Development of a Tonometric Sensor with a Decoupled Circular Array for Precisely Measuring Radial Artery Pulse
Abstract
:1. Introduction
2. Design and Experimental Section
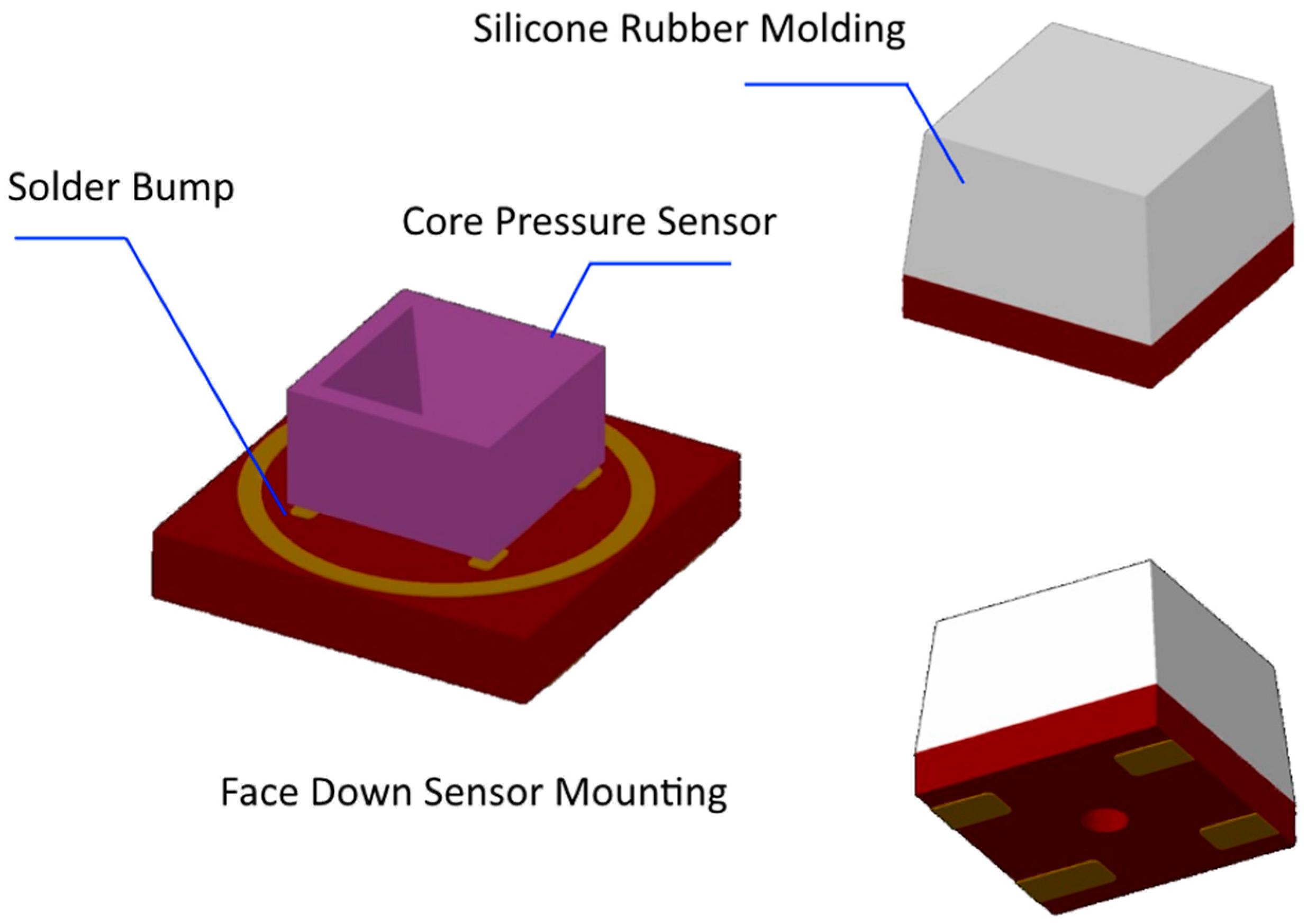
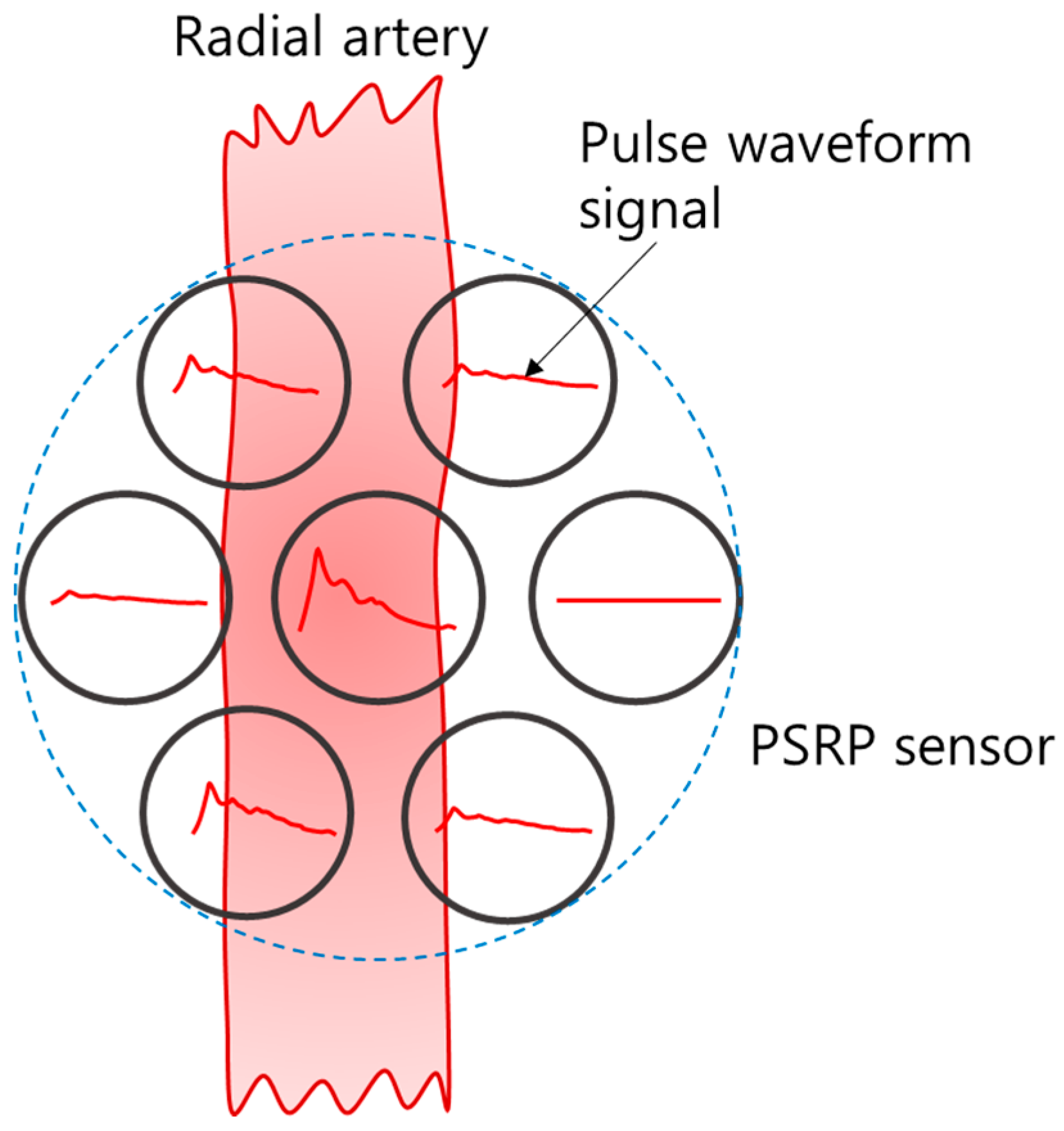
2.1. FDB-Type Pressure Sensor for Radial Pulse (PSRP)
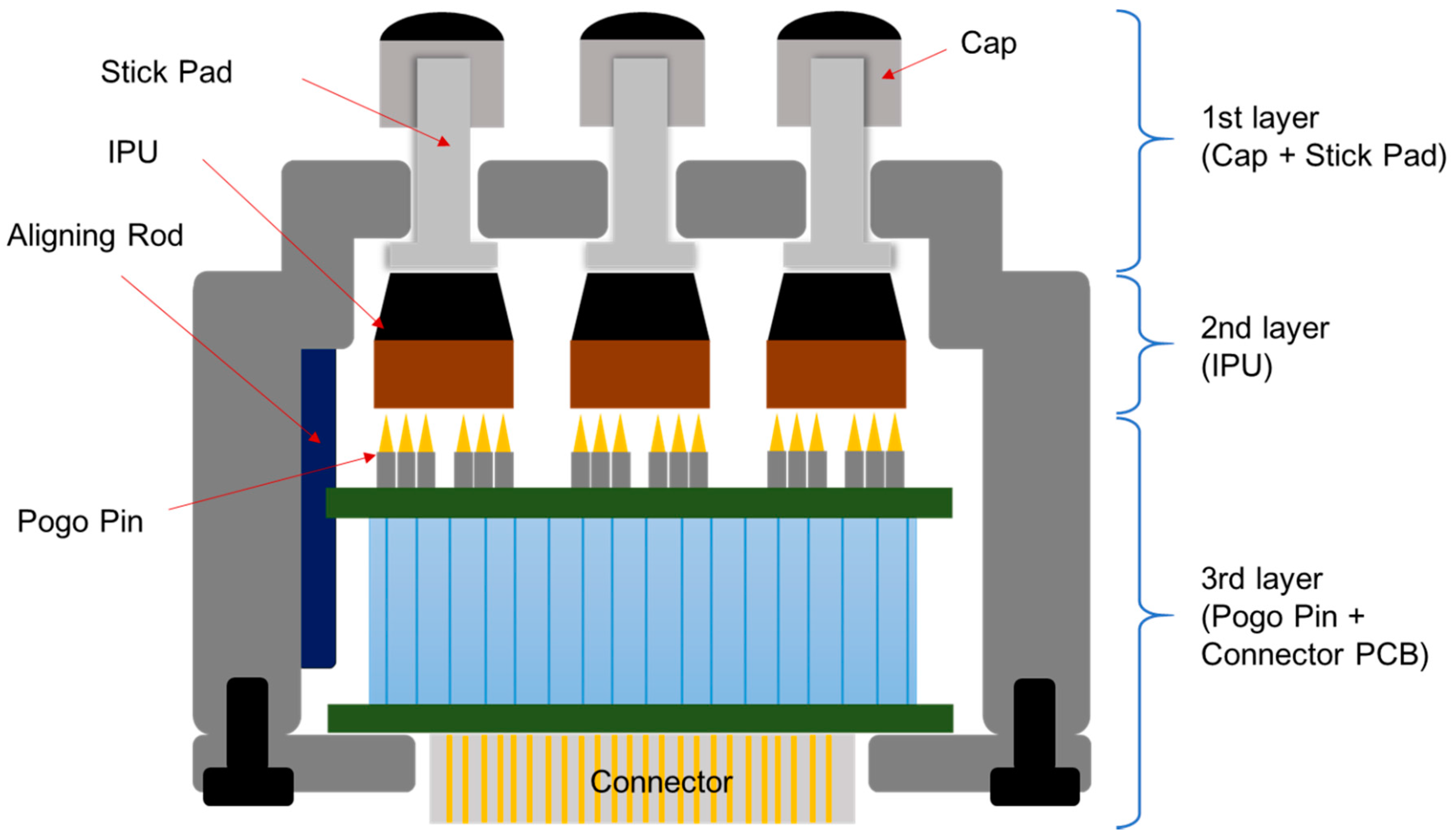
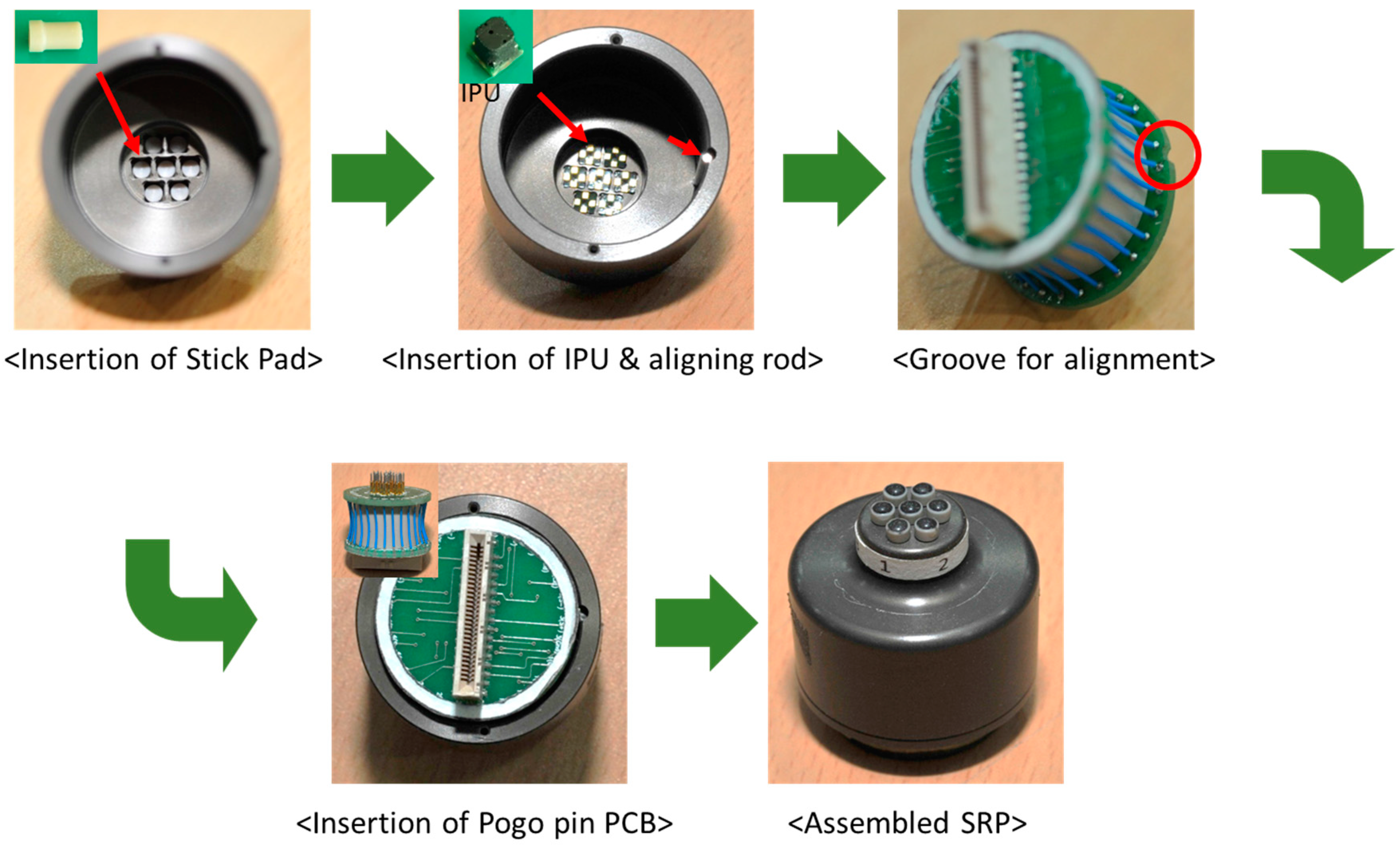
2.1.1. The Structure and Assembly of PSRP
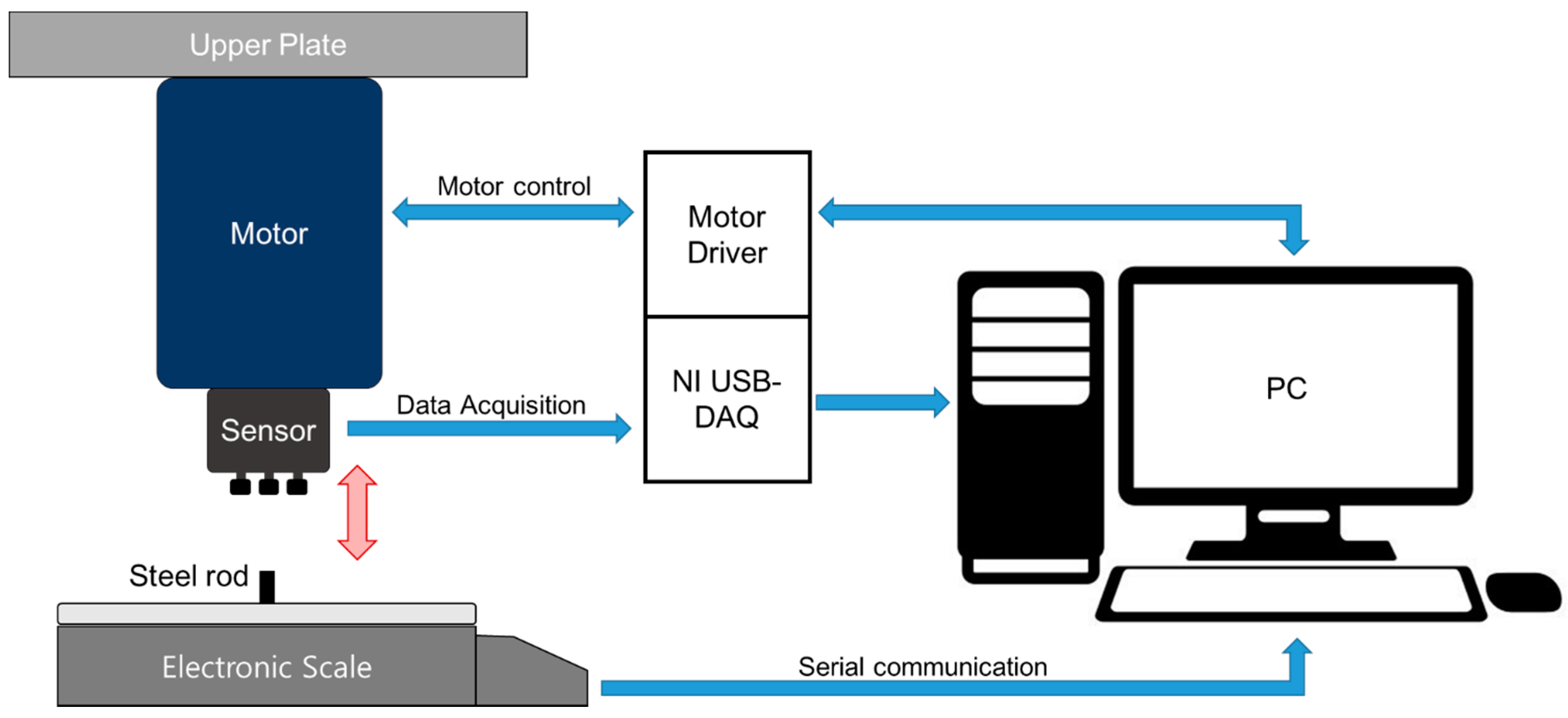
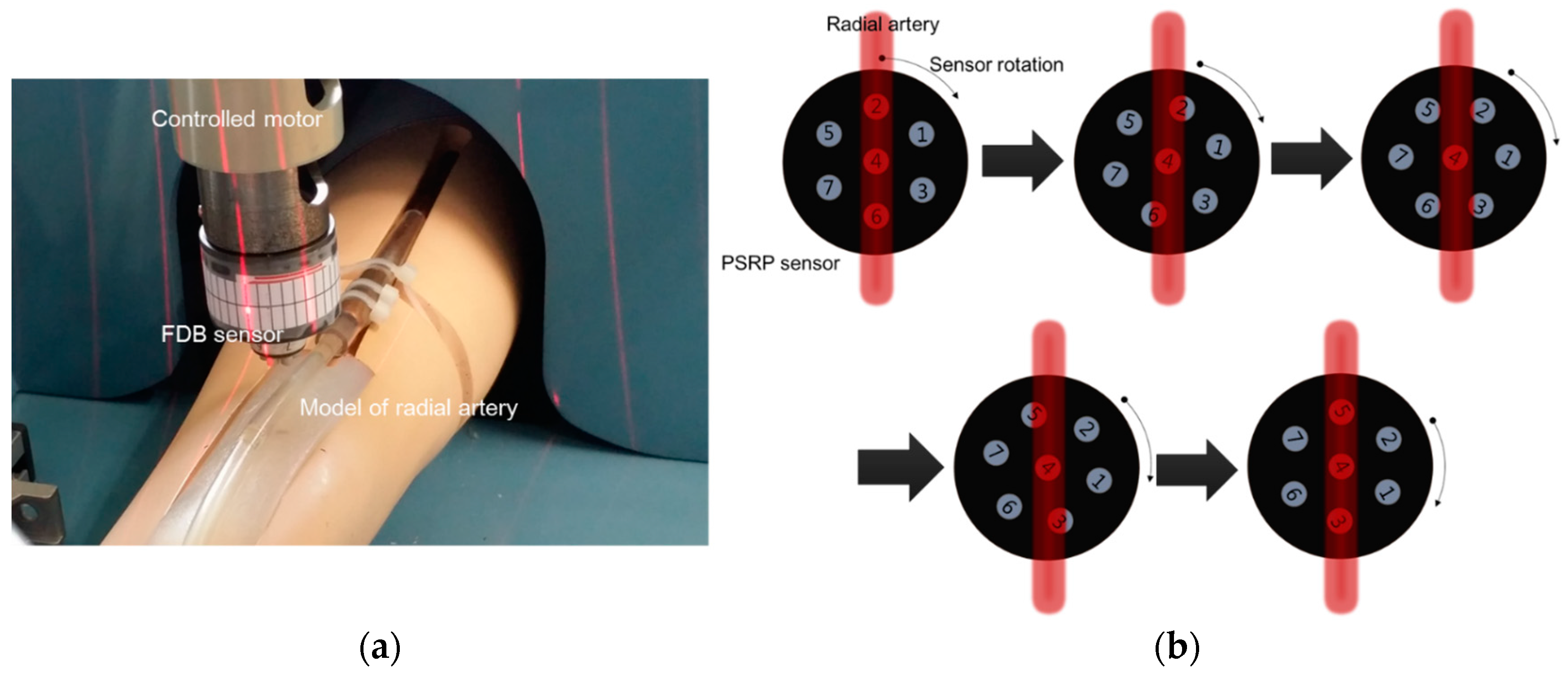
2.1.2. PSRP Evaluation
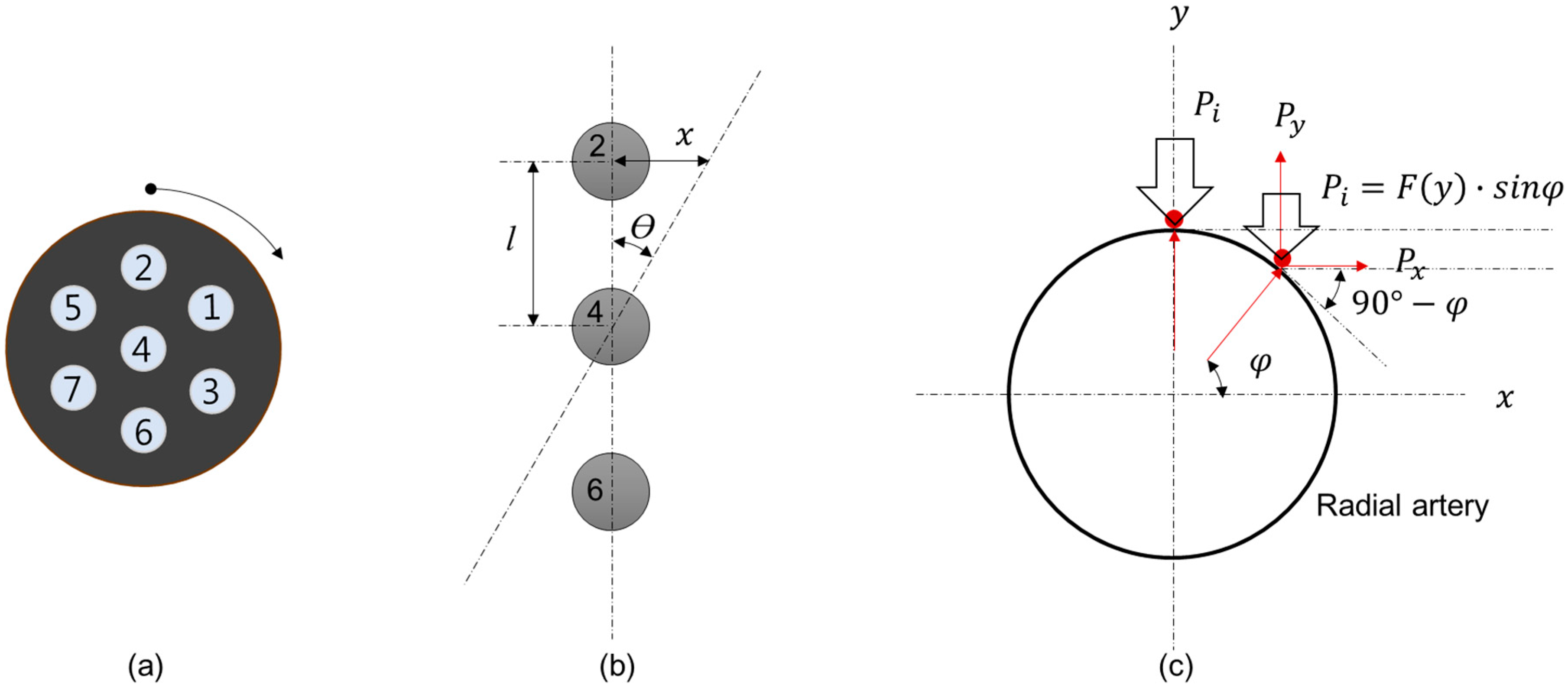
2.2. Method for Estimating Blood Vessel Direction
3. Results and Discussion
3.1. Characteristics of PSRP Sensor
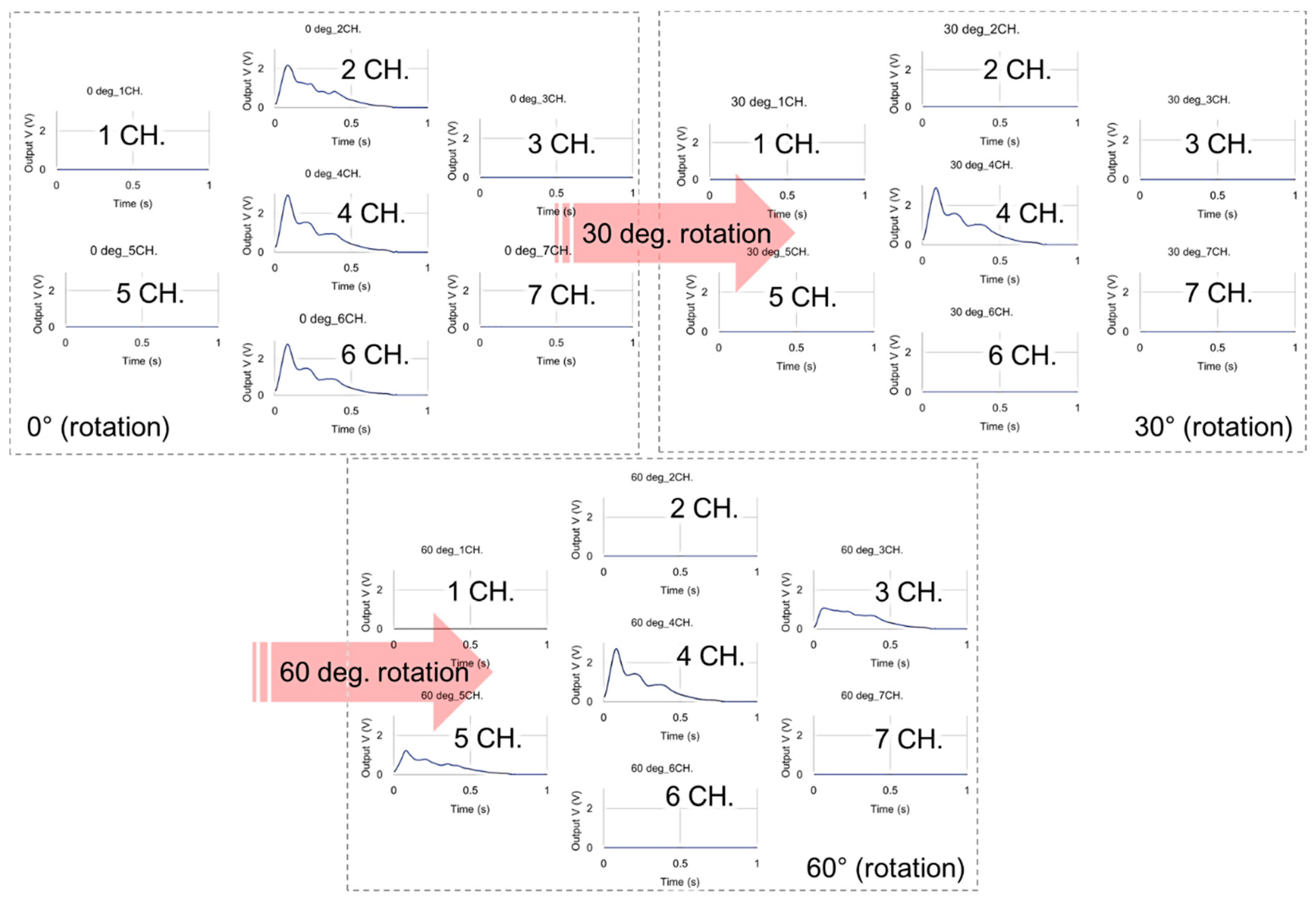
3.2. Experimental Result for Estimating Blood Vessel Direction
4. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nichols, W.W.; O’Rourke, M.F. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles, 5th ed.; Hodder Arnold: London, UK, 2005. [Google Scholar]
- Westerhof, N.; Stergiopulos, N.; Noble, M.I.M. Snapshots of Hemodynamics: An Aid for Clinical Research and Graduate Education, 2nd ed.; Springer Science & Business Media: New York, NY, USA, 2010. [Google Scholar]
- Ryu, H.H.; Lee, S.W.; Lee, J.; Lee., Y.; Kim, J.Y. Analysis of physical quantification of pulse types by pulse diagnosis. Korean J. Oriental Physiol. Pathol. 2007, 21, 1381–1387. [Google Scholar]
- Nichols, W.W. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am. J. Hypertens. 2005, 18, 3S–10S. [Google Scholar] [CrossRef] [PubMed]
- Kohara, K.; Tabara, Y.; Oshiumi, A.; Miyawaki, Y.; Kobayashi, T.; Miki, T. Radial augmentation index: A useful and easily obtainable parameter for vascular aging. Am. J. Hypertens. 2005, 18, 11S–14S. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Auer, J.; O’Rourke, M.F.; Kvas, E.; Lassnig, E.; Berent, R.; Eber, B. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation 2004, 109, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.J.; Kim, J.U.; Lee, H.J.; Ryu, H.H.; Lee, Y.J.; Kim, J.Y. A clinical study of the pulse wave characteristics at the three pulse diagnosis positions of chon, gwan and cheok. Evid. Based Complement. Altern. Med. 2011. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.Y.; Park, Y.-J.; Park, Y.-B. Development of pulse diagnostic devices in Korea. Integr. Med. Res. 2013, 2, 7–17. [Google Scholar] [CrossRef]
- Alastruey, J.; Passerini, T.; Formaggia, L.; Peiró, J. Physical determining factors of the arterial pulse waveform: Theoretical analysis and calculation using the 1-D formulation. J. Eng. Math. 2012, 77, 19–37. [Google Scholar] [CrossRef]
- Zuo, W.; Wang, P.; Zhang, D. Comparison of Three Different Types of Wrist Pulse Signals by Their Physical Meanings and Diagnosis Performance. IEEE J. Biomed. Health Inform. 2016, 20, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.L.; Chiu, W.B.; Jan, M.Y.; Bau, J.G.; Li, S.P.; Wang, W.K. Analysis of transverse wave as a propagation mode for the pressure pulse in large arteries. J. Appl. Phys. 2007, 102, 064702. [Google Scholar] [CrossRef]
- Bae, J.H.; Jeon, Y.J.; Kim, J.Y.; Kim, J.U. New assessment model of pulse depth based on sensor displacement in pulse diagnostic devices. Evid. Based Complement. Altern. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Tyan, C.C. Quantitative analysis of sensor for pressure waveform measurement. Biomed. Eng. Online 2010, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Nam, D.H.; Hong, Y.S.; Lee, W.B.; Son, I.H.; Kim, K.H.; Choi, J.G. Measurement of blood pressure using an arterial pulsimeter equipped with a Hall device. Sensors 2011, 11, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-S.; Chung, Y.-F.; Yeh, C.-C.; Luo, C.H. Temporal and spatial properties of arterial pulsation measurement using pressure sensor array. Evid. Based Complement. Altern. Med. 2012, 2012, 745127. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.K.; Shin, K.Y.; Lee, T.B.; Jin, S.O.; Kim, J.U. Development of a radial pulse tonometric (RPT) sensor with a temperature compensation mechanism. Sensors 2013, 13, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.U.; Jeon, Y.J.; Kim, Y.M.; Lee, H.J.; Kim, J.Y. Novel diagnostic model for the deficient and excess pulse qualities. Evid. Based Complement. Altern. Med. 2012, 2012, 563958. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.U.; Jeon, Y.J.; Lee, Y.J.; Kim, K.H.; Kim, J.Y. Novel diagnostic algorithm for the floating and sunken pulse qualities and its clinical test. Evid. Based Complement. Altern. Med. 2011, 2011, 813427. [Google Scholar] [CrossRef] [PubMed]



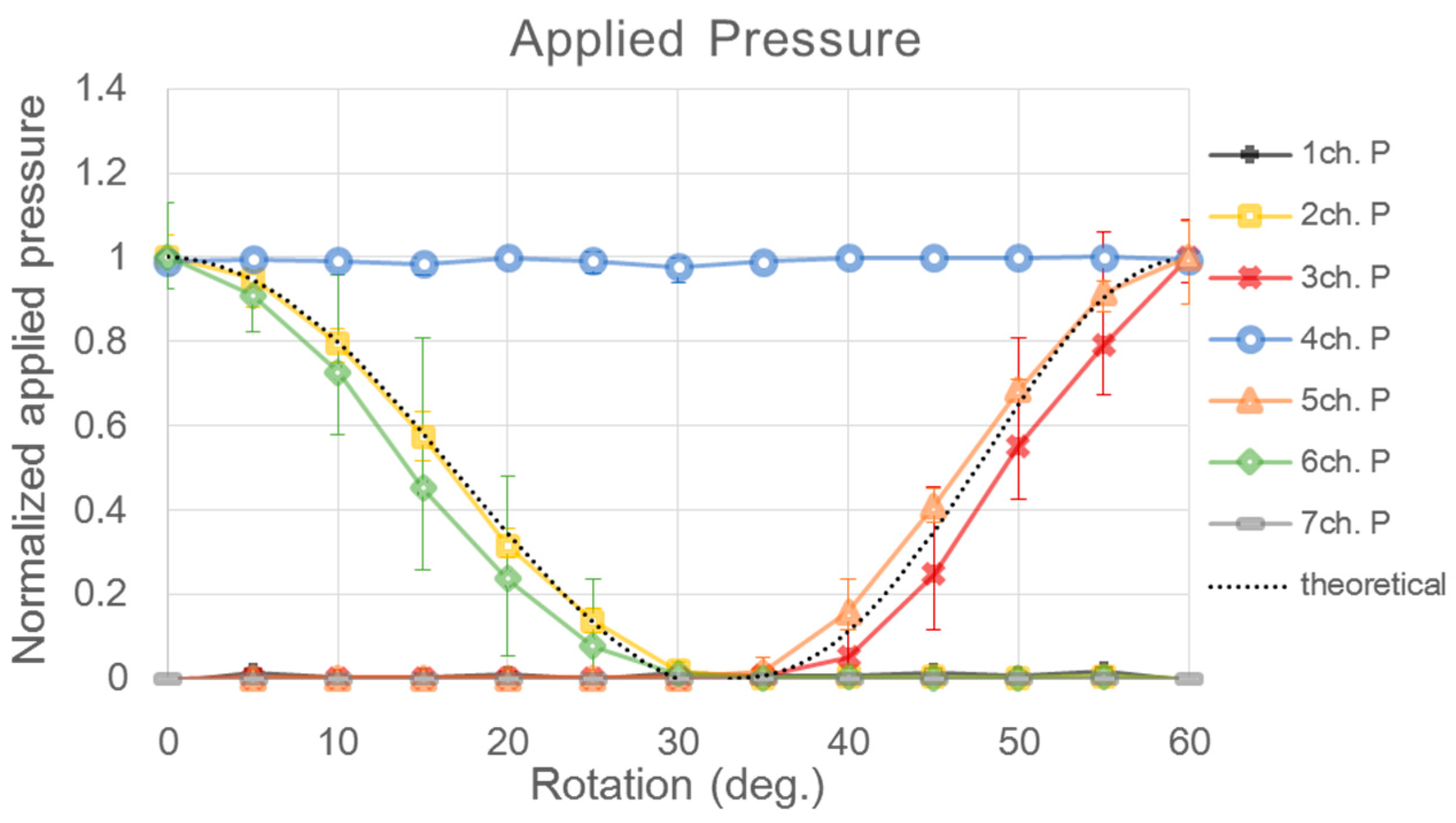
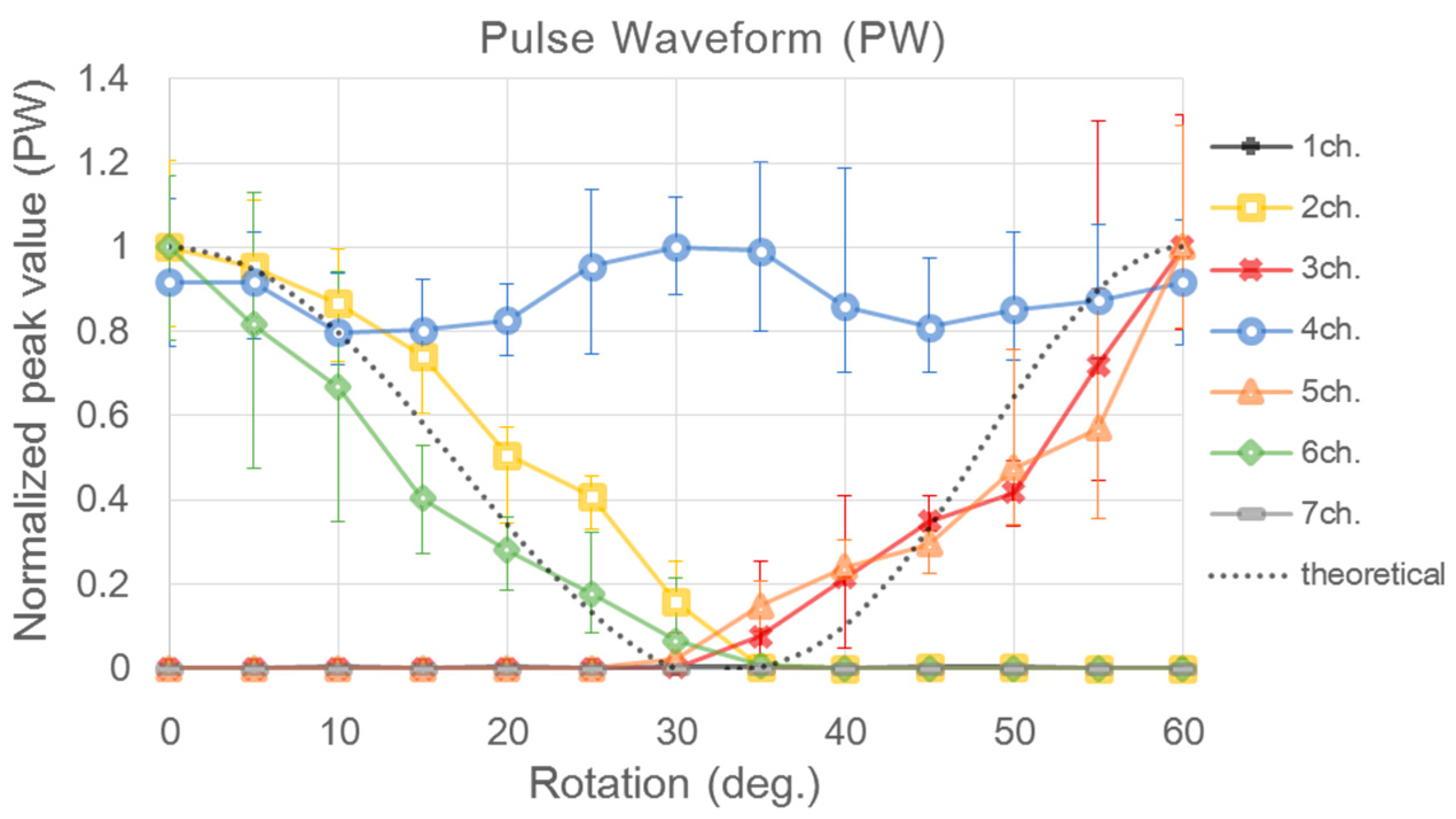
| Sensor | CH1 | CH2 | CH3 | CH4 | CH5 | CH6 | CH7 | |
|---|---|---|---|---|---|---|---|---|
| Sensor #1 | Sensitivity (V/gf) | 0.32294 | 0.37252 | 0.36359 | 0.35303 | 0.32533 | 0.31089 | 0.32765 |
| CV (%) | 0.57954 | 0.03916 | 0.73674 | 0.48756 | 0.05013 | 3.05291 | 0.88291 | |
| Sensor #2 | Sensitivity (V/gf) | 0.32178 | 0.32949 | 0.33023 | 0.35977 | 0.35673 | 0.3545 | 0.34215 |
| CV (%) | 2.38609 | 0.22454 | 0.40759 | 0.04822 | 0.08609 | 0.06177 | 0.13737 | |
| Sensor #3 | Sensitivity (V/gf) | 0.32415 | 0.35095 | 0.32455 | 0.3523 | 0.36408 | 0.32426 | 0.348 |
| CV (%) | 0.22802 | 0.177 | 0.05883 | 0.07506 | 0.45001 | 0.21664 | 0.24718 | |
| Sensor #4 | Sensitivity (V/gf) | 0.33615 | 0.32551 | 0.25834 | 0.31423 | 0.30729 | 0.3162 | 0.3162 |
| CV (%) | 0.22001 | 0.05361 | 0.31644 | 0.45993 | 0.43231 | 1.08301 | 0.15042 | |
| Sensor #5 | Sensitivity (V/gf) | 0.33256 | 0.30244 | 0.28766 | 0.33387 | 0.29701 | 0.27744 | 0.29768 |
| CV (%) | 2.69237 | 0.43782 | 1.07538 | 2.37248 | 0.82258 | 0.07627 | 0.13191 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jun, M.-H.; Kim, Y.-M.; Bae, J.-H.; Jung, C.J.; Cho, J.-H.; Jeon, Y.J. Development of a Tonometric Sensor with a Decoupled Circular Array for Precisely Measuring Radial Artery Pulse. Sensors 2016, 16, 768. https://doi.org/10.3390/s16060768
Jun M-H, Kim Y-M, Bae J-H, Jung CJ, Cho J-H, Jeon YJ. Development of a Tonometric Sensor with a Decoupled Circular Array for Precisely Measuring Radial Artery Pulse. Sensors. 2016; 16(6):768. https://doi.org/10.3390/s16060768
Chicago/Turabian StyleJun, Min-Ho, Young-Min Kim, Jang-Han Bae, Chang Jin Jung, Jung-Hee Cho, and Young Ju Jeon. 2016. "Development of a Tonometric Sensor with a Decoupled Circular Array for Precisely Measuring Radial Artery Pulse" Sensors 16, no. 6: 768. https://doi.org/10.3390/s16060768





