A Fast Strategy for Determination of Vitamin B9 in Food and Pharmaceutical Samples Using an Ionic Liquid-Modified Nanostructure Voltammetric Sensor
Abstract
:1. Introduction
2. Experimental Section
2.1. Apparatus and Chemicals
2.2. Preparation of the Electrode
2.3. Preparation of Real Samples
3. Results and Discussion
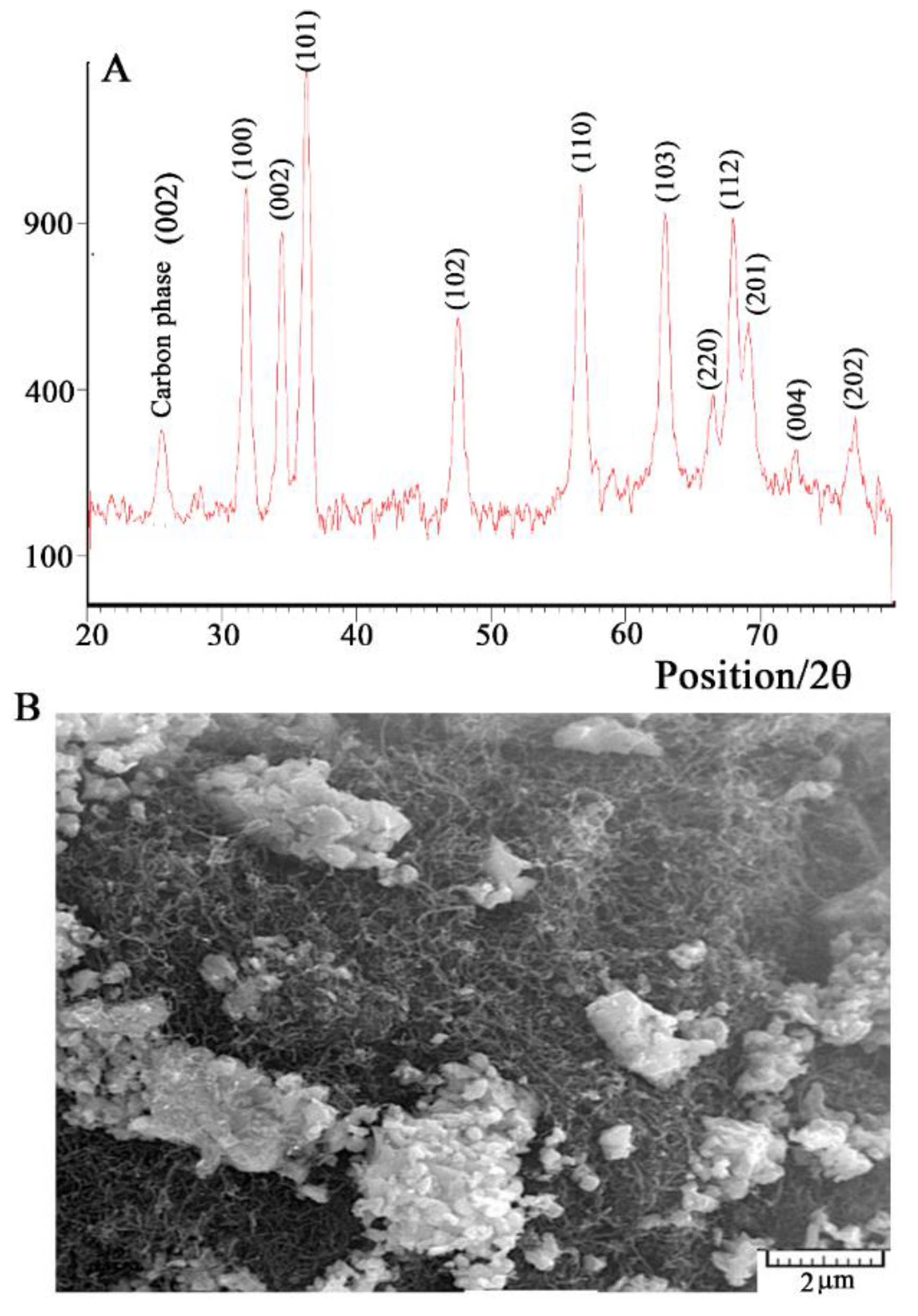
3.1. ZnO/CNTs Characterization
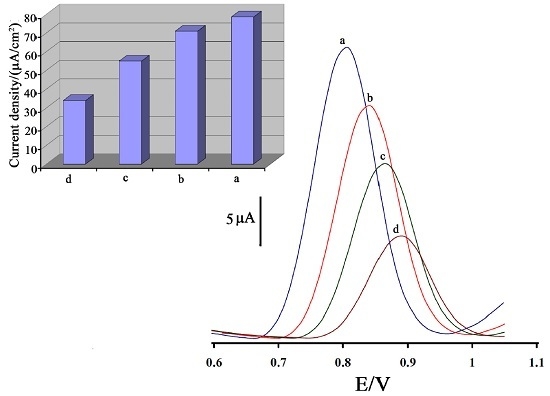
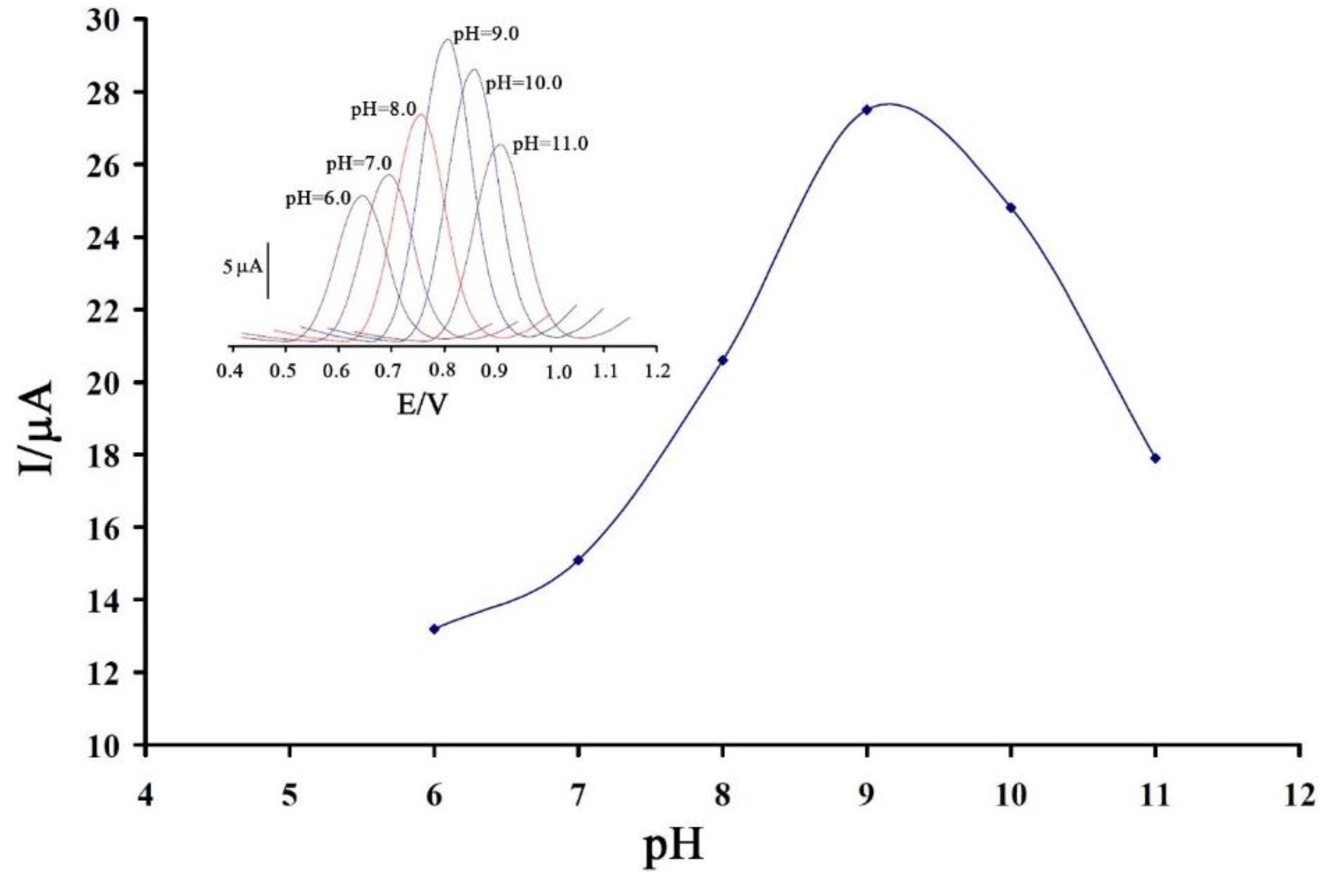
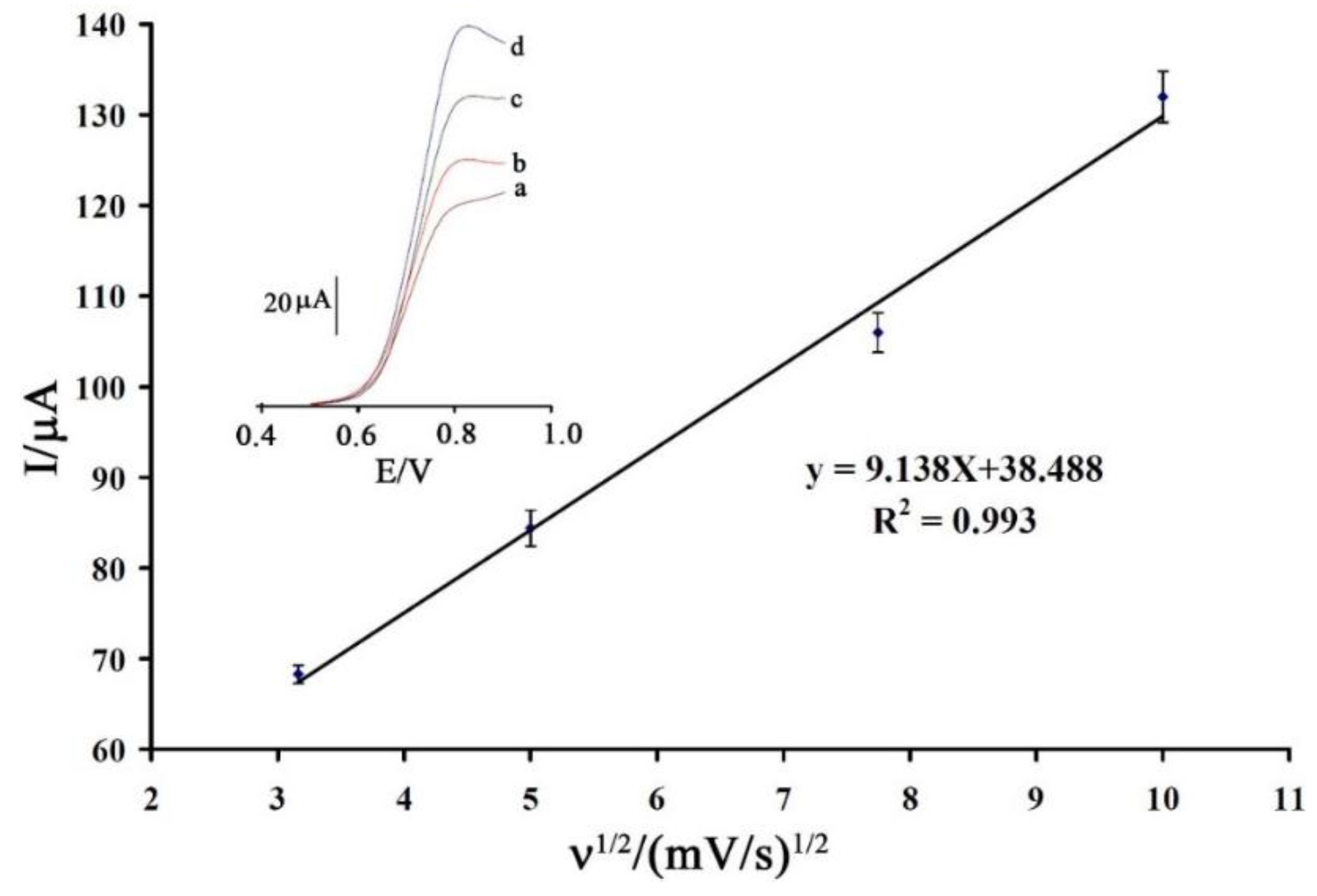
3.2. Voltammetric Investigation
3.3. Analytical Parameters for Determination of Vitamin B9
3.4. Stability and Reproducibility
3.5. Interference Study
3.6. Real Sample Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Karimi-Maleh, H.; Tahernejad-Javazmi, F.; Ensafi, A.A.; Moradi, R.; Mallakpour, S.; Beitollahi, H. A high sensitive biosensor based on fept/cnts nanocomposite/N-(4-hydroxyphenyl)-3,5-dinitrobenzamide modified carbon paste electrode for simultaneous determination of glutathione and piroxicam. Biosens. Bioelectron. 2014, 60, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Biparva, P.; Hatami, M. A novel modified carbon paste electrode based on nio/cnts nanocomposite and (9,10-dihydro-9,10-ethanoanthracene-11,12-dicarboximido)-4-ethylbenzene-1,2-diol as a mediator for simultaneous determination of cysteamine, nicotinamide adenine dinucleotide and folic acid. Biosens. Bioelectron. 2013, 48, 270–275. [Google Scholar] [PubMed]
- Eren, T.; Atar, N.; Yola, M.L.; Karimi-Maleh, H. A sensitive molecularly imprinted polymer based quartz crystal microbalance nanosensor for selective determination of lovastatin in red yeast rice. Food Chem. 2015, 185, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Sanghavi, B.J.; Mobin, S.M.; Mathur, P.; Lahiri, G.K.; Srivastava, A.K. Biomimetic sensor for certain catecholamines employing copper (II) complex and silver nanoparticle modified glassy carbon paste electrode. Biosens. Bioelectron. 2013, 39, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Mobin, S.M.; Sanghavi, B.J.; Srivastava, A.K.; Mathur, P.; Lahiri, G.K. Biomimetic sensor for certain phenols employing a copper (II) complex. Anal. Chem. 2010, 82, 5983–5992. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubian, H.; Karimi-Maleh, H.; Khalilzadeh, M.A.; Karimi, F. Electrocatalytic oxidation of levodopa at a ferrocene modified carbon nanotube paste electrode. Int. J. Electrochem. Sci. 2009, 4, 993–1003. [Google Scholar]
- Raoof, J.B.; Ojani, R.; Karimi-Maleh, H. Carbon paste electrode incorporating 1-[4-(ferrocenyl ethynyl) phenyl]-1-ethanone for electrocatalytic and voltammetric determination of tryptophan. Electroanalysis 2008, 20, 1259–1262. [Google Scholar] [CrossRef]
- Yola, M.L.; Atar, N.; Eren, T.; Karimi-Maleh, H.; Wang, S. Sensitive and selective determination of aqueous triclosan based on gold nanoparticles on polyoxometalate/reduced graphene oxide nanohybrid. RSC Adv. 2015, 5, 65953–65962. [Google Scholar] [CrossRef]
- Atar, N.; Eren, T.; Yola, M.L.; Karimi-Maleh, H.; Demirdögen, B. Magnetic iron oxide and iron oxide@ gold nanoparticle anchored nitrogen and sulfur-functionalized reduced graphene oxide electrocatalyst for methanol oxidation. RSC Adv. 2015, 5, 26402–26409. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Karimi-Maleh, H.; Mallakpour, S.; Hatami, M. Simultaneous determination of N-acetylcysteine and acetaminophen by voltammetric method using N-(3, 4-dihydroxyphenethyl)-3, 5-dinitrobenzamide modified multiwall carbon nanotubes paste electrode. Sens. Actuators B Chem. 2011, 155, 464–472. [Google Scholar] [CrossRef]
- Sanghavi, B.J.; Hirsch, G.; Karna, S.P.; Srivastava, A.K. Potentiometric stripping analysis of methyl and ethyl parathion employing carbon nanoparticles and halloysite nanoclay modified carbon paste electrode. Anal. Chim. Acta 2012, 735, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Sanghavi, B.J.; Sitaula, S.; Griep, M.H.; Karna, S.P.; Ali, M.F.; Swami, N.S. Real-time electrochemical monitoring of adenosine triphosphate in the picomolar to micromolar range using graphene-modified electrodes. Anal. Chem. 2013, 85, 8158–8165. [Google Scholar] [CrossRef] [PubMed]
- Moradi, R.; Sebt, S.; Karimi-Maleh, H.; Sadeghi, R.; Karimi, F.; Bahari, A.; Arabi, H. Synthesis and application of FePt/CNTs nanocomposite as a sensor and novel amide ligand as a mediator for simultaneous determination of glutathione, nicotinamide adenine dinucleotide and tryptophan. Phys. Chem. Chem. Phys. 2013, 15, 5888–5897. [Google Scholar] [CrossRef] [PubMed]
- Shahmiri, M.R.; Bahari, A.; Karimi-Maleh, H.; Hosseinzadeh, R.; Mirnia, N. Ethynylferrocene-NiO/MWCNT nanocomposite modified carbon paste electrode as a novel voltammetric sensor for simultaneous determination of glutathione and acetaminophen. Sens. Actuators B Chem. 2013, 177, 70–77. [Google Scholar] [CrossRef]
- Afsharmanesh, E.; Karimi-Maleh, H.; Pahlavan, A.; Vahedi, J. Electrochemical behavior of morphine at zno/cnt nanocomposite room temperature ionic liquid modified carbon paste electrode and its determination in real samples. J. Mol. Liq. 2013, 181, 8–13. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Karimi-Maleh, H. Modified multiwall carbon nanotubes paste electrode as a sensor for simultaneous determination of 6-thioguanine and folic acid using ferrocenedicarboxylic acid as a mediator. J. Electroanal. Chem. 2010, 640, 75–83. [Google Scholar] [CrossRef]
- Bavandpour, R.; Karimi-Maleh, H.; Asif, M.; Gupta, V.K.; Atar, N.; Abbasghorbani, M. Liquid phase determination of adrenaline uses a voltammetric sensor employing CuFe2O4 nanoparticles and room temperature ionic liquids. J. Mol. Liq. 2016, 213, 369–373. [Google Scholar] [CrossRef]
- Beitollah, H.; Goodarzian, M.; Khalilzadeh, M.A.; Karimi-Maleh, H.; Hassanzadeh, M.; Tajbakhsh, M. Electrochemical behaviors and determination of carbidopa on carbon nanotubes ionic liquid paste electrode. J. Mol. Liq. 2012, 173, 137–143. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, Y.; Zhan, D.; Liu, H.; Zhao, Q.; Kou, Y.; Shao, Y.; Li, M.; Zhuang, Q.; Zhu, Z. Selective detection of dopamine in the presence of ascorbic acid and uric acid by a carbon nanotubes-ionic liquid gel modified electrode. Talanta 2005, 66, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Tavana, T.; Khalilzadeh, M.A.; Karimi-Maleh, H.; Ensafi, A.A.; Beitollahi, H.; Zareyee, D. Sensitive voltammetric determination of epinephrine in the presence of acetaminophen at a novel ionic liquid modified carbon nanotubes paste electrode. J. Mol. Liq. 2012, 168, 69–74. [Google Scholar] [CrossRef]
- Elyasi, M.; Khalilzadeh, M.A.; Karimi-Maleh, H. High sensitive voltammetric sensor based on Pt/CNTs nanocomposite modified ionic liquid carbon paste electrode for determination of Sudan I in food samples. Food Chem. 2013, 141, 4311–4317. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Khalilzadeh, M.A.; Karimi-Maleh, H. A new strategy for determination of bisphenol A in the presence of Sudan I using a ZnO/CNTs/ionic liquid paste electrode in food samples. Food Chem. 2014, 158, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yang, M.; Jiao, K. Electrocatalytic oxidation of dopamine at an ionic liquid modified carbon paste electrode and its analytical application. Anal. Bioanal. Chem. 2007, 389, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Safavi, A.; Maleki, N.; Moradlou, O.; Tajabadi, F. Simultaneous determination of dopamine, ascorbic acid, and uric acid using carbon ionic liquid electrode. Anal. Biochem. 2006, 359, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Bijad, M.; Karimi-Maleh, H.; Khalilzadeh, M.A. Application of ZnO/CNTs nanocomposite ionic liquid paste electrode as a sensitive voltammetric sensor for determination of ascorbic acid in food samples. Food Anal. Methods 2013, 6, 1639–1647. [Google Scholar] [CrossRef]
- Baghizadeh, A.; Karimi-Maleh, H.; Khoshnama, Z.; Hassankhani, A.; Abbasghorbani, M. A voltammetric sensor for simultaneous determination of vitamin C and vitamin B6 in food samples using ZrO2 nanoparticle/ionic liquids carbon paste electrode. Food Anal. Methods 2015, 8, 549–557. [Google Scholar] [CrossRef]
- Arabali, V.; Ebrahimi, M.; Abbasghorbani, M.; Gupta, V.K.; Farsi, M.; Ganjali, M.; Karimi, F. Electrochemical determination of vitamin C in the presence of NADH using a CdO nanoparticle/ionic liquid modified carbon paste electrode as a sensor. J. Mol. Liq. 2016, 213, 312–316. [Google Scholar] [CrossRef]
- Shayeh, J.S.; Ehsani, A.; Ganjali, M.; Norouzi, P.; Jaleh, B. Conductive polymer/reduced graphene oxide/Au nanoparticles as efficient composite materials in electrochemical supercapacitors. Appl. Surf. Sci. 2015, 353, 594–599. [Google Scholar] [CrossRef]
- Tizfahm, J.; Aghazadeh, M.; Maragheh, M.G.; Ganjali, M.R.; Norouzi, P.; Faridbod, F. Electrochemical preparation and evaluation of the supercapacitive performance of MnO2 nanoworms. Mater. Lett. 2016, 167, 153–156. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Maragheh, M.G.; Ganjali, M.R.; Norouzi, P.; Faridbod, F. Electrochemical preparation of MnO2 nanobelts through pulse base-electrogeneration and evaluation of their electrochemical performance. Appl. Surf. Sci. 2016, 364, 141–147. [Google Scholar] [CrossRef]
- Naderi, H.R.; Norouzi, P.; Ganjali, M.R. Electrochemical study of a novel high performance supercapacitor based on MnO2/nitrogen-doped graphene nanocomposite. Appl. Surf. Sci. 2016, 366, 552–560. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Larijani, B.; Pourbasheer, E. Fabrication of an all solid state (ASS) polymeric membrane sensor (PME) for tramadol and its application. Int. J. Electrochem. Sci. 2016, 11, 2119–2129. [Google Scholar]
- Aghazadeh, M.; Asadi, M.; Maragheh, M.G.; Ganjali, M.R.; Norouzi, P.; Faridbod, F. Facile preparation of MnO2 nanorods and evaluation of their supercapacitive characteristics. Appl. Surf. Sci. 2016, 364, 726–731. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Maragheh, M.G.; Ganjali, M.; Norouzi, P. One-step electrochemical preparation and characterization of nanostructured hydrohausmannite as electrode material for supercapacitors. RSC Adv. 2016, 6, 10442–10449. [Google Scholar] [CrossRef]
- Ariga, K.; Yamauchi, Y.; Ji, Q.; Yonamine, Y.; Hill, J.P. Research update: Mesoporous sensor nanoarchitectonics. APL Mater. 2014, 2. [Google Scholar] [CrossRef]
- Nakanishi, W.; Minami, K.; Shrestha, L.K.; Ji, Q.; Hill, J.P.; Ariga, K. Bioactive nanocarbon assemblies: Nanoarchitectonics and applications. Nano Today 2014, 9, 378–394. [Google Scholar] [CrossRef]
- Goyal, R.N.; Gupta, V.K.; Chatterjee, S. Simultaneous determination of adenosine and inosine using single-wall carbon nanotubes modified pyrolytic graphite electrode. Talanta 2008, 76, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.N.; Gupta, V.K.; Chatterjee, S. Voltammetric biosensors for the determination of paracetamol at carbon nanotube modified pyrolytic graphite electrode. Sens. Actuators B Chem. 2010, 149, 252–258. [Google Scholar] [CrossRef]
- Jamali, T.; Karimi-Maleh, H.; Khalilzadeh, M.A. A novel nanosensor based on Pt:Co nanoalloy ionic liquid carbon paste electrode for voltammetric determination of vitamin B9 in food samples. LWT-Food Sci. Technol. 2014, 57, 679–685. [Google Scholar] [CrossRef]
- Ariga, K.; Li, J.; Fei, J.; Ji, Q.; Hill, J.P. Nanoarchitectonics for dynamic functional materials from atomic-/molecular-level manipulation to macroscopic action. Adv. Mater. 2016, 28, 1251–1286. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Ishihara, S.; Abe, H.; Li, M.; Hill, J.P. Materials nanoarchitectonics for environmental remediation and sensing. J. Mater. Chem. 2012, 22, 2369–2377. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Ahanjan, K.; Taghavi, M.; Ghaemy, M. A novel voltammetric sensor employing zinc oxide nanoparticles and a new ferrocene-derivative modified carbon paste electrode for determination of captopril in drug samples. Anal. Methods 2016, 8, 1780–1788. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Tahernejad-Javazmi, F.; Atar, N.; Yola, M.L.T.; Gupta, V.K.; Ensafi, A.A. A novel DNA biosensor based on a pencil graphite electrode modified with polypyrrole/functionalized multiwalled carbon nanotubes for determination of 6-mercaptopurine anticancer drug. Ind. Eng. Chem. Res. 2015, 54, 3634–3639. [Google Scholar] [CrossRef]
- Jafari, S.; Faridbod, F.; Norouzi, P.; Dezfuli, A.S.; Ajloo, D.; Mohammadipanah, F.; Ganjali, M.R. Detection of aeromonas hydrophila DNA oligonucleotide sequence using a biosensor design based on ceria nanoparticles decorated reduced graphene oxide and fast Fourier transform square wave voltammetry. Anal. Chim. Acta 2015, 895, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, T.; Ganjali, M.R.; Akhoundian, M.; Norouzi, P. Voltammetric determination of ultratrace levels of Cerium (III) using a carbon paste electrode modified with nano-sized cerium-imprinted polymer and multiwalled carbon nanotubes. Microchim. Acta 2016, 183, 1123–1130. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Rostami, S.; Gupta, V.K.; Fouladgar, M. Evaluation of ZnO nanoparticle ionic liquid composite as a voltammetric sensing of isoprenaline in the presence of aspirin for liquid phase determination. J. Mol. Liq. 2015, 201, 102–107. [Google Scholar] [CrossRef]
- Cheraghi, S.; Taher, M.A.; Karimi-Maleh, H. A novel strategy for determination of paracetamol in the presence of morphine using a carbon paste electrode modified with CdO nanoparticles and ionic liquids. Electroanalysis 2016, 28, 366–371. [Google Scholar] [CrossRef]
- Atta, N.F.; El-Kady, M.F.; Galal, A. Simultaneous determination of catecholamines, uric acid and ascorbic acid at physiological levels using poly(N-methylpyrrole)/Pd-nanoclusters sensor. Anal. Biochem. 2010, 400, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Atta, N.F.; El-Kady, M.F.; Galal, A. Palladium nanoclusters-coated polyfuran as a novel sensor for catecholamine neurotransmitters and paracetamol. Sens. Actuators B Chem. 2009, 141, 566–574. [Google Scholar] [CrossRef]
- Taherkhani, A.; Jamali, T.; Hadadzadeh, H.; Karimi-Maleh, H.; Beitollahi, H.; Taghavi, M.; Karimi, F. ZnO nanoparticle-modified ionic liquid-carbon paste electrode for voltammetric determination of folic acid in food and pharmaceutical samples. Ionics 2014, 20, 421–429. [Google Scholar] [CrossRef]
- Unnikrishnan, B.; Yang, Y.-L.; Chen, S.-M. Amperometric determination of folic acid at multi-walled carbon nanotube-polyvinyl sulfonic acid composite film modified glassy carbon electrode. Int. J. Electrochem. Sci 2011, 6, 3224–3237. [Google Scholar]
- Xiao, F.; Ruan, C.; Liu, L.; Yan, R.; Zhao, F.; Zeng, B. Single-walled carbon nanotube-ionic liquid paste electrode for the sensitive voltammetric determination of folic acid. Sens. Actuators B Chem. 2008, 134, 895–901. [Google Scholar] [CrossRef]
- Nagaraja, P.; Vasantha, R.A.; Yathirajan, H.S. Spectrophotometric determination of folic acid in pharmaceutical preparations by coupling reactions with iminodibenzyl or 3-aminophenol or sodium molybdate-pyrocatechol. Anal. Biochem. 2002, 307, 316–321. [Google Scholar] [CrossRef]
- Song, Z.; Zhou, X. Chemiluminescence flow sensor for folic acid with immobilized reagents. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 2567–2574. [Google Scholar] [CrossRef]
- Zhao, S.; Yuan, H.; Xie, C.; Xiao, D. Determination of folic acid by capillary electrophoresis with chemiluminescence detection. J. Chromatogr. A 2006, 1107, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Amidžić, R.; Brborić, J.S.; Čudina, O.A.; Vladimirov, S.M. Rp-HPLC determination of vitamins, folic acid and B12 in multivitamin tablets. J. Serb. Chem. Soc. 2005, 70, 1229–1235. [Google Scholar] [CrossRef]
- Sadeghi, R.; Karimi-Maleh, H.; Bahari, A.; Taghavi, M. A novel biosensor based on ZnO nanoparticle/1,3-dipropylimidazolium bromide ionic liquid-modified carbon paste electrode for square-wave voltammetric determination of epinephrine. Phys. Chem. Liq. 2013, 51, 704–714. [Google Scholar] [CrossRef]
- Wan, Q.; Yang, N. The direct electrochemistry of folic acid at a 2-mercaptobenzothiazole self-assembled gold electrode. J. Electroanal. Chem. 2002, 527, 131–136. [Google Scholar] [CrossRef]
- Arvand, M.; Dehsaraei, M. A simple and efficient electrochemical sensor for folic acid determination in human blood plasma based on gold nanoparticles-modified carbon paste electrode. Mater. Sci. Eng. C 2013, 33, 3474–3480. [Google Scholar] [CrossRef] [PubMed]




| Method | Electrode | Modifier | pH | LOD a | LDR b | Reference |
|---|---|---|---|---|---|---|
| DPV c | GCE d | SWCNT/IL e | 5.5 | 0.001 | 0.002–1.0 | [52] |
| Cyclic voltammetry | Au | MBT/SAM f | 7.4 | 0.004 | 0.008–1.0 | [58] |
| SWV | CPE | ZnO/NPs/IL | 9.0 | 0.01 | 0.05–550 | [50] |
| SWV | CPE | Pt:Co nanoalloy/IL | 9.0 | 0.04 | 0.1–500 | [39] |
| SWV | CPE | Gold nanoparticles | 6.5 | 0.0027 | 0.006–80 | [59] |
| SWV | CPE | 1,3-DIBr/ZnO/CNTs | 9.0 | 0.05 | 0.08–650 | This work |
| Species | Tolerance Limits (WSubstance/Wvitamin B9) |
|---|---|
| Glucose, leucine, glycine, methionine, alanine, valine, histidine | 900 |
| Uric acid and ascorbic acid, vitamin B6 | 400 |
| Starch | Saturation |
| Sample | Found (Vitamin B9) Proposed Method (μM) | Found (Vitamin B9) Other Method (μM) | Fex | Ftab | tex | ttab |
|---|---|---|---|---|---|---|
| Tablet | 10.22 ± 0.55 | 9.22 ± 0.65 | 7.8 | 19.0 | 1.5 | 3.8 |
| Mint vegetable | 4.87 ± 0.25 | 5.32 ± 0.35 | 6.7 | 19.0 | 1.1 | 3.8 |
| Orange juice | 14.98 ± 0.75 | 15.78 ± 0.95 | 12.9 | 19.0 | 2.6 | 3.8 |
| Apple juice | 13.42 ± 0.69 | 12.98 ± 0.75 | 8.3 | 19.0 | 1.8 | 3.8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaleghi, F.; Irai, A.E.; Sadeghi, R.; Gupta, V.K.; Wen, Y. A Fast Strategy for Determination of Vitamin B9 in Food and Pharmaceutical Samples Using an Ionic Liquid-Modified Nanostructure Voltammetric Sensor. Sensors 2016, 16, 747. https://doi.org/10.3390/s16060747
Khaleghi F, Irai AE, Sadeghi R, Gupta VK, Wen Y. A Fast Strategy for Determination of Vitamin B9 in Food and Pharmaceutical Samples Using an Ionic Liquid-Modified Nanostructure Voltammetric Sensor. Sensors. 2016; 16(6):747. https://doi.org/10.3390/s16060747
Chicago/Turabian StyleKhaleghi, Fatemeh, Abolfazl Elyasi Irai, Roya Sadeghi, Vinod Kumar Gupta, and Yangping Wen. 2016. "A Fast Strategy for Determination of Vitamin B9 in Food and Pharmaceutical Samples Using an Ionic Liquid-Modified Nanostructure Voltammetric Sensor" Sensors 16, no. 6: 747. https://doi.org/10.3390/s16060747