organic dyes from waste water using hierarchical NiO spheres with high surface area. J. Phys. Chem. C 2012, 116, 6873–6878. [Google Scholar] [CrossRef]
- Ge, J.P.; Hu, Y.X.; Biasini, M.; Beyermann, W.P.; Yin, Y.D. Superparamagnetic magnetite colloidal nanocrystal clusters. Angew. Chem. Int. Ed. 2007, 46, 4342–4345. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; You, Z.; Sha, H.L.; Sun, Z.L.; Sun, W. Electrochemical myoglobin biosensor based on carbon ionic liquid electrode modified with Fe3O4@SiO2 microsphere. J. Solid State Electr. 2014, 18, 207–213. [Google Scholar] [CrossRef]
- Hung, D.Q.; Nekrassova, O.; Compton, R.G. Analytical methods for inorganic arsenic in water: A review. Talanta 2004, 64, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yang, R.; Liu, J.H.; Huang, X.J. Selective detection toward Hg(II) and Pb(II) using polypyrrole/carbonaceous nanospheres modified screen-printed electrode. Electrochim. Acta 2013, 105, 218–223. [Google Scholar] [CrossRef]
- Feeney, R.; Kounaves, S.P. On-Site Analysis of arsenic in groundwater using a microfabricated gold ultramicroelectrode array. Anal. Chem. 2000, 72, 2222–2228. [Google Scholar] [CrossRef] [PubMed]
- Zen, J.M.; Liou, S.L.; Kumar, A.S.; Hsia, M.S. An efficient and selective photocatalytic system for the oxidation of sulfides to sulfoxides. Angew. Chem. Int. Ed. 2003, 42, 577–599. [Google Scholar] [CrossRef] [PubMed]
- Simm, A.O.; Banks, C.E.; Wilkins, S.J.; Karousos, N.G.; Davis, J.; Compton, R.G. A comparison of different types of gold-carbon composite electrode for detection of arsenic(III). Anal. Bioanal. Chem. 2005, 381, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Profumo, A.; Fagnoni, M.; Merli, D.; Quartarone, E.; Protti, S.; Dondi, D.; Albini, A. Multiwalled carbon nanotube chemically modified gold electrode for inorganic as speciation and Bi(III) determination. Anal. Chem. 2006, 78, 4194–4199. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Yao, X.Z.; Guo, Z.; Liu, J.H.; Huang, X.J. Fe3O4 with novel nanoplate-stacked structure: Surfactant-free hydrothermal synthesis and application in detection of heavy metal ions. J. Electroanal. Chem. 2015, 749, 75–82. [Google Scholar] [CrossRef]
| Electrode | Sensitivity (μA/ppb) | LOD (ppb) | Refs. |
|---|---|---|---|
| Au-UMEA | 0.044 | 0.013 | [38] |
| Au NPs/GCE | 0.24 | 0.0096 | [3] |
| PBSPE | 0.000387 | 1.875 | [39] |
| Gold-carbon composite electrode | 0.133 | 0.375 | [40] |
| MWCNTs/gold electrode | 0.236 | - | [41] |
| Co3Ox/GCE | 0.00148 | 0.825 | [17] |
| MnFe2O4/gold electrode | 0.295 | 1.95 | [25] |
| MnFe2O4/Au modified GCE | 0.315 | 3.37 | This work |
| Fe3O4-RTIL modified SPCE | 4.91 | 8 × 10−4 | [18] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Han, X.; Fan, H.; Liu, Y. Electrochemical Sensing toward Trace As(III) Based on Mesoporous MnFe2O4/Au Hybrid Nanospheres Modified Glass Carbon Electrode. Sensors 2016, 16, 935. https://doi.org/10.3390/s16060935
Zhou S, Han X, Fan H, Liu Y. Electrochemical Sensing toward Trace As(III) Based on Mesoporous MnFe2O4/Au Hybrid Nanospheres Modified Glass Carbon Electrode. Sensors. 2016; 16(6):935. https://doi.org/10.3390/s16060935
Chicago/Turabian StyleZhou, Shaofeng, Xiaojuan Han, Honglei Fan, and Yaqing Liu. 2016. "Electrochemical Sensing toward Trace As(III) Based on Mesoporous MnFe2O4/Au Hybrid Nanospheres Modified Glass Carbon Electrode" Sensors 16, no. 6: 935. https://doi.org/10.3390/s16060935