Novel Wearable Device for Blood Leakage Detection during Hemodialysis Using an Array Sensing Patch
Abstract
:1. Introduction
2. Materials and Methods
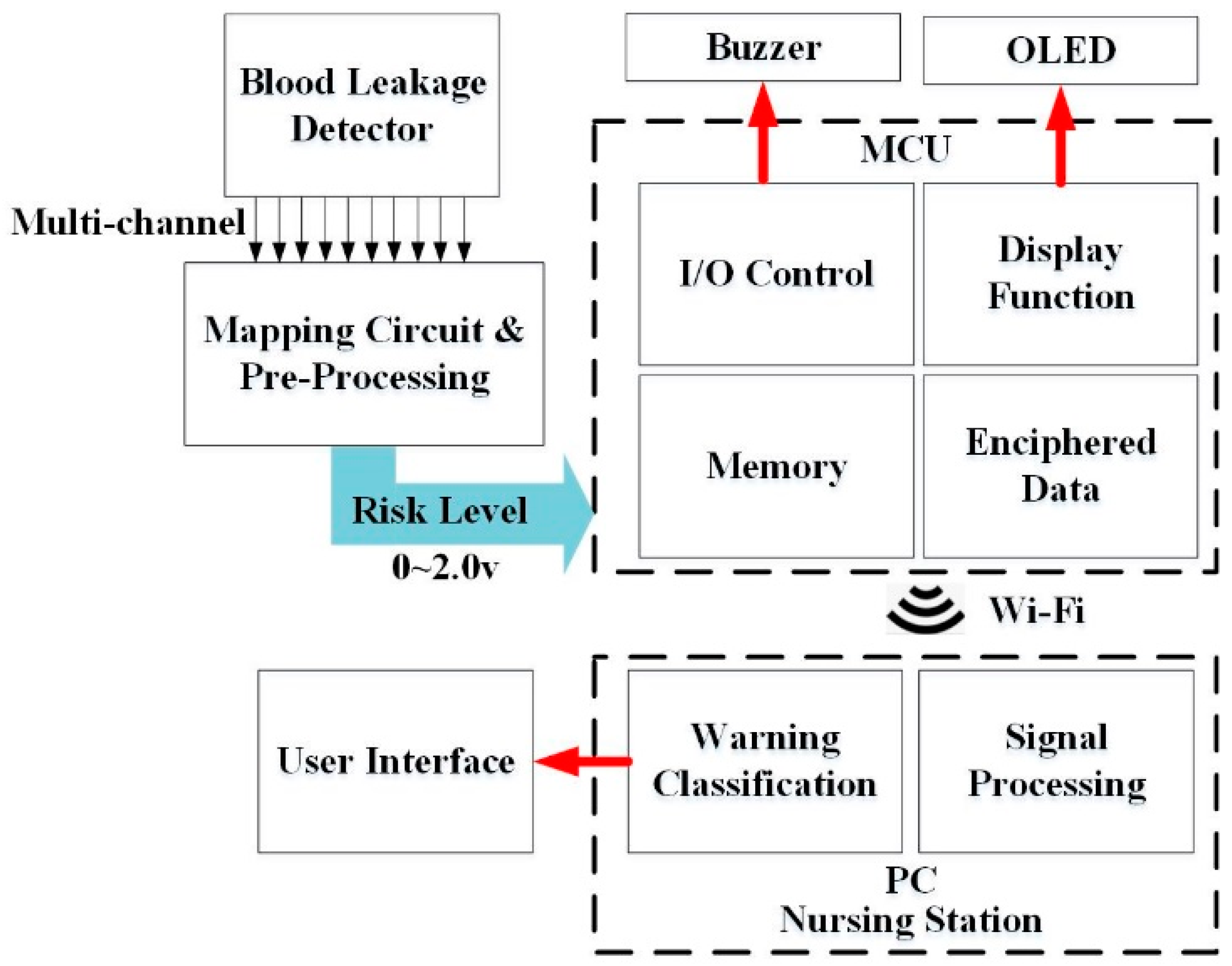
2.1. Architecture of the Blood Leakage Detector
2.2. Blood Leakage Detector Components
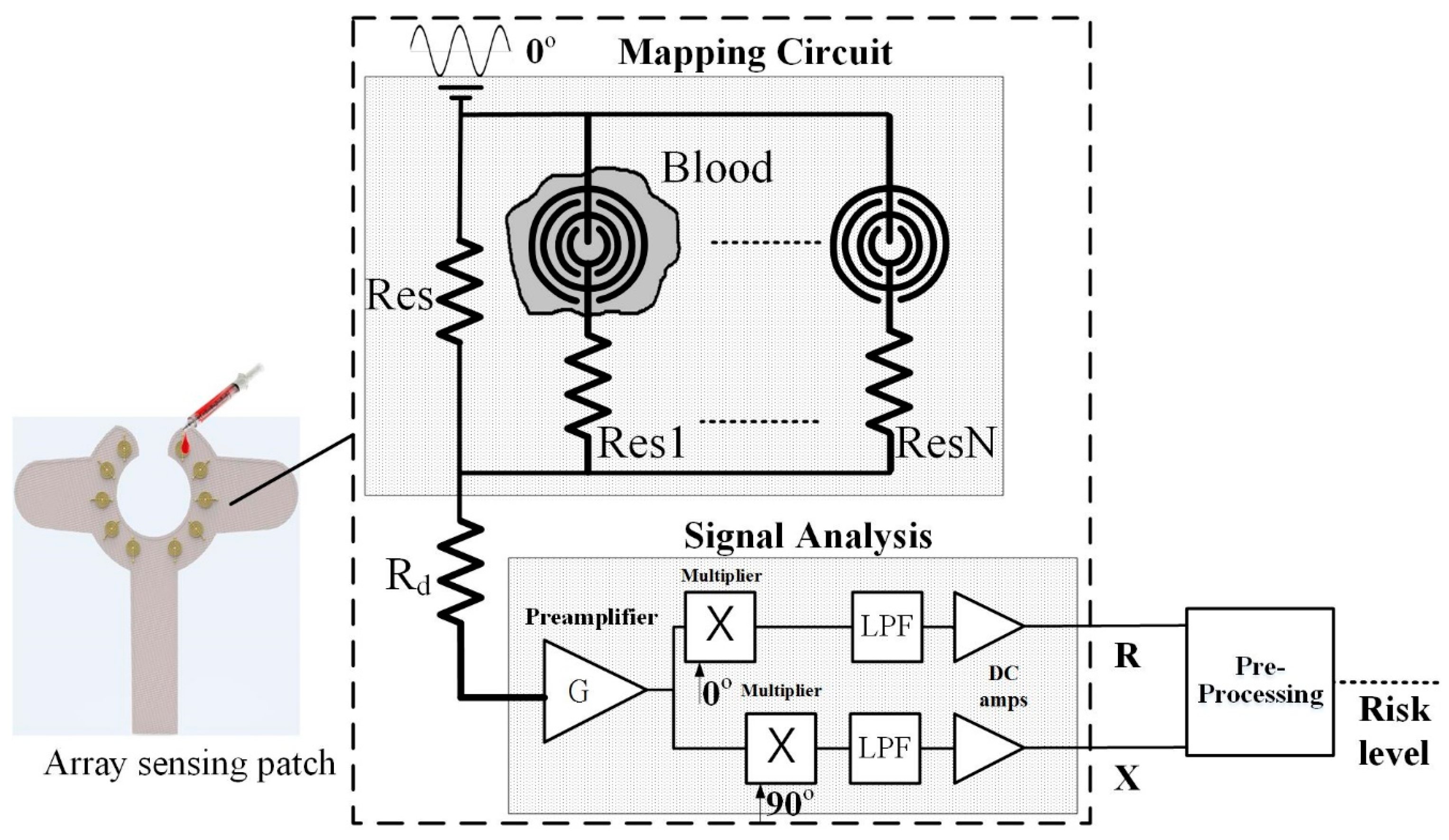
2.2.1. Array Sensor Patch and Mapping Circuit
2.2.2. MCU and Wi-Fi Module
2.2.3. Alert Components
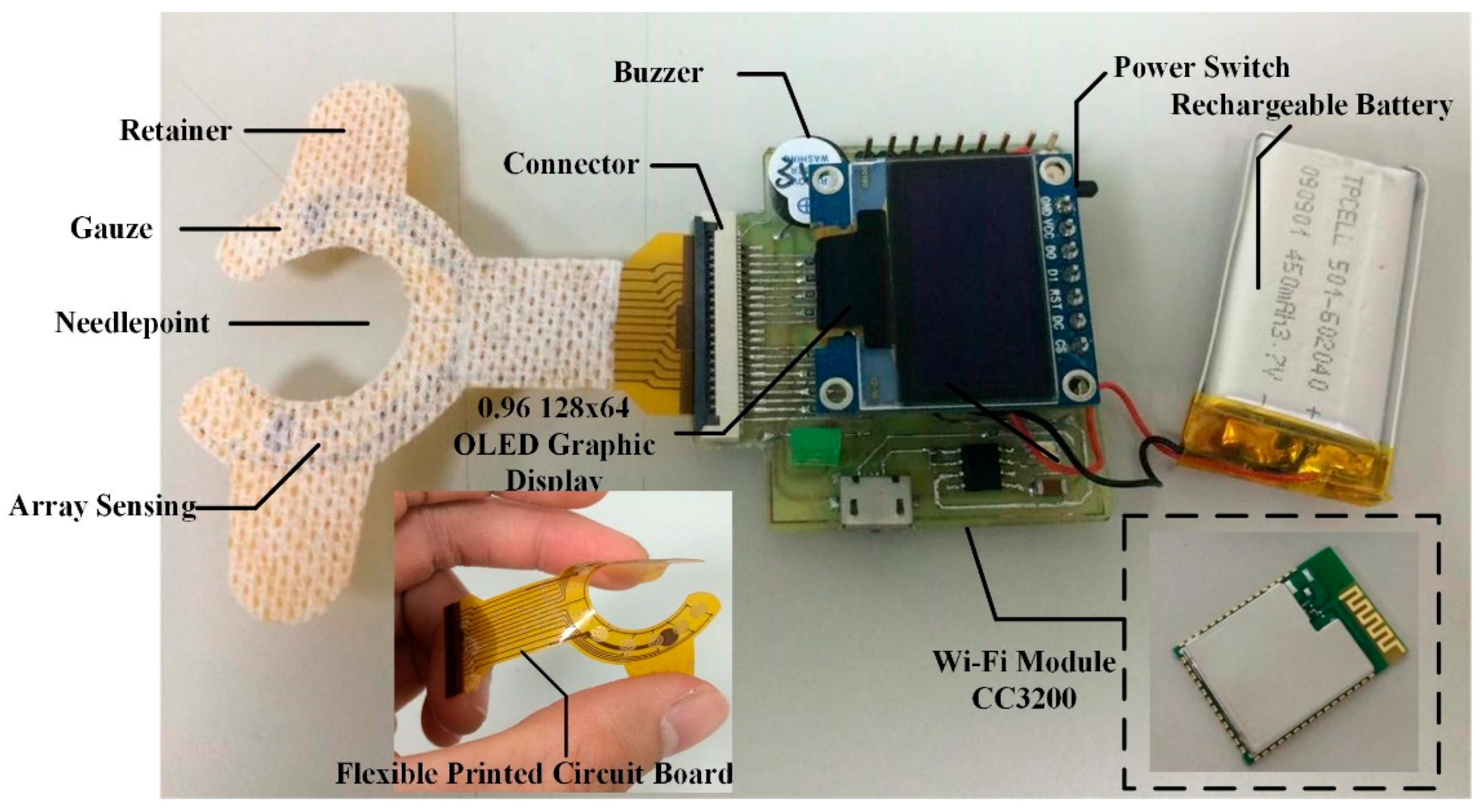
- OLED Graphic Display: The device proposed in this study, a 0.96” 128 × 64 organic light-emitting diode (OLED) is used in the blood leakage detector to show the device’s power level and the visual warning signal. Compared to conventional LCD panels, OLED has the advantages of high brightness, flexibility, and wide viewing range which saves the overall circuit space of the detector. During dialysis, it could provide users with an immediate warning signal and shows the device’s power level [12,13].
- Buzzer: A buzzer as an integrated electronic signal warning device has been widely used in medical devices. It is classified into two types: electromagnetic and piezoelectric element. We used the smaller-sized electromagnetic buzzer as the sound warning device. When used clinically, it may produce noise and disturb other dialysis patients; therefore, this device is designed with sound alert on/off features and can be used according to user preference.
2.2.4. Package and Wearable Device
2.3. Signal Processing and User Interface
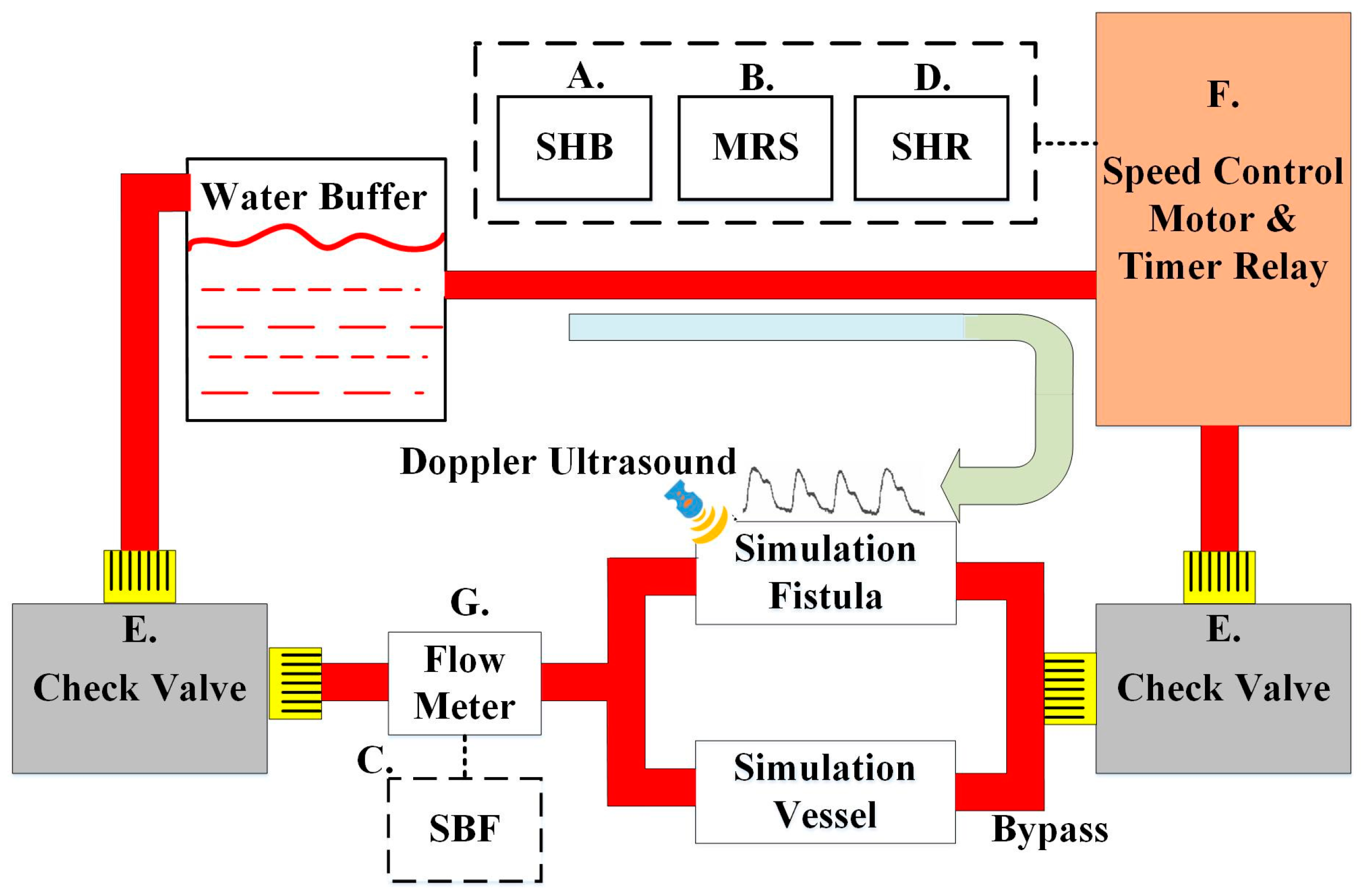
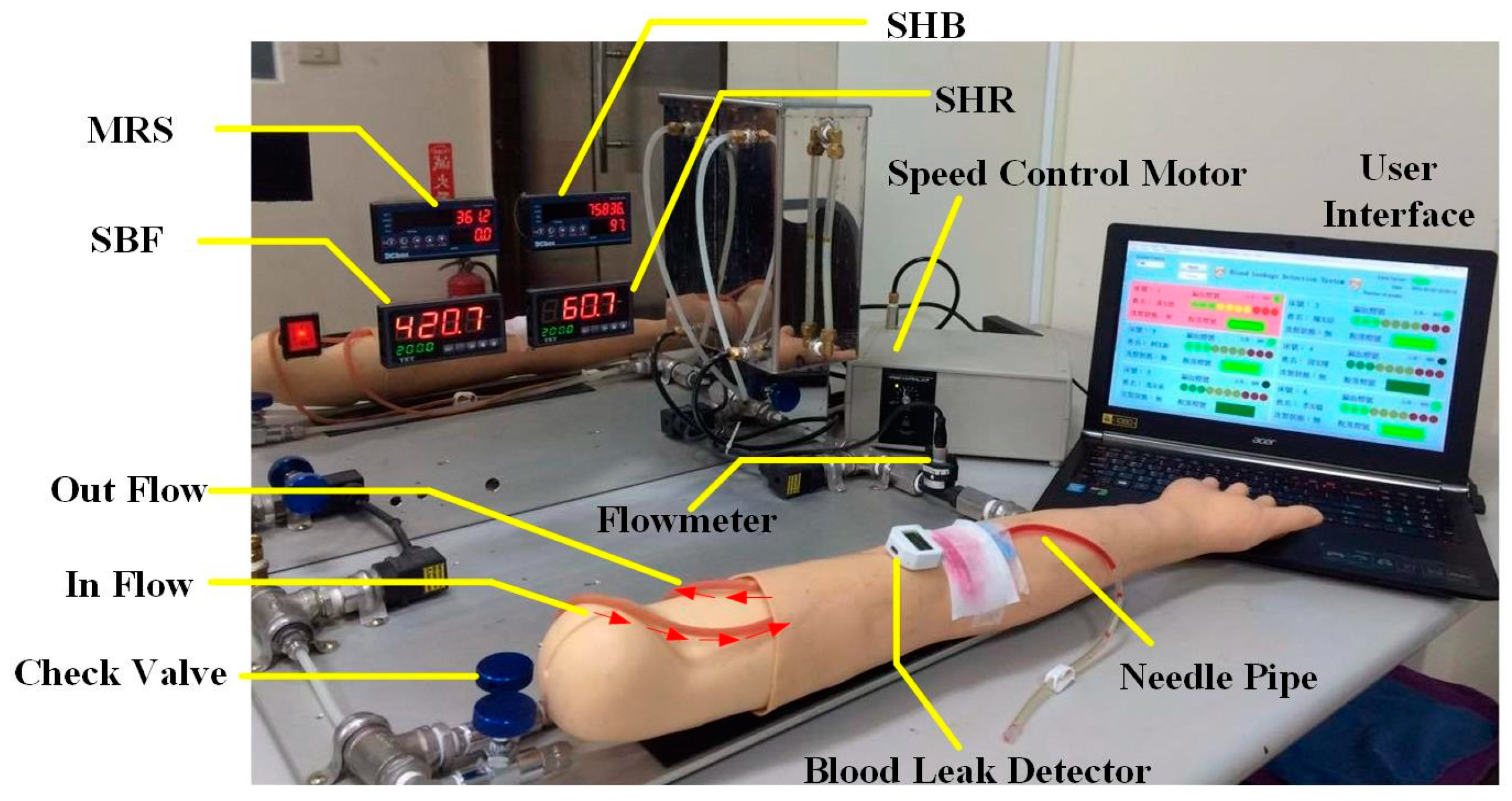
2.4. System Design of the Circulation Simulation Test
3. Experimental Design and Results
3.1. Single-Point Response Time
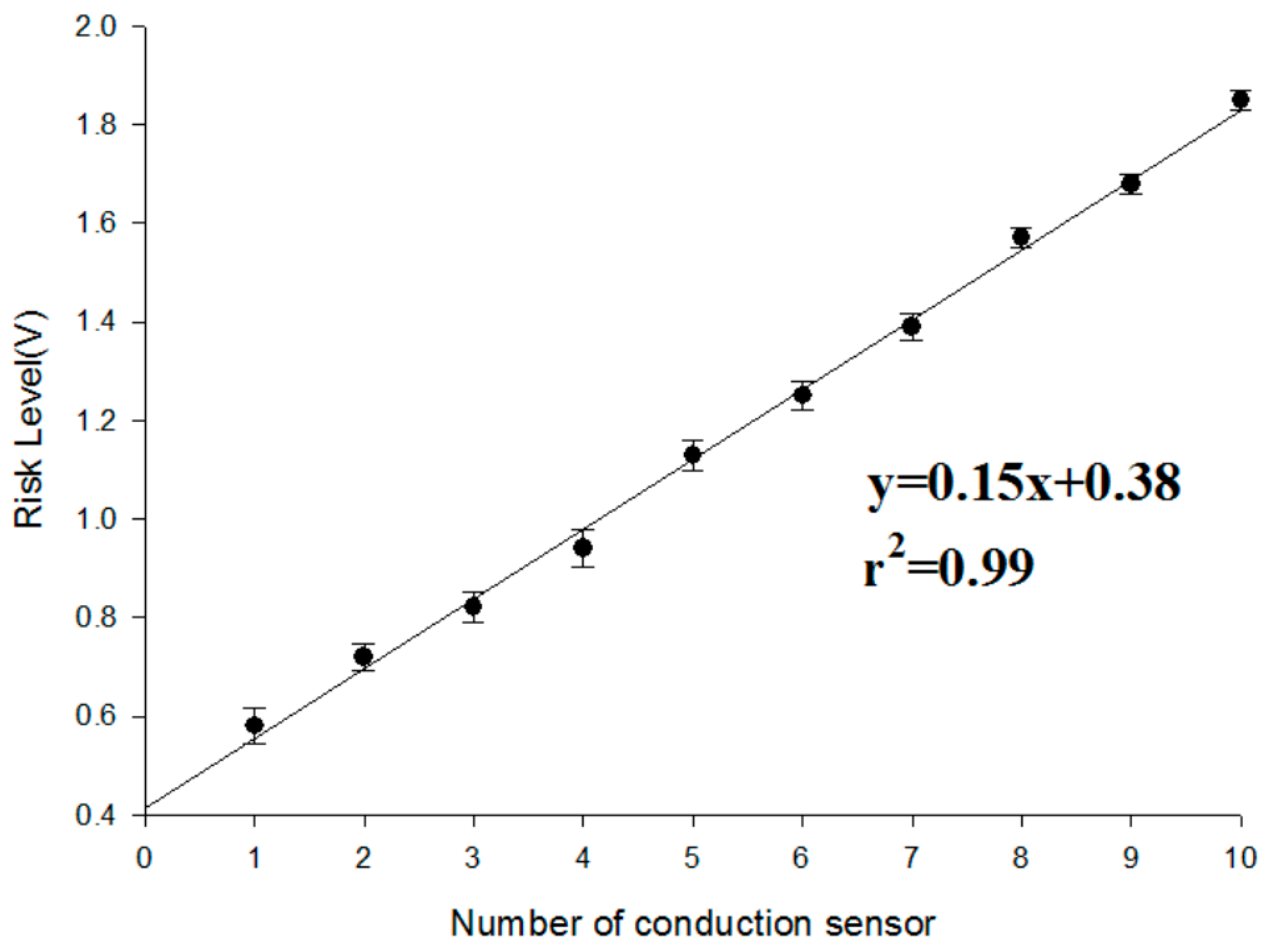
3.2. Risk Level Experiment
- (a)
- First, in the experiment with the same weight ratio, we dropped 0.1 mL of blood on each sensing point in random order until all the sensor points were covered. This experiment was repeated 10 times. The results of the experiment are shown in Figure 10. With an increase in the number of conducting sensing points, the risk level voltage linearly increased. Based on the Student’s t-test analysis, they displayed significant differences between each other (p < 0.05) as shown in Table 1.
- (b)
- Because each sensing point could have different weight ratios for the risk level, we designed a symmetrical weight ratio distribution using two parallel points to test different conditions of clinical blood leakage. Ten sensing points were divided into five groups, namely 1 (A + B), 2 (C + D), 3 (E + F), 4 (G + H), and 5 (I + J). The weight ratio was proportionally allocated in accordance with the distance from the tip of the needle. The weight ratio of 1 (A, B), which is the closest the needle, and 5 (I, H), which is the farthest from the needle, is 1:10. During the start of the experiment, the conduction for one group was done at fixed times to determine whether the difference in voltage level conforms to the weight ratio. Test results are shown in Figure 11. Group 1 (A and B), which is closer to the tip of the needle, shows a small change in voltage level difference after conduction. When the degree of blood leakage increased, Group 5 (I and J), which is located farthest from the tip of the needle, showed an increase in voltage level difference. The data ratio is about 1:10 and is consistent with the weight ratio values. The results indicated the risk level is highly reproducible and consistent with the blood leakage level. In addition, during conduction, the average response time of the system is less than 100 ms. Based on the Student’s t-test analysis, there were significant differences between the tests as shown in Table 2.
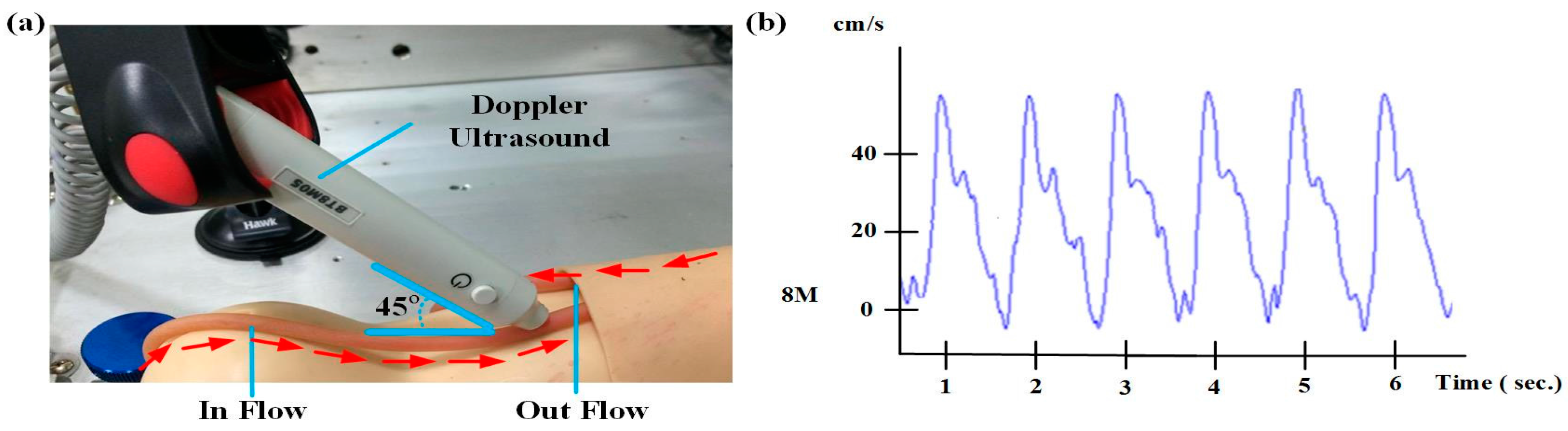
3.3. Circulatory System Simulation Test
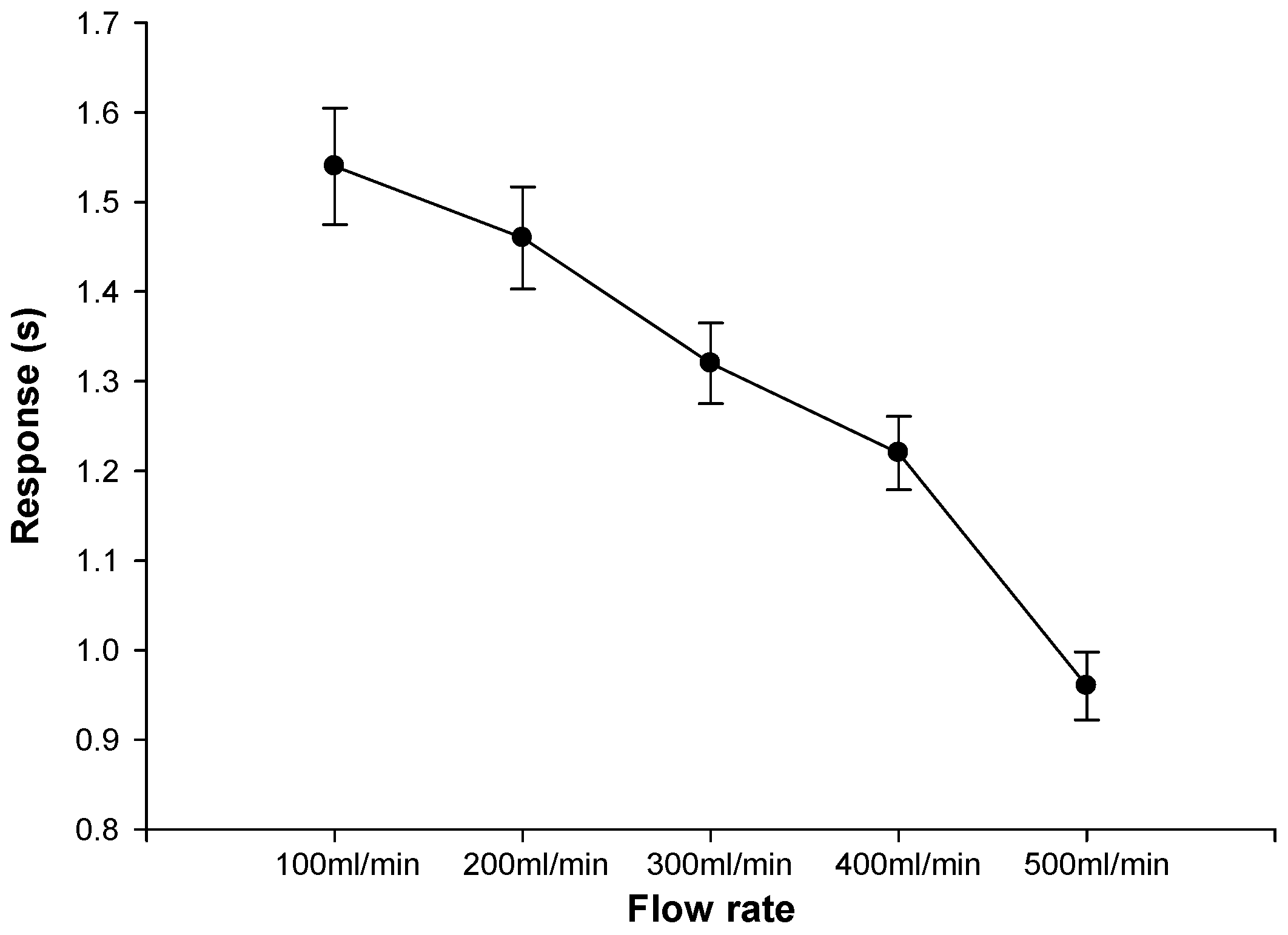
3.4. Gauze Diffusion Time Test for Different Flow Rates
3.5. Wireless Signal Attenuation Test of Wi-Fi
3.6. Battery Life Experiments
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HD | Hemodialysis |
| CKD | Chronic kidney disease |
| ESRD | End-stage renal disease |
| VND | Venous needle dislodgement |
| FPCB | Flexible Printed Circuit Board |
| SHB | Simulation heart beat |
| MRS | Motor rotational speed |
| SBF | Simulation blood flow |
| SHR | Simulation heart rate |
| IoT | Internet of Things |
References
- USRDS. 2015 Annual Data Report; USRDS: New York, NY, USA, 2015. [Google Scholar]
- MacRaei, J.M.; Ahmed, S.B.; Hemmelgarn, B.R.; Alberta Kidney Disease Network. Arteriovenous fistula survival and needling technique: Long-term results from a randomized buttonhole trial. Am. J. Kidney Dis. 2014, 63, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chen, W.L.; Kan, C.D.; Wu, M.J.; Mai, Y.C. Detection of venous needle dislodgement during hemodialysis using fractional order shape index ratio and fuzzy colour relation analysis. Healthc. Technol. Lett. 2015, 2, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Ribitsch, W.; Schilcher, G.; Hafner-Giessauf, H.; Krisper, P.; Horina, J.H.; Rosenkranz, A.R.; Schneditz, D. Prevalence of detectable venous pressure drops expected with venous needle dislodgement. In Seminars in Dialysis; Wiley online library: Graz City, Austria, 2014; Volume 27, pp. 507–511. [Google Scholar]
- Anzacare Ltd. New Zealand. Device and apparatus for detecting moisture. Available online: http://www.hemodialert.com/ (accessed on 12 May 2005).
- Redsense. Blood Leakage Detection Device. Available online: http://www.redsensemedical.com/startpage.html/ (accessed on 13 February 2014).
- Ahlmén, J.; Gydell, K.H.; Hadimeri, H.; Hernandez, I.; Rogland, B.; Strömbom, U. A new safety device for hemodialysis. Hemodial. Int. 2008, 12, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Wolff, H.; Ritter, K.U. Apparatus and Method for Detecting Venous Needle Dislodgement. U.S. Patent 20,150,246,171, 28 February 2015. [Google Scholar]
- Lay-Ekuakille, A.; Vergallo, P. Decimated signal diagonalization method for improved spectral leakage detection in pipelines. IEEE Sens. J. 2014, 14, 1741–1748. [Google Scholar] [CrossRef]
- Chuang, H.C.; Shih, C.Y.; Chou, C.H.; Huang, J.T.; Wu, C.J. The Development of a Blood Leakage Monitoring System for the Applications in Hemodialysis Therapy. IEEE Sens. J. 2015, 15, 1515–1522. [Google Scholar] [CrossRef]
- Chuang, C.H.; Du, Y.C.; Wu, T.F.; Chen, C.H.; Lee, D.H.; Chen, S.M.; Shaikh, M.O. Immunosensor for the ultrasensitive and quantitative detection of bladder cancer in point of care testing. Biosens. Bioelectron. 2016, 84, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, T. OLED Display Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Gaspar, D.J. Materials, Processing and Applications. In OLED Fundamentals: Materials, Devices, and Processing of Organic Light-Emitting Diodes; CRC Press: Boca Raton, FL, USA, 2015; p. 437. [Google Scholar]
- Saucedo-Zeni, N.; Mewes, S.; Niestroj, R.; Gasiorowski, L.; Murawa, D.; Nowaczyk, P.; Tomasi, T.; Weber, E.; Dworacki, G.; Morgenthaler, N.G.; et al. A novel method for the in vivo isolation of circulating tumor cells from peripheral blood of cancer patients using a functionalized and structured medical wire. Int. J. Oncol. 2012, 41, 1241–1250. [Google Scholar] [PubMed]
- Cao, T.S.; Yuan, L.J. Factors Influencing Doppler Blood Flow and Its Measurements; INTECH: Xi’an, China, 2011. [Google Scholar]
- Xu, T.; Bashford, G.R. Two-dimensional blood flow velocity estimation using ultrasound speckle pattern dependence on scan direction and A-line acquisition velocity. IEEE Tran. Ultrason. Ferroelectr. Freq. Control 2013, 60, 898–908. [Google Scholar]
- Hernández, N.; Alonso, J.M.; Ocaña, M.; Marina, M.K. Impact of Signal Representations on the Performance of Hierarchical WiFi Localization Systems. In Computer Aided Systems Theory-EUROCAST 2013; Springer: Berlin/Heidelberg, Germany, 2013; pp. 17–24. [Google Scholar]
- Saghezchi, F.B.; Radwan, A.; Rodriguez, J. Energy efficiency performance of WiFi/WiMedia relaying in hybrid ad-hoc networks. In Proceedings of the 2013 Third International Conference on Communications and Information Technology (ICCIT), Beirut, France, 19–21 June 2013; pp. 285–289.
- Demirci, H.; Satman, I.; Bilgel, N.; Çınar, Y. Essentials of Diabetes Care in Family Practice; INTECH Open Access Publisher: Lincoln City, OR, USA, 2013. [Google Scholar]
- Leverett, J. A Low-Power, Lightweight, Wireless Neural Recording and Stimulating Headstage for Brain Machine Interfaces; UC Berkeley Master’s Report; University of California: Berkeley, CA, USA, 1 December 2014. [Google Scholar]






| Number of Conduction Sensor | Mean (V) | Number of Conduction Sensor | Mean (V) |
|---|---|---|---|
| 1 | 0.58 ± 0.054 | 6 | 1.25 ± 0.051 |
| 2 | 0.72 ± 0.052 | 7 | 1.39 ± 0.045 |
| 3 | 0.82 ± 0.048 | 8 | 1.57 ± 0.042 |
| 4 | 0.94 ± 0.051 | 9 | 1.68 ± 0.021 |
| 5 | 1.13 ± 0.057 | 10 | 1.85 ± 0.025 |
| Group | Mean ± SD |
|---|---|
| 1 (A + B) | 0.18 ± 0.041 |
| 2 (C + D) | 0.52 ± 0.039 |
| 3 (E + F) | 0.94 ± 0.031 |
| 4 (G + H) | 1.48 ± 0.045 |
| 5 (I + J) | 1.92 ± 0.052 |
| Distance (m) | Wi-Fi (dBm) | Data Loss (%) | |
|---|---|---|---|
| Detector | 20 m | −72 | 0% |
| 40 m | −76 | 0% | |
| 60 m | −83 | <1% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.-C.; Lim, B.-Y.; Ciou, W.-S.; Wu, M.-J. Novel Wearable Device for Blood Leakage Detection during Hemodialysis Using an Array Sensing Patch. Sensors 2016, 16, 849. https://doi.org/10.3390/s16060849
Du Y-C, Lim B-Y, Ciou W-S, Wu M-J. Novel Wearable Device for Blood Leakage Detection during Hemodialysis Using an Array Sensing Patch. Sensors. 2016; 16(6):849. https://doi.org/10.3390/s16060849
Chicago/Turabian StyleDu, Yi-Chun, Bee-Yen Lim, Wei-Siang Ciou, and Ming-Jui Wu. 2016. "Novel Wearable Device for Blood Leakage Detection during Hemodialysis Using an Array Sensing Patch" Sensors 16, no. 6: 849. https://doi.org/10.3390/s16060849






