A Multidisciplinary Approach to High Throughput Nuclear Magnetic Resonance Spectroscopy
Abstract
:1. Introduction
2. Related Works
2.1. NMR Challenges
2.1.1. SNR Challenge
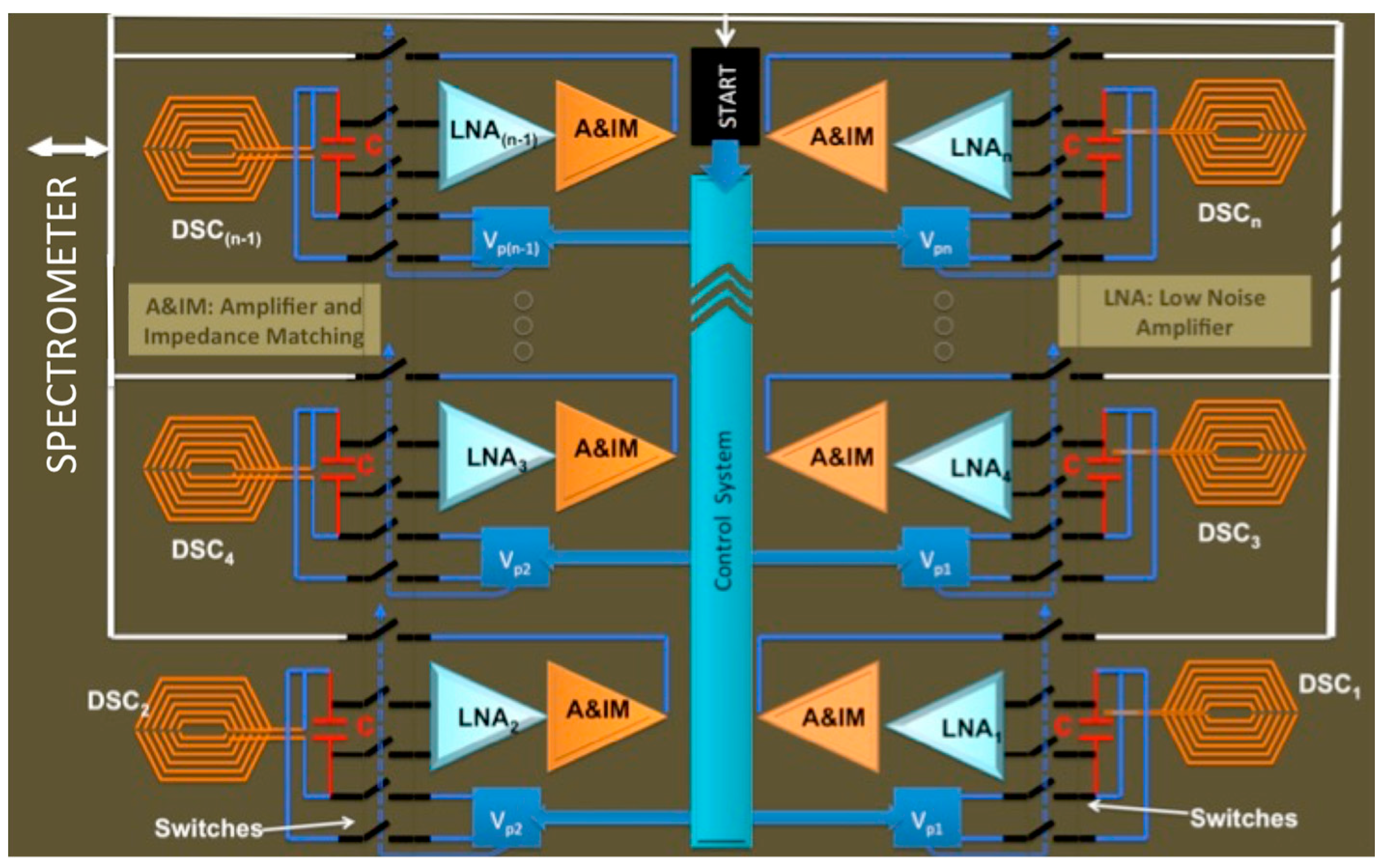
2.1.2. Towards High Throughput Spectroscopy
2.2. Emerging NMR Technologies
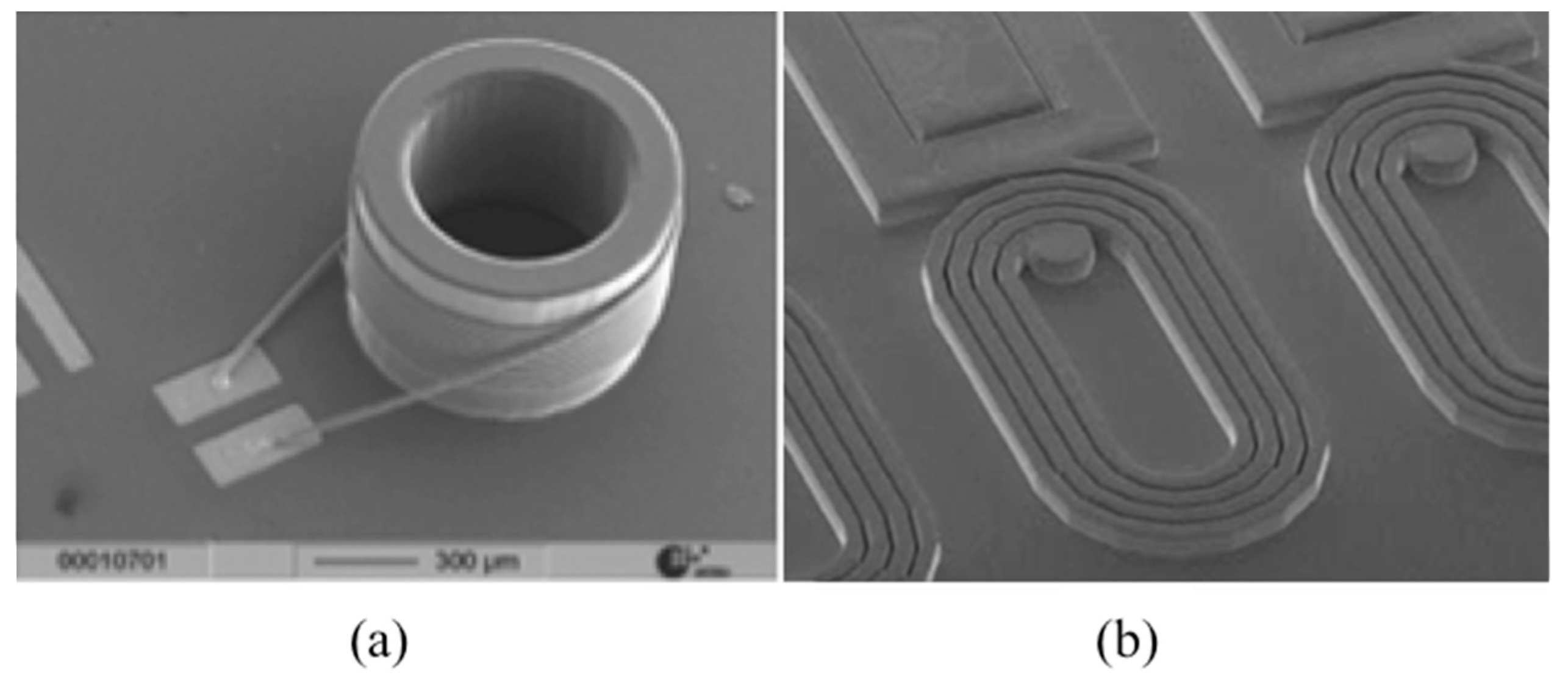
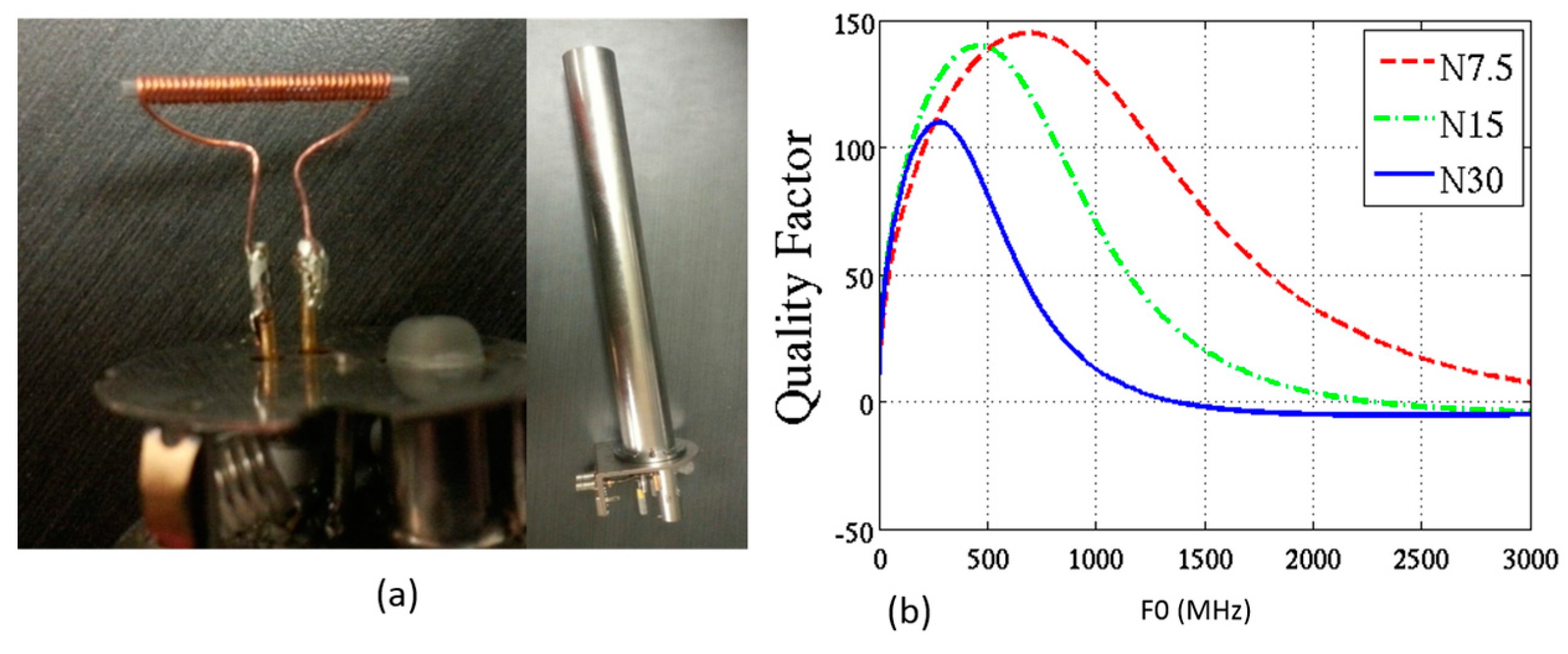
2.2.1. NMR µCoils
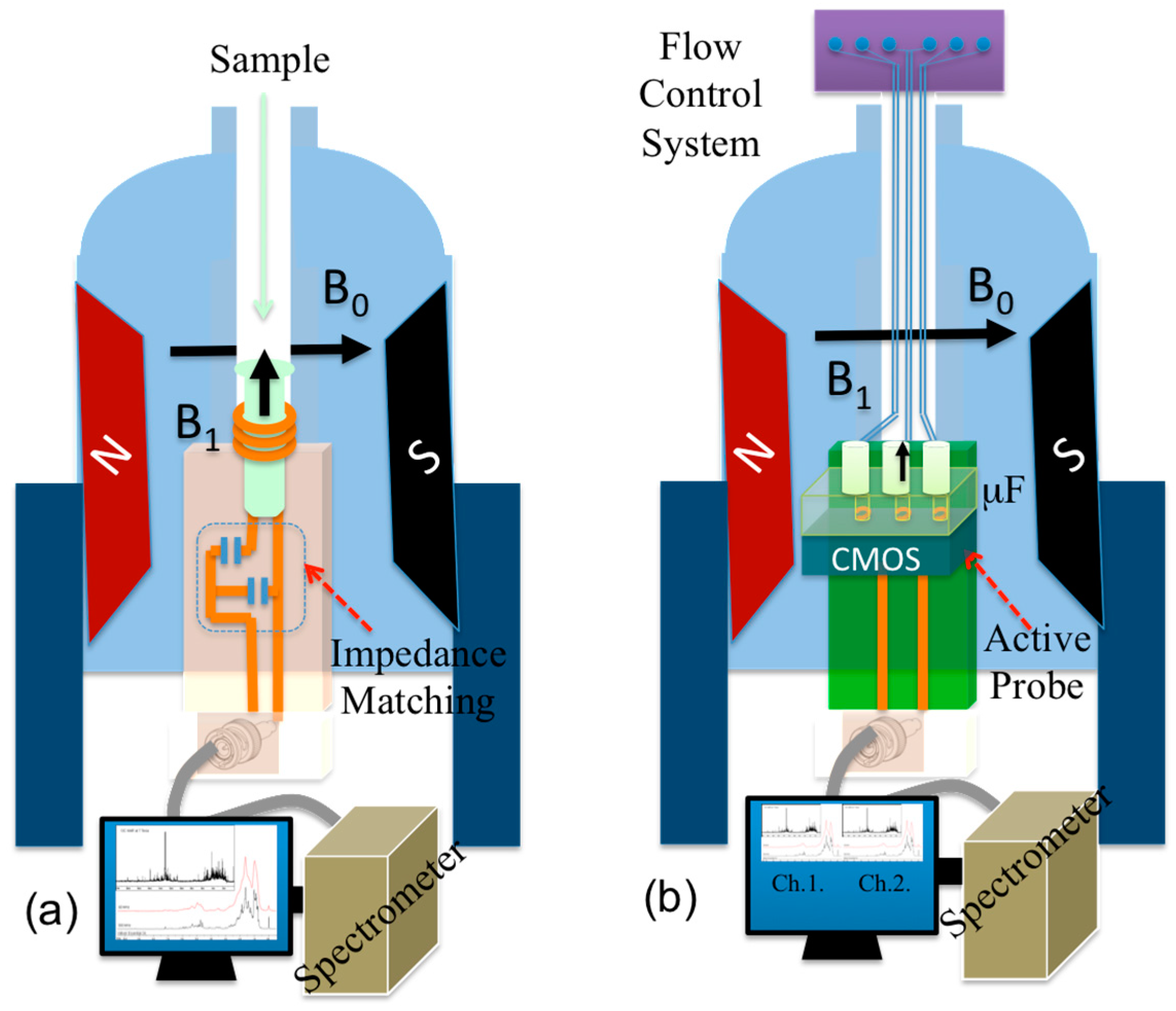
2.2.2. Integrated CMOS NMR Probe
3. Design Strategy
3.1. Design Criteria
3.1.1. High Static Magnetic Field B0
3.1.2. Homogeneity
3.1.3. Ultra-Low Noise Voltage Amplifier
3.1.4. NMR Compatible Active Probe
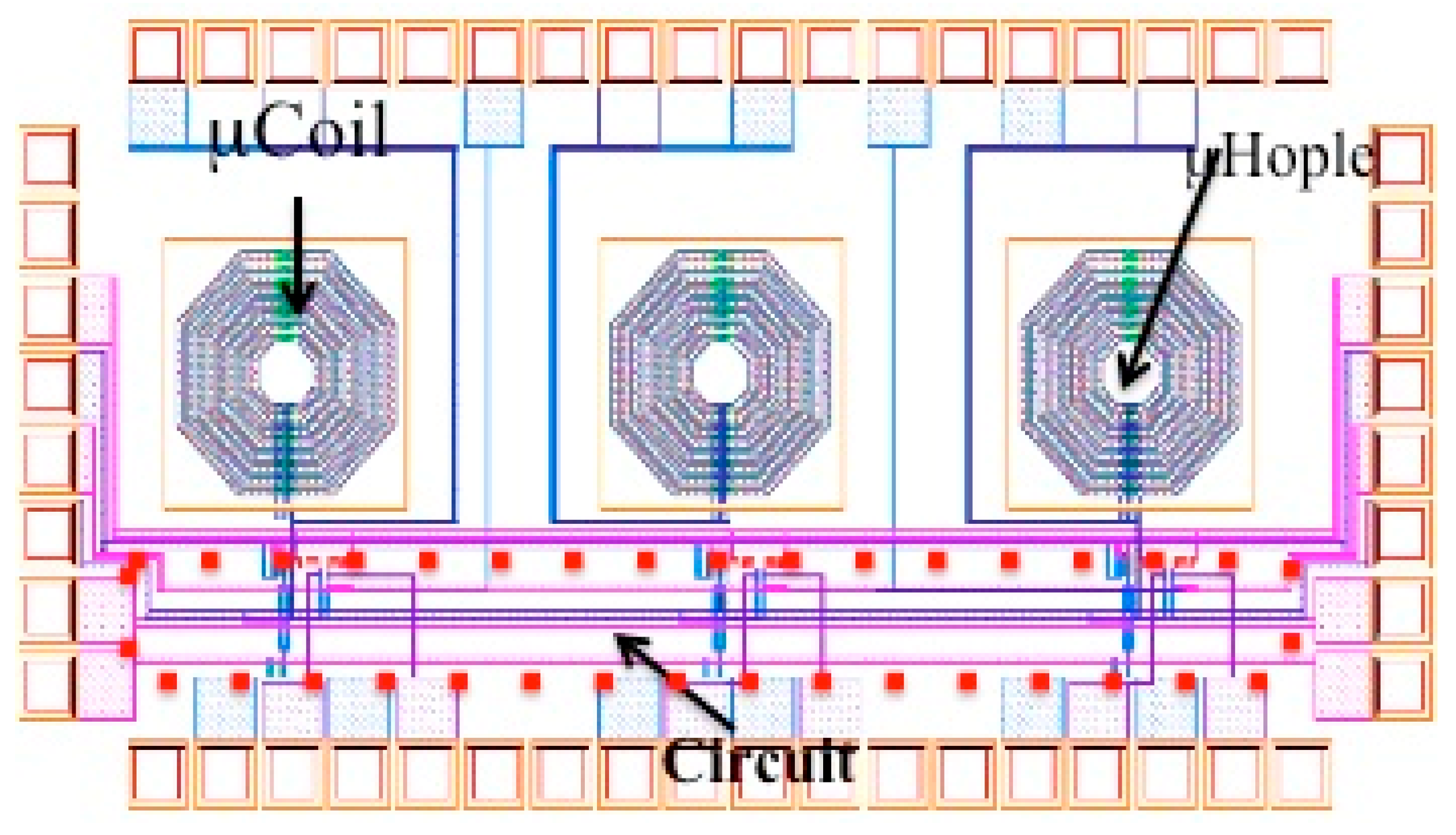
3.2. µCoils
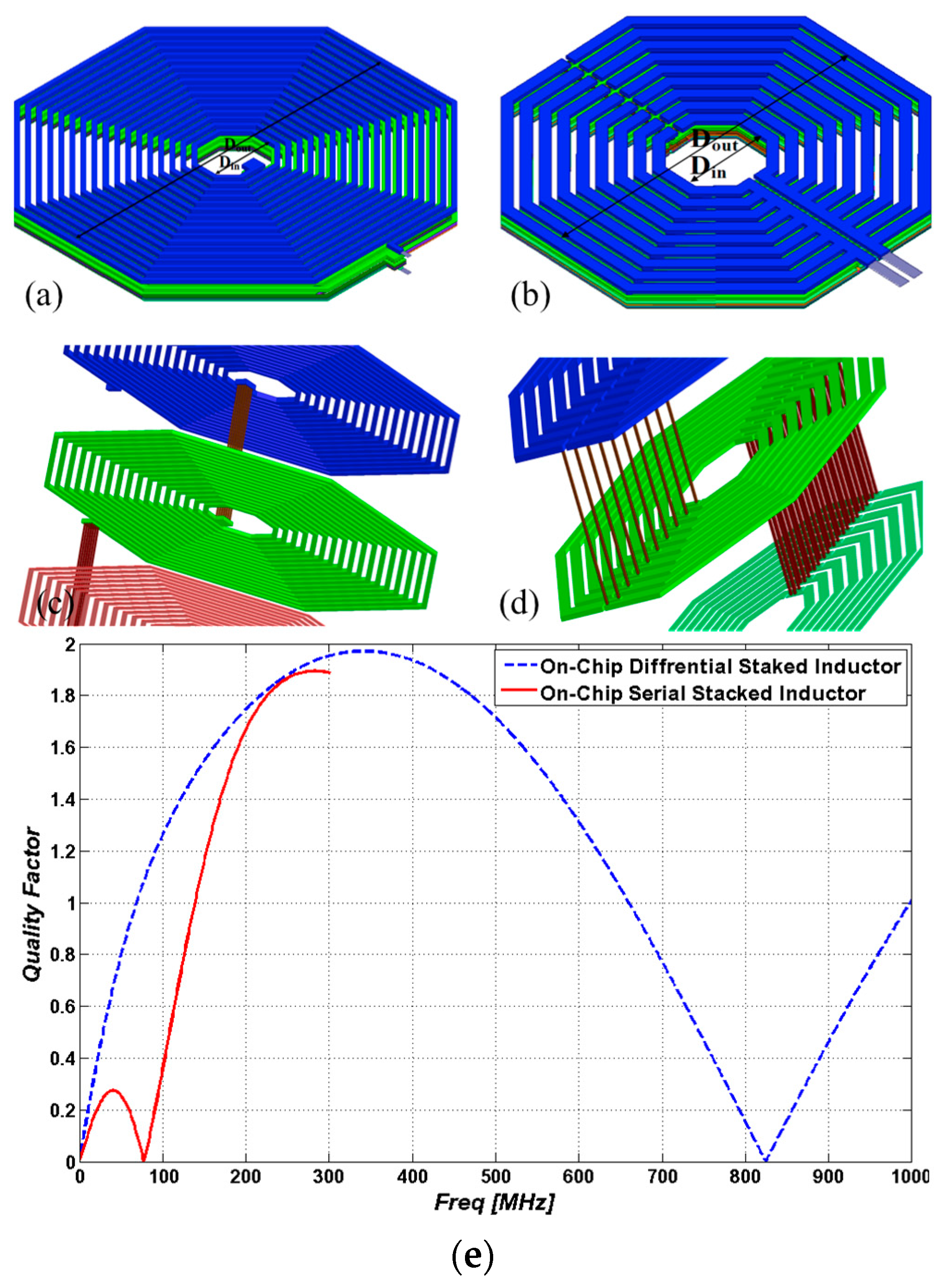
3.2.1. Serial Stacked Coil (SSC) vs. Differential Stacked Coil (DSC)
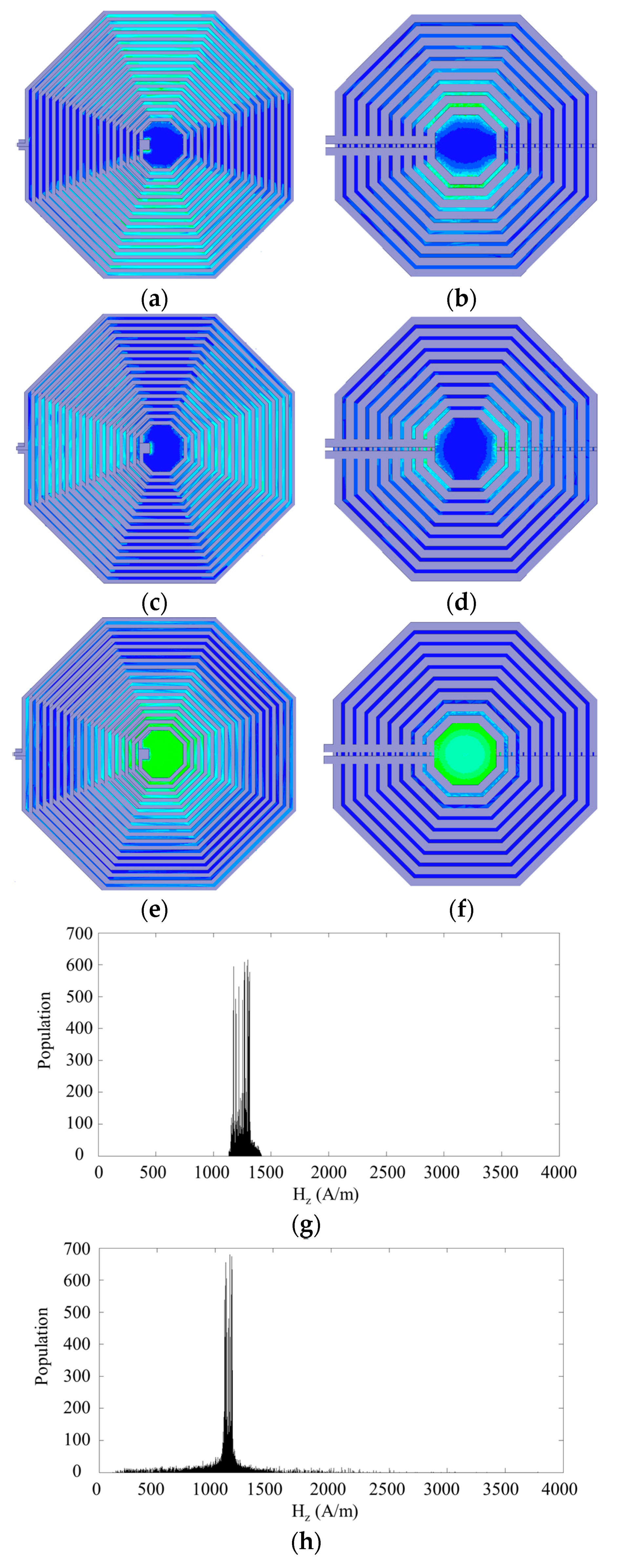
3.2.2. Geometry Design, Modeling and Optimization
3.2.3. Homogenous Magnetic Field
3.3. Low Noise Amplifier (LNA)
3.3.1. Front-End Pre-amplifier
3.3.2. Post Amplifier
4. Results
4.1. LNA Post-Layout Simulation
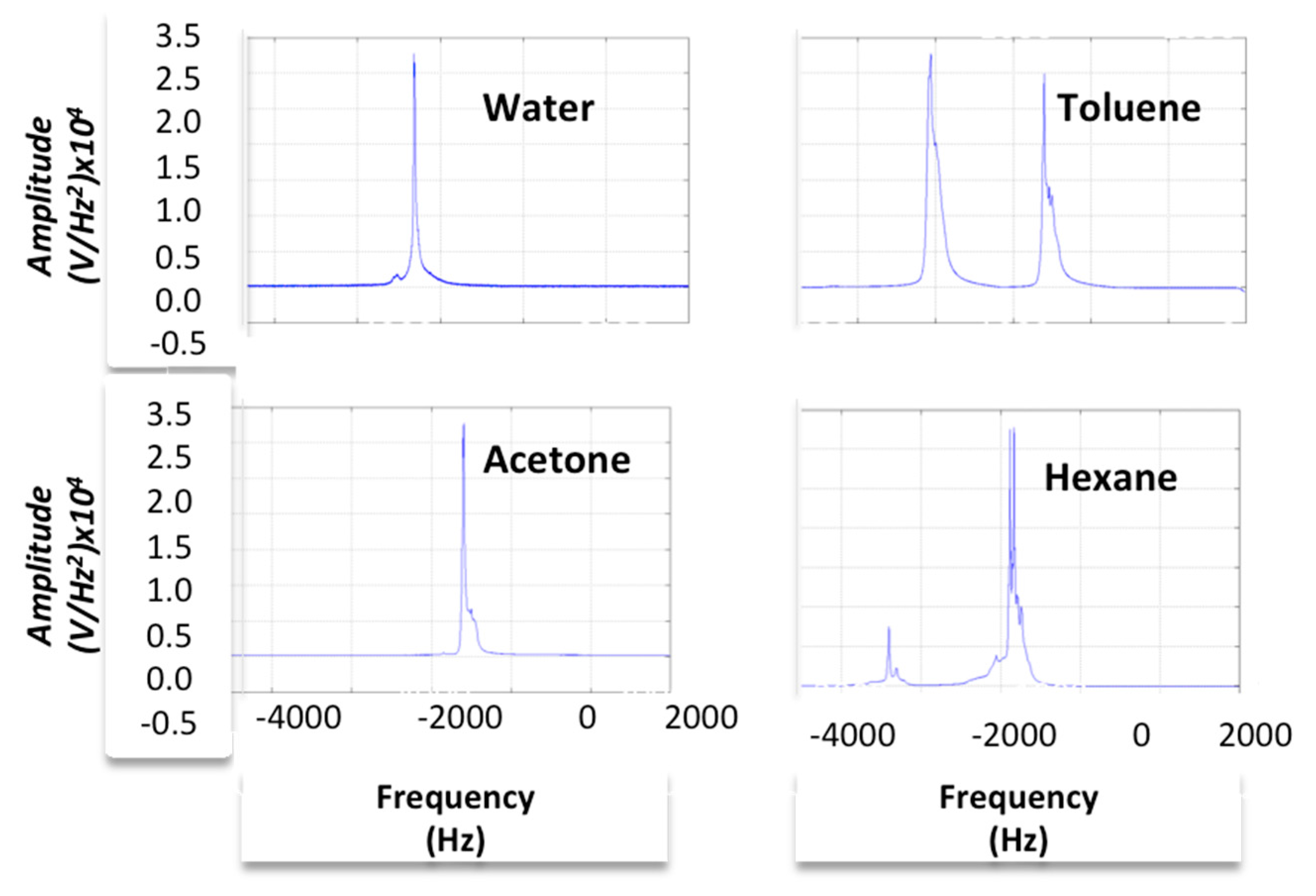
4.2. Spectral Analysis
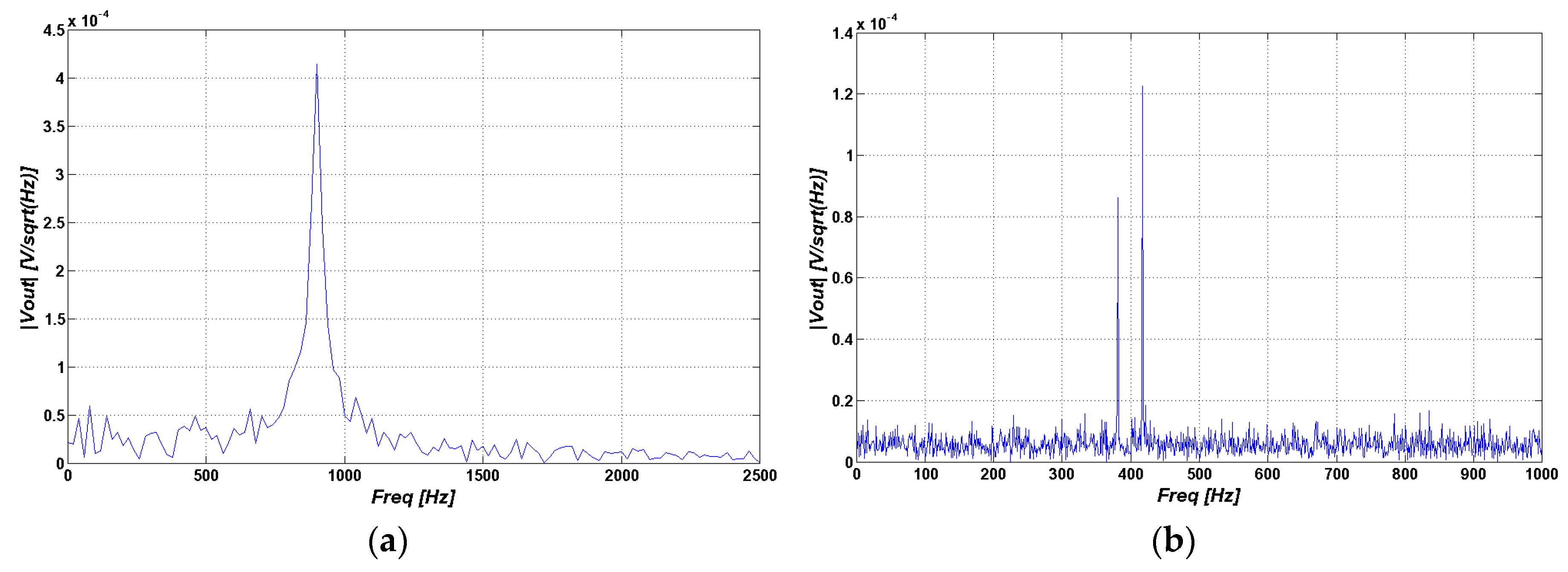
4.2.1. Simulation Model
4.2.2. Experimental Model
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| NMR | Nuclear Magnetic Resonance |
| CMOS | Complementary Metal Oxide Semiconductor |
| RF | Radio Frequency |
| LNA | Low Noise Amplifier |
References
- Pellecchia, M.; Sem, D.S.; Wüthrich, K. NMR in drug discovery. Nat. Rev. Drug Discov. 2002, 1, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Novak, T.J.; Grinberg, N.; Hartman, B.; Marcinko, S.; DiMichele, L.; Mao, B. LCMS using a hybrid quadrupole time of flight mass spectrometer. J. Pharm. Biomed. Anal. 2010, 51, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Wendy, L. UV area imaging analysis to gain additional insight into the dissolutional behavior of pharmaceutical ingredients. Int. J. Pharm. 2012, 434, 133–139. [Google Scholar]
- Elkins, P.; Coleman, D.; Burgess, J.; Gardner, M.; Hines, J.; Scott, B.; Kroenke, M.; Larson, J.; Lightner, M.; Turner, G.; et al. Characterization of the isomeric configuration and impurities of (Z)-endoxifen by 2D NMR, high resolution LC–MS, and quantitative HPLC analysis. J. Pharm. Biomed. Anal. 2014, 88, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Zalesskiy, S.S.; Danieli, E.; Blümich, B.; Ananikov, V.P. Miniaturization of NMR Systems: Desktop Spectrometers, Microcoil Spectroscopy, and “NMR on a Chip” for Chemistry, Biochemistry, and Industry. Chem. Rev. 2014, 114, 5641–5694. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Yoon, T.-J.; Lee, H.; Andress, W.; Weissleder, R.; Ham, D. Palm NMR and 1-Chip NMR. IEEE J. Solid State Circuits 2011, 46, 342–352. [Google Scholar] [CrossRef]
- Maguire, Y.; Chuang, I.L.; Zhang, S.; Gershenfeld, N. Ultra-small-sample molecular structure detection using microslot waveguide nuclear spin resonance. Proc. Natl. Acad. Sci. USA 2007, 104. [Google Scholar] [CrossRef] [PubMed]
- Pourmodheji, H.; Ghafar-Zadeh, E.; Magierowski, S. Active nuclear magnetic resonance probe: A new multidiciplinary approach toward highly sensitive biomolecoular spectroscopy. In Proceedings of the IEEE International Symposium on Circuits and Systems, Lisbon, Portugal, 24–27 May 2015.
- Hoult, D.I.; Richards, R.E. The signal-to-noise ratio of the nuclear magnetic resonance experiment. J. Magn. Reson. 1976, 24, 71–85. [Google Scholar] [CrossRef]
- Cabrita, L.D.; Hsu, S.-T.D.; Launay, H.; Dobson, C.M.; Christodoulou, J. Probing ribosome–nascent chain complexes produced in vivo by NMR spectroscopy. Proc. Natl. Acad. Sci. USA 2009, 106, 22239–22244. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, A.; Beerbaum, M.; Rajagopalan, N.; Fiaux, J.; Schmieder, P.; Kramer, G.; Oschkinat, H.; Bukau, B. Large-scale purification of ribosome–nascent chain complexes for biochemical and structural studies. FEBS Lett. 2009, 583, 2407–2413. [Google Scholar] [CrossRef] [PubMed]
- Eichmann, C.; Preissler, S.; Riek, R.; Deuerling, E. Cotranslational structureacquisition of nascent polypeptides monitored by NMR spectroscopy. Proc. Natl. Acad. Sci. USA 2010, 107, 9111–9116. [Google Scholar] [CrossRef] [PubMed]
- Voehler, M.W.; Collier, G.; Young, J.K.; Stone, M.P.; Germann, M.W. Performance of cryogenic probes as a function of ionic strength and sample tube geometry. J. Magn. Reson. 2006, 183, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, H. Cryogenically cooled probes a leap in NMR technology. Prog. Nucl. Magn. Reson. Spectrosc. 2005, 46, 131–156. [Google Scholar] [CrossRef]
- Chidambaram, R. Signal to Noise Ratio in Nuclear Magnetic Resonance. Proc. Phys. Soc. 1960, 75, 163–164. [Google Scholar] [CrossRef]
- De Swiet, T.M. Optimal electric fields for different sample shapes in high resolution NMR spectroscopy. J. Magn. Reson. 2005, 174, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Giraudeau, P.; Akoka, S. Sensitivity losses and line shape modifications due to molecular diffusion in continuous encoding ultrafast 2D NMR experiments. J. Magn. Reson. 2008, 195, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Schanda, P.; Brutscher, B. Very fast two-dimensional NMR spectroscopy for real-time investigation of dynamic events in proteins on the time scale of seconds. J. Am. Chem. Soc. 2005, 127, 8014–8015. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Guo, D.; Cao, X.; Cai, S.; Chen, Z. Reconstruction of Self-Sparse 2D NMR Spectra from Undersampled Data in the Indirect Dimension. Sensors 2011, 11, 8888–8909. [Google Scholar] [CrossRef] [PubMed]
- Shrot, Y.; Frydman, L. Compressed sensing and the reconstruction of ultrafast 2D NMR data: Principles and biomolecular applications. J. Magn. Reson. 2011, 209, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Haken, R.; Blümich, B. Anisotropy in tendon investigation in vivo by a portable NMR scanner, the NMR-MOUSE. J. Magn. Reson. 2000, 144, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Coil-on-a-Chip, Broker Inc. Available online: https://www.bruker.com/products/mr/nm/probes/probes/coil-on-a-chip/overview.html (accessed on 1 June 2016).
- Fratila, R.M.; Gomez, M.V.; Sýkora, S.; Velders, A.H. Multinuclear nanoliter one-dimensional and two-dimensional NMR spectroscopy with a single non-resonant microcoil. Nat. Commun. 2014, 5, 3025. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Chung, J.; Balaj, L.; Charest, A.; Bigner, D.D.; Carter, B.S.; Hochberg, F.H.; Breakefield, X.O.; Weissleder, R.; Lee, H. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat. Med. 2012, 18, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Kratt, K.; Badilita, V.; Burger, T.; Wallrabe, U. A fully MEMS-compatible process for 3D high aspect ratio micro coils obtained with an automatic wire bonder. J. Micromech. Microeng. 2010, 20, 015021. [Google Scholar] [CrossRef]
- Badilita, V.; Kratt, K.; Baxan, N.; Mohmmadzadeh, M.; Burger, T.; Weber, H.; Elverfeldt, D.V.; Hennig, J.; Korvinkcd, J.G.; Wallrabead, U. On-chip three dimensional microcoils for MRI at the microscale. Lab Chip 2010, 10, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Cherifi, T.; Abouchi, N.; Lu, G.N.; Bouchet-Fakri, L.; Quiquerez, L.; Sorli, B.; Chateaux, J.F.; Pitaval, M.; Morin, P. A CMOS microcoil-associated preamplifier for NMR spectroscopy. IEEE Trans. Circuits Syst. 2005, 52, 2576–2583. [Google Scholar] [CrossRef]
- Lee, H.; Sun, E.; Ham, D.; Weissleder, R. Chip-NMR biosensor for detection and molecular analysis of cells. Nat. Med. 2008, 14, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Benesi, A.J. A Primer of NMR Theory with Calculation in Mathematica. Wiley: New York City, NY, USA, 2015. [Google Scholar]
- Watzlaw, J.; Müntjes, J.; Mokwa, W.; Schnakenberg, U. Multilayer Micro Coils for Thin Film Analysis with Mobile NMR Arrays. Proc. Eng. 2011, 25, 395–398. [Google Scholar] [CrossRef]
- Zolfaghari, A.; Chan, A.; Razavi, B. Stacked inductors and transformers in CMOS technology. IEEE J. Solid-State Circuits 2001, 36, 620–628. [Google Scholar] [CrossRef]
- Lin, L.; Yin, W.-Y.; Mao, J.-F.; Wang, Y.-Y. Implementation of new CMOS differential stacked spiral inductor for VCO design. Microw. Wirel. Compon. Lett. 2007, 17, 727–729. [Google Scholar] [CrossRef]
- Anders, J.; SanGiorgio, P.; Boero, G. A fully integrated IQ-receiver for NMR microscopy. J. Magn. Reson. 2011, 209, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Uddin, A.; Yemenicioglu, S.; Chen, C.H.; Corigliano, E.; Milaninia, K.; Theogarajan, L. Integration of solid-state nanopores in a 0.5 μm CMOS foundry process. Nanotechnology 2013, 24, 15. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.; Gaitan, M.; Locascio, L.E.; Zaghloul, M.E. Fabrication techniques to realize CMOS-compatible microfluidic microchannels. J. Microelectromech. Syst. 2001, 10, 286–297. [Google Scholar] [CrossRef]
- Yilmaz, A.; Utz, M. Characterisation of oxygen permeation into a microfluidic device for cell culture by in-situ NMR spectroscopy. Lab on a Chip 2016, 16, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Spengler, N.; Höfflin, J.; Moazenzadeh, A.; Mager, D.; MacKinnon, N.; Badilita, V.; Wallrabe, U.; Korvink, J.G. Heteronuclear Micro-Helmholtz Coil Facilitates µm-Range Spatial and Sub-Hz Spectral Resolution NMR of nL-Volume Samples on Customisable Microfluidic Chips. PLoS ONE 2016, 11, e0146384. [Google Scholar] [CrossRef] [PubMed]
- Massin, C.; Vincent, F.; Homsy, A.; Ehrmann, K.; Boero, G.; Besse, P.-A.; Daridon, A.; Verpoorte, E.; de Rooij, N.F.; Popovi, R.S. Planar microcoil-based microfluidic NMR probes. J. Magn. 2003, 164, 242–255. [Google Scholar] [CrossRef]
- Erin, E. NMR analysis on microfluidic devices by remote detection. Anal. Chem. 2005, 77, 8109–8114. [Google Scholar]
- Bart, J.; Kolkman, A.J.; Oosthoek-de Vries, A.J.O.; Koch, K.; Nieuwland, P.J.; Janssen, H.; van Bentum, J.; Ampt, K.A.M.; Rutjes, F.P.J.T.; Wijmenga, S.S.; et al. A Microfluidic High-Resolution NMR Flow Probe. J. Am. Chem. Soc. 2009, 131, 5014–5015. [Google Scholar] [CrossRef] [PubMed]




| Technology | CMOS 0.13 µm |
| Area (mm2) | 1 × 2 |
| Vdd (V) | 1.6 |
| DC current of the LNA core (mA) | 3.4 |
| LNA 3-dB BW (MHz) | 800 |
| Voltage Gain (dB) @ 300 MHz | 42.85 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pourmodheji, H.; Ghafar-Zadeh, E.; Magierowski, S. A Multidisciplinary Approach to High Throughput Nuclear Magnetic Resonance Spectroscopy. Sensors 2016, 16, 850. https://doi.org/10.3390/s16060850
Pourmodheji H, Ghafar-Zadeh E, Magierowski S. A Multidisciplinary Approach to High Throughput Nuclear Magnetic Resonance Spectroscopy. Sensors. 2016; 16(6):850. https://doi.org/10.3390/s16060850
Chicago/Turabian StylePourmodheji, Hossein, Ebrahim Ghafar-Zadeh, and Sebastian Magierowski. 2016. "A Multidisciplinary Approach to High Throughput Nuclear Magnetic Resonance Spectroscopy" Sensors 16, no. 6: 850. https://doi.org/10.3390/s16060850