Reduction of Motion Artifacts and Improvement of R Peak Detecting Accuracy Using Adjacent Non-Intrusive ECG Sensors
Abstract
:1. Introduction
2. Materials and Method
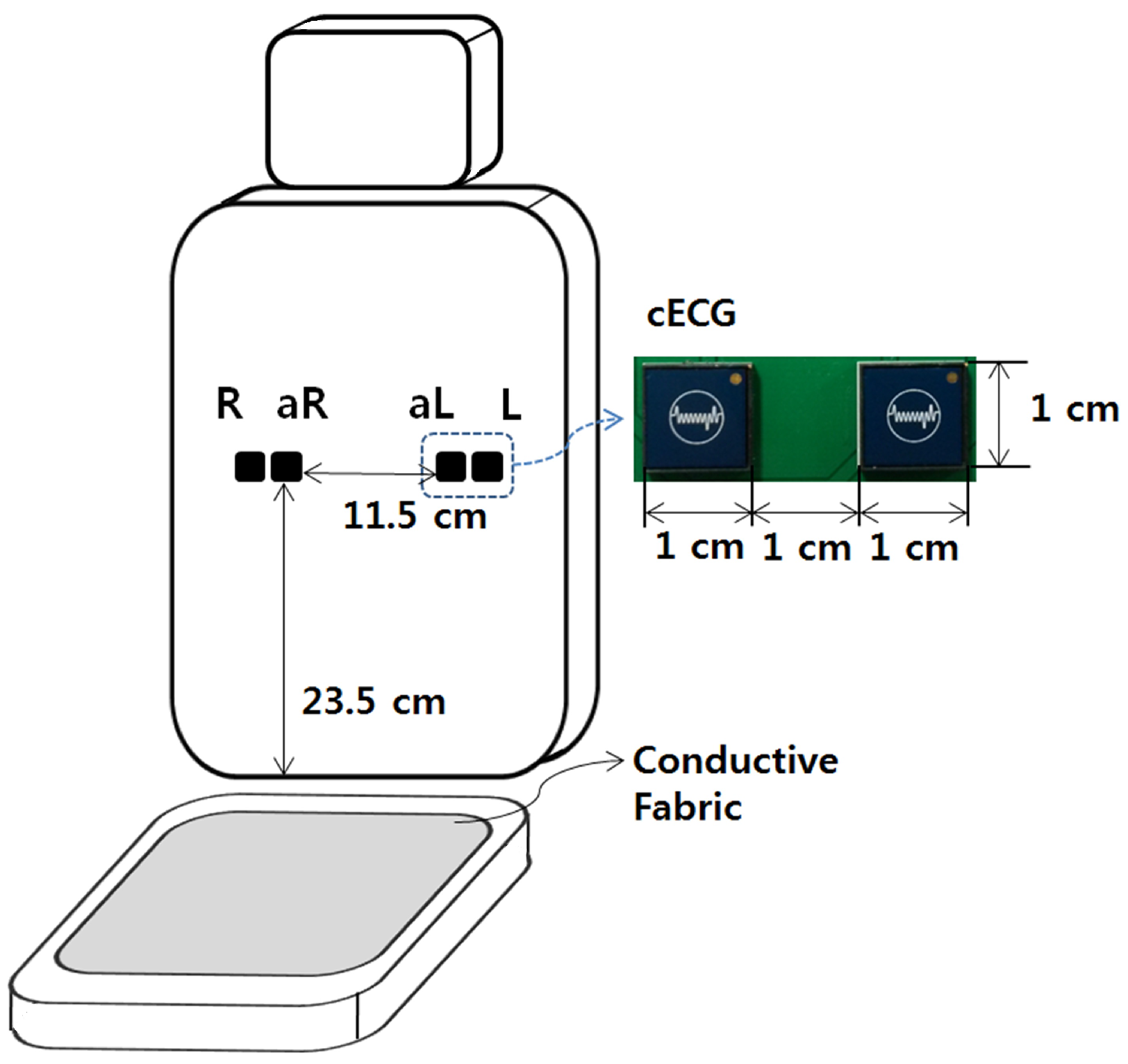
2.1. Measurement System
2.2. Data Acquisition
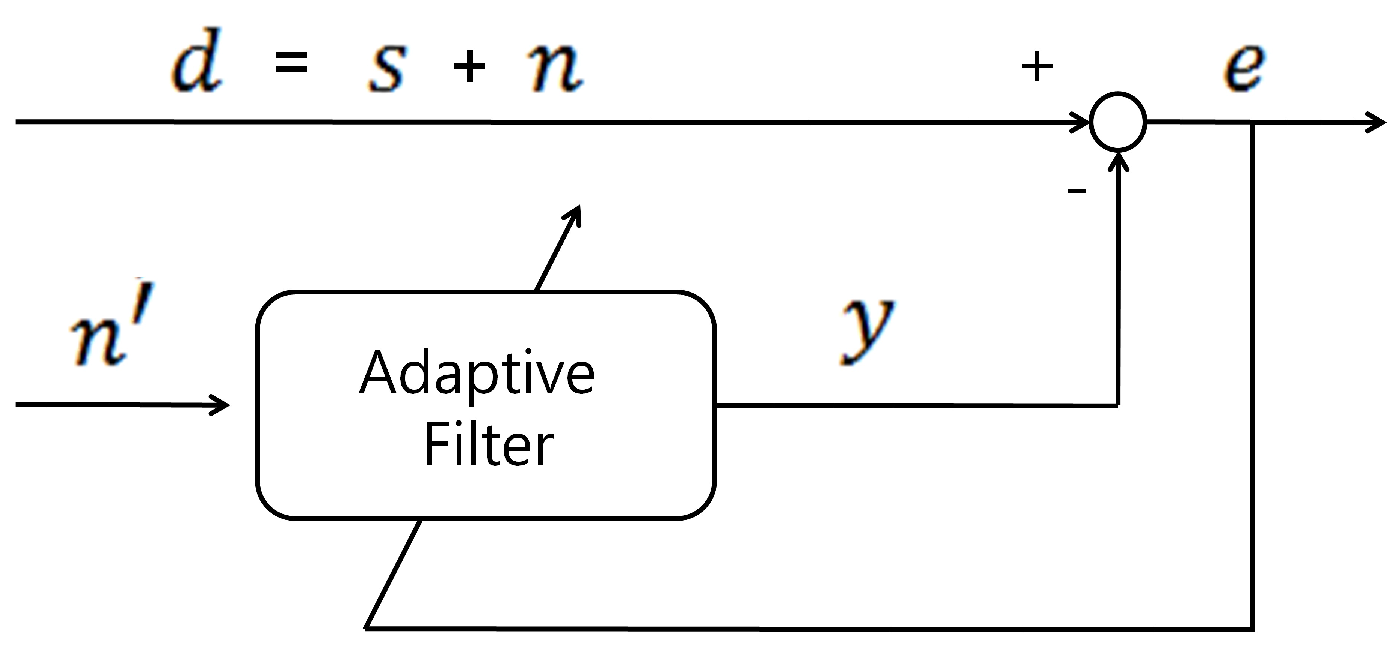
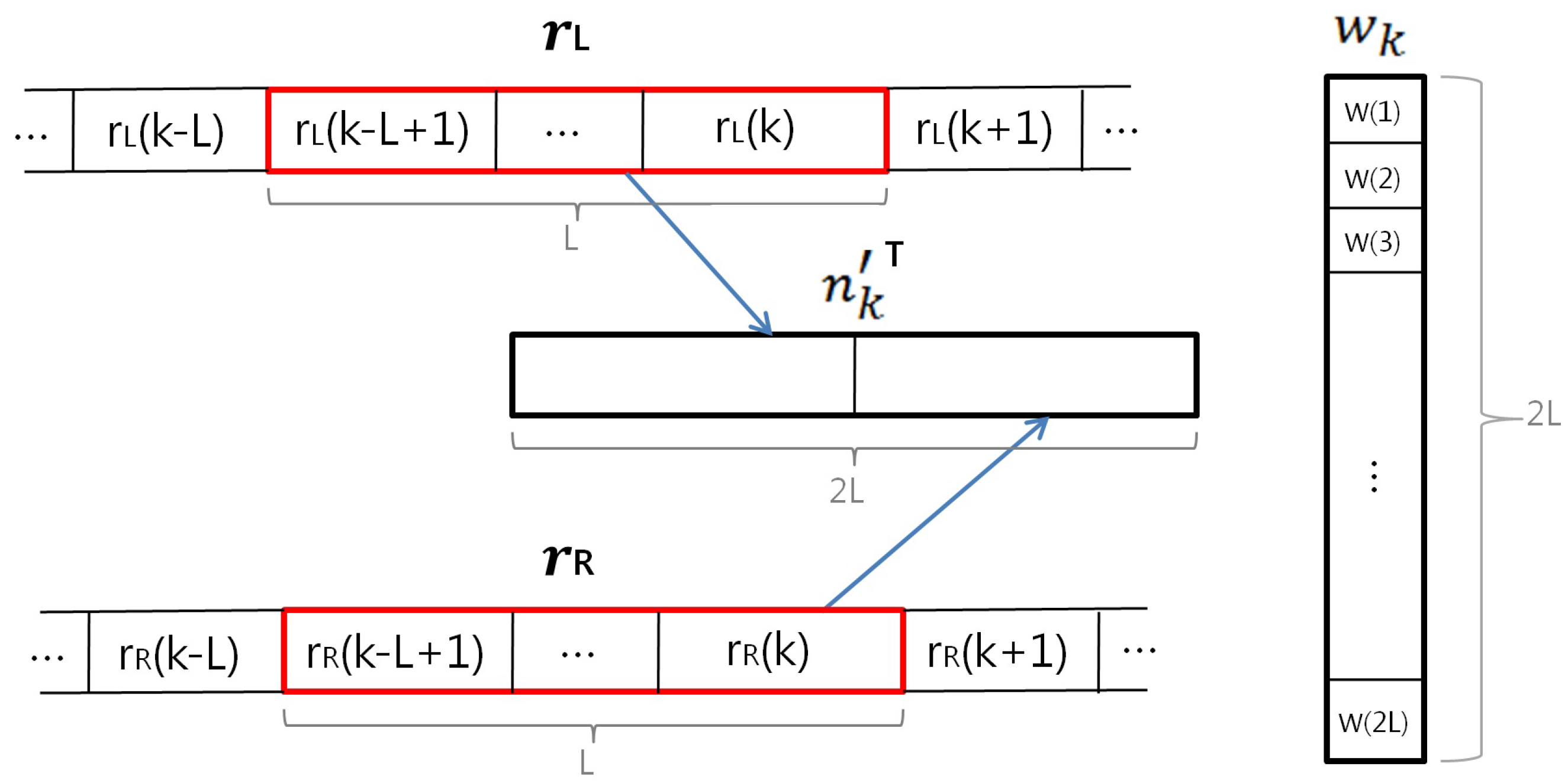
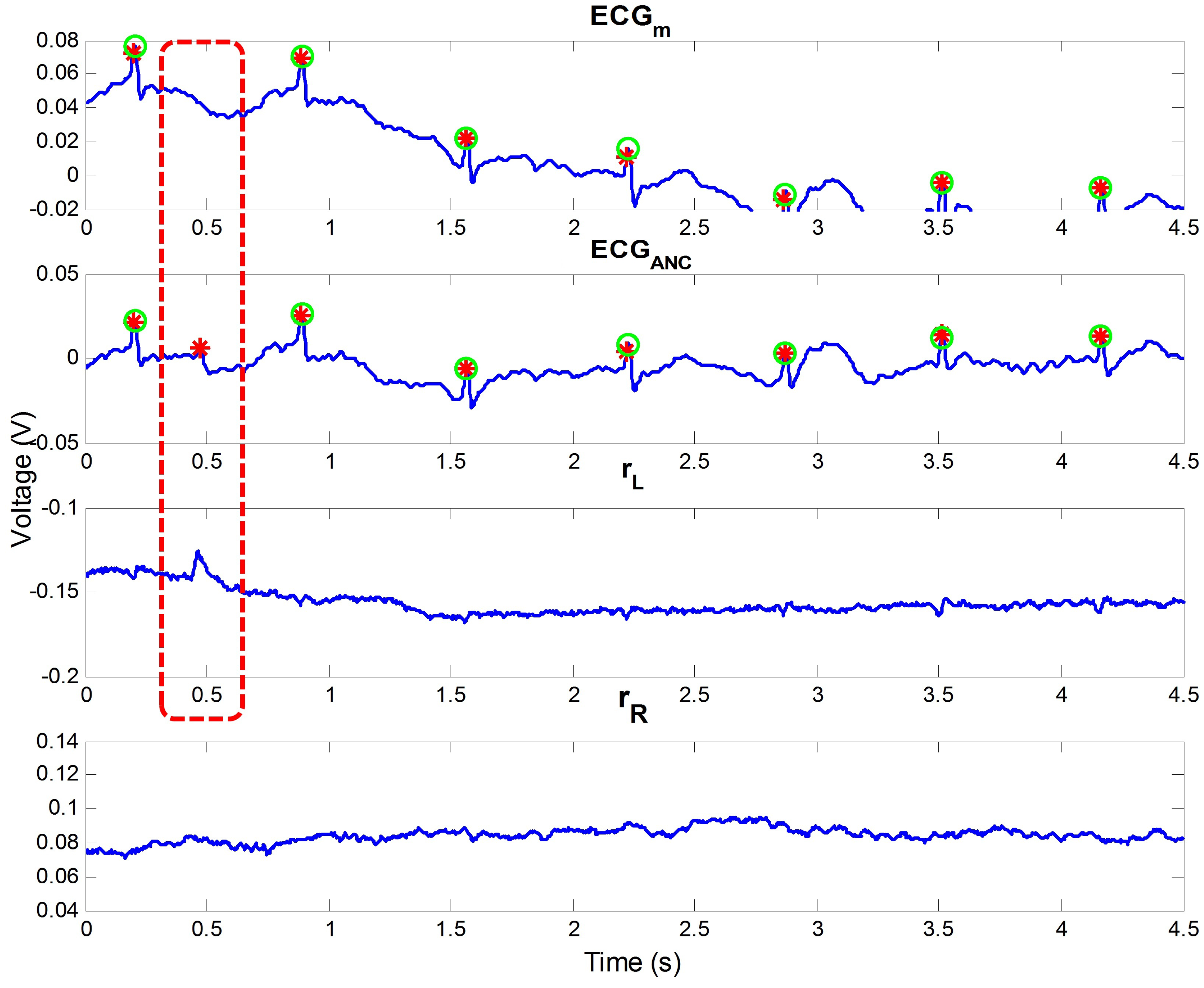
2.3. ANC and Proposed Reference Signal
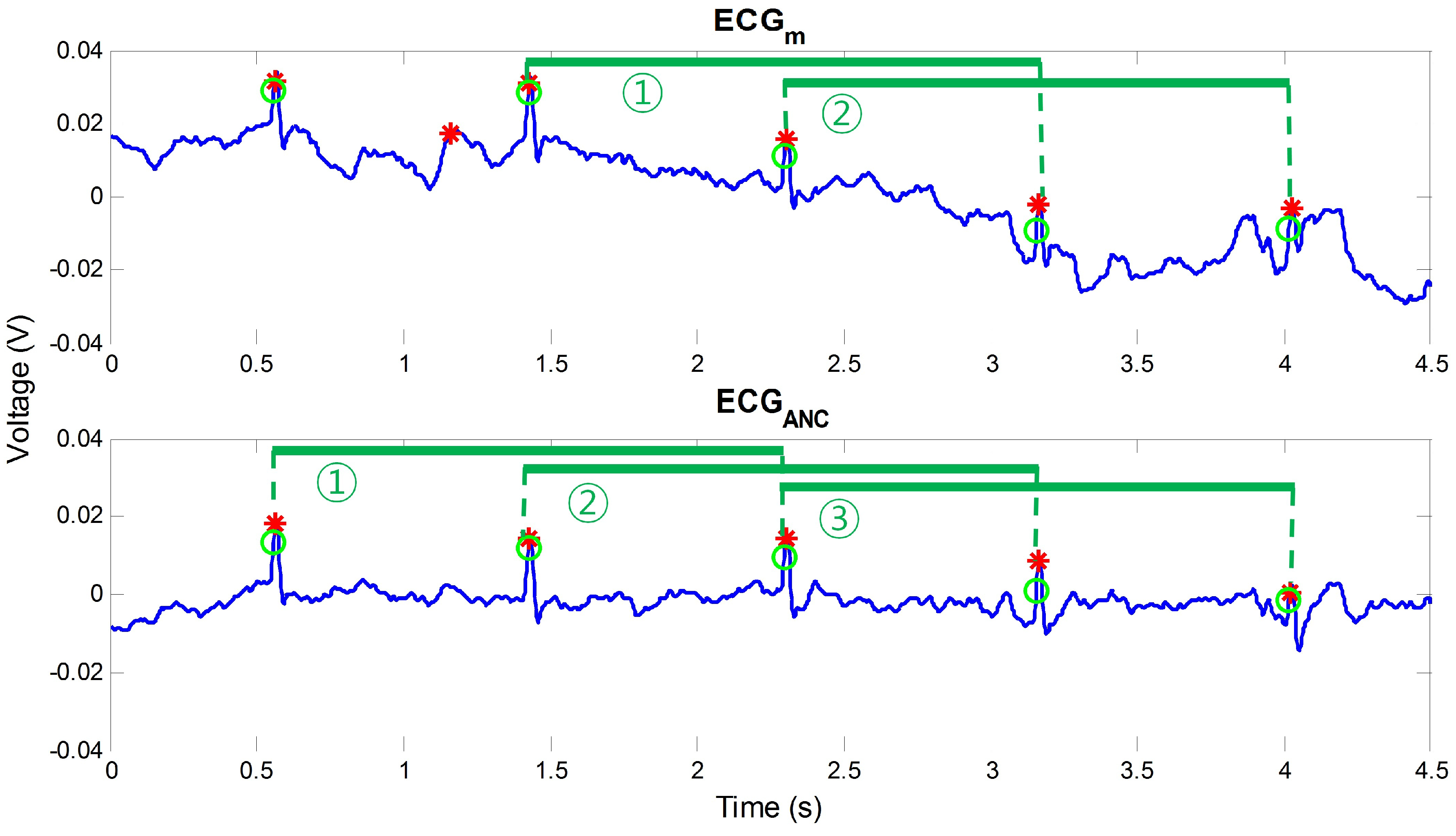
2.4. Post-Processing
3. Experimental Results
3.1. Performance Index
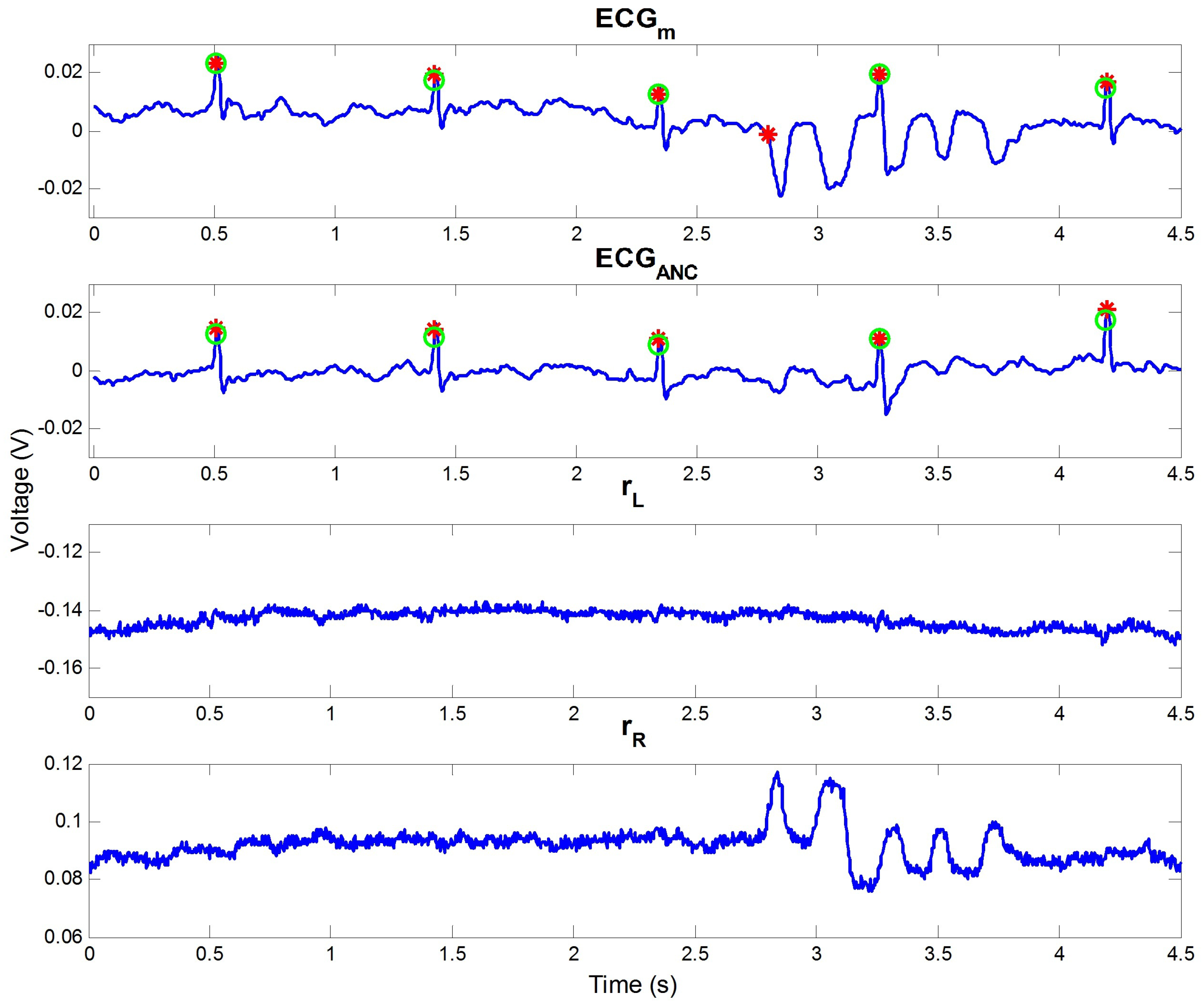
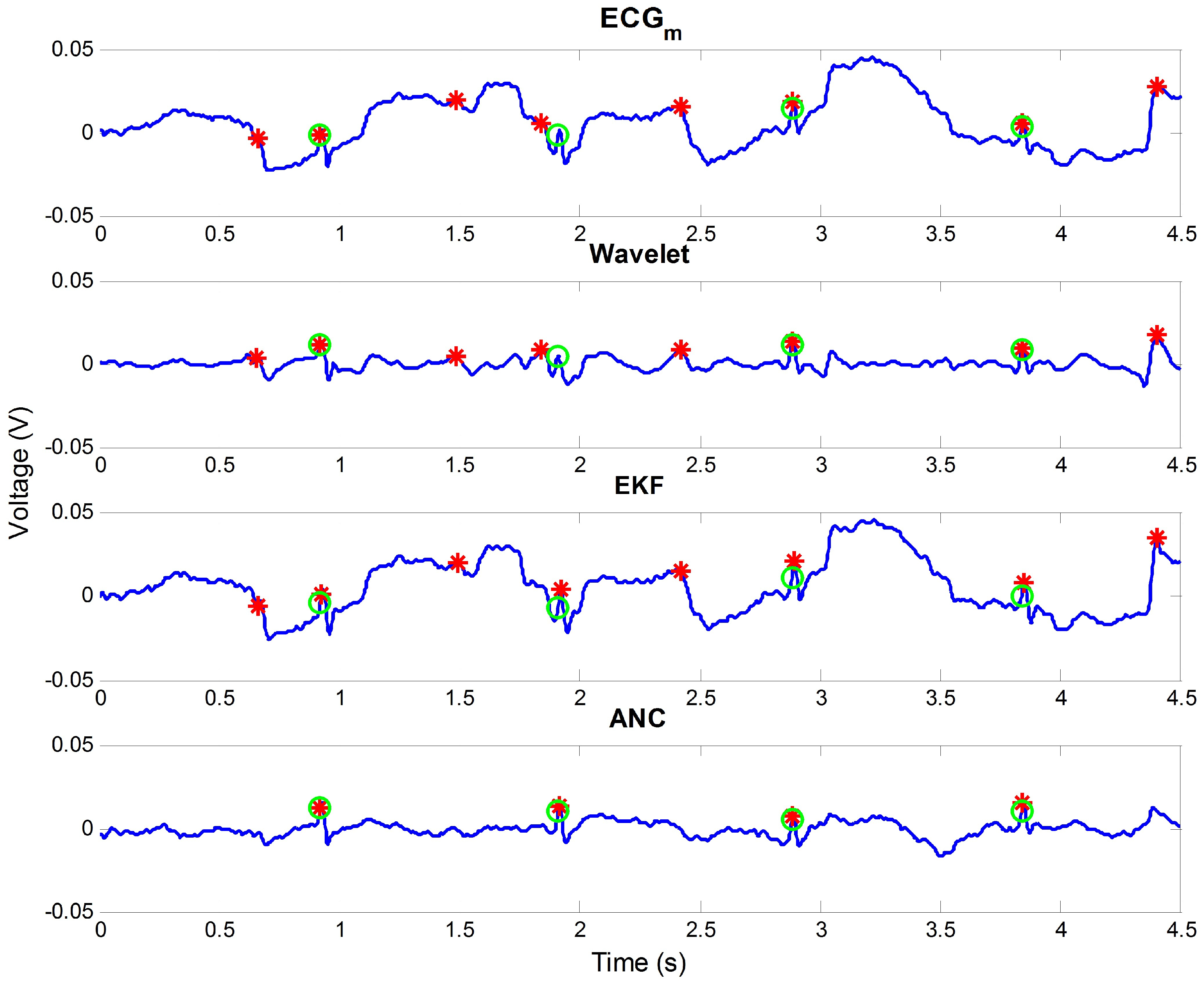
3.2. Comparison of Results
4. Discussion
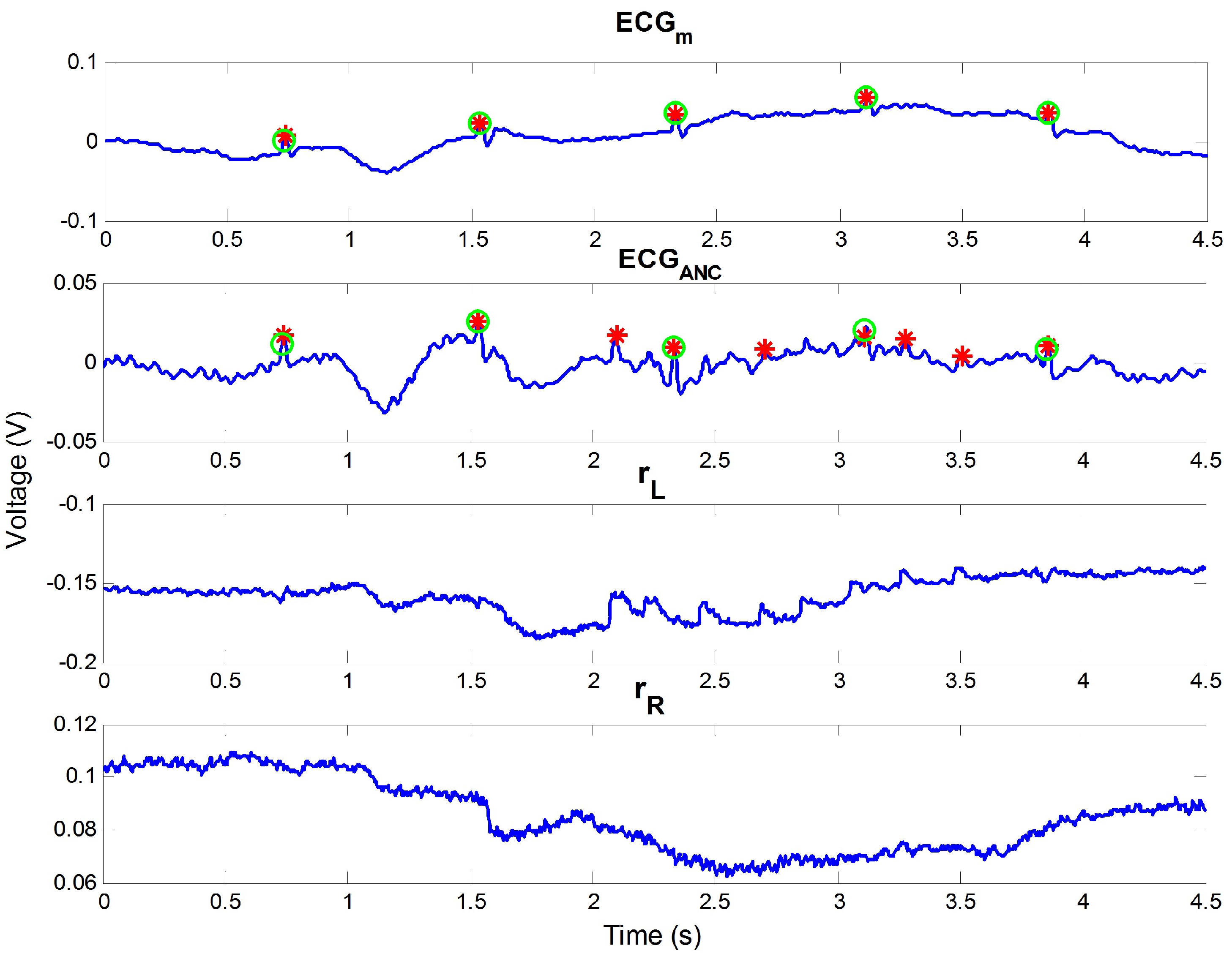
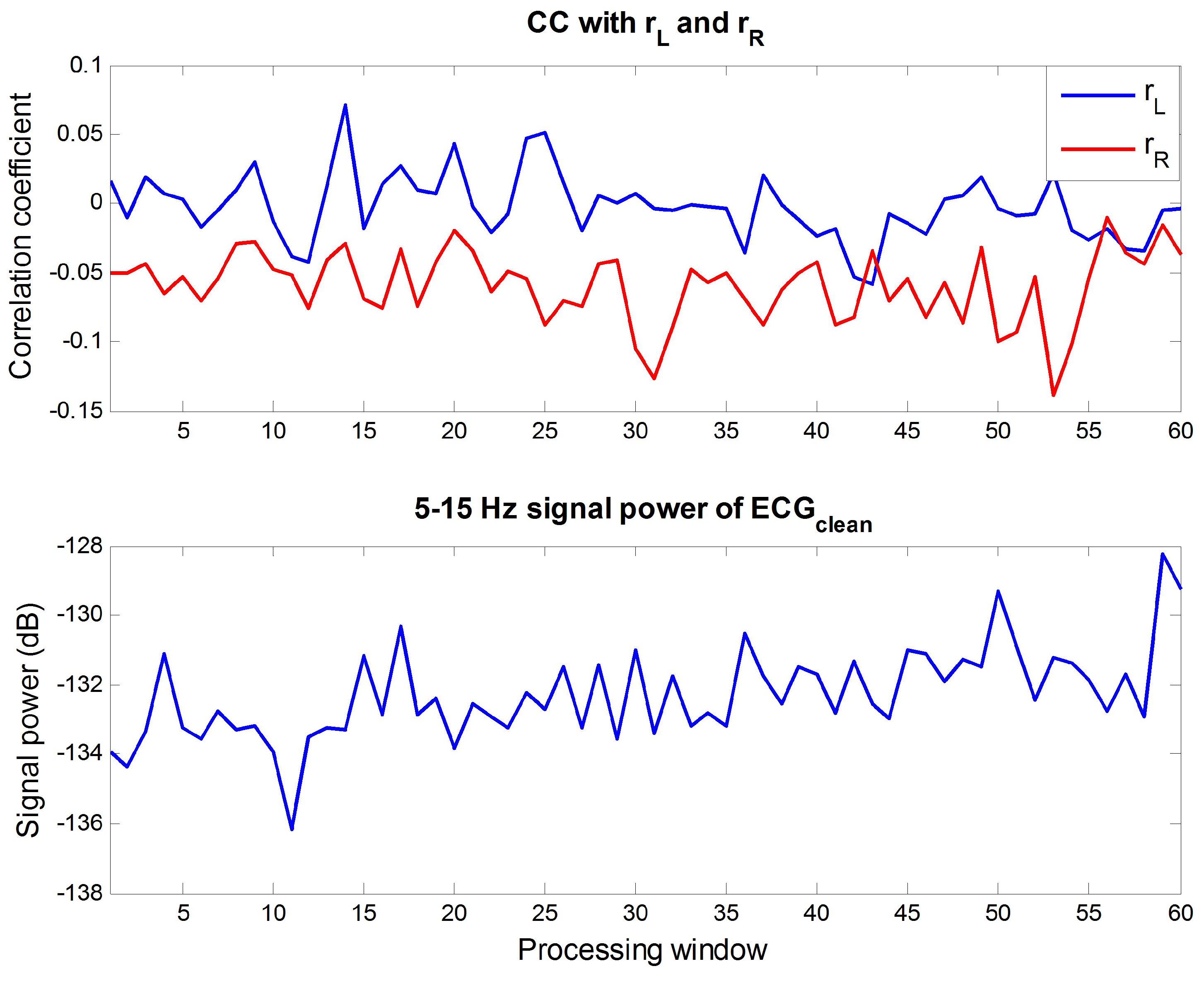
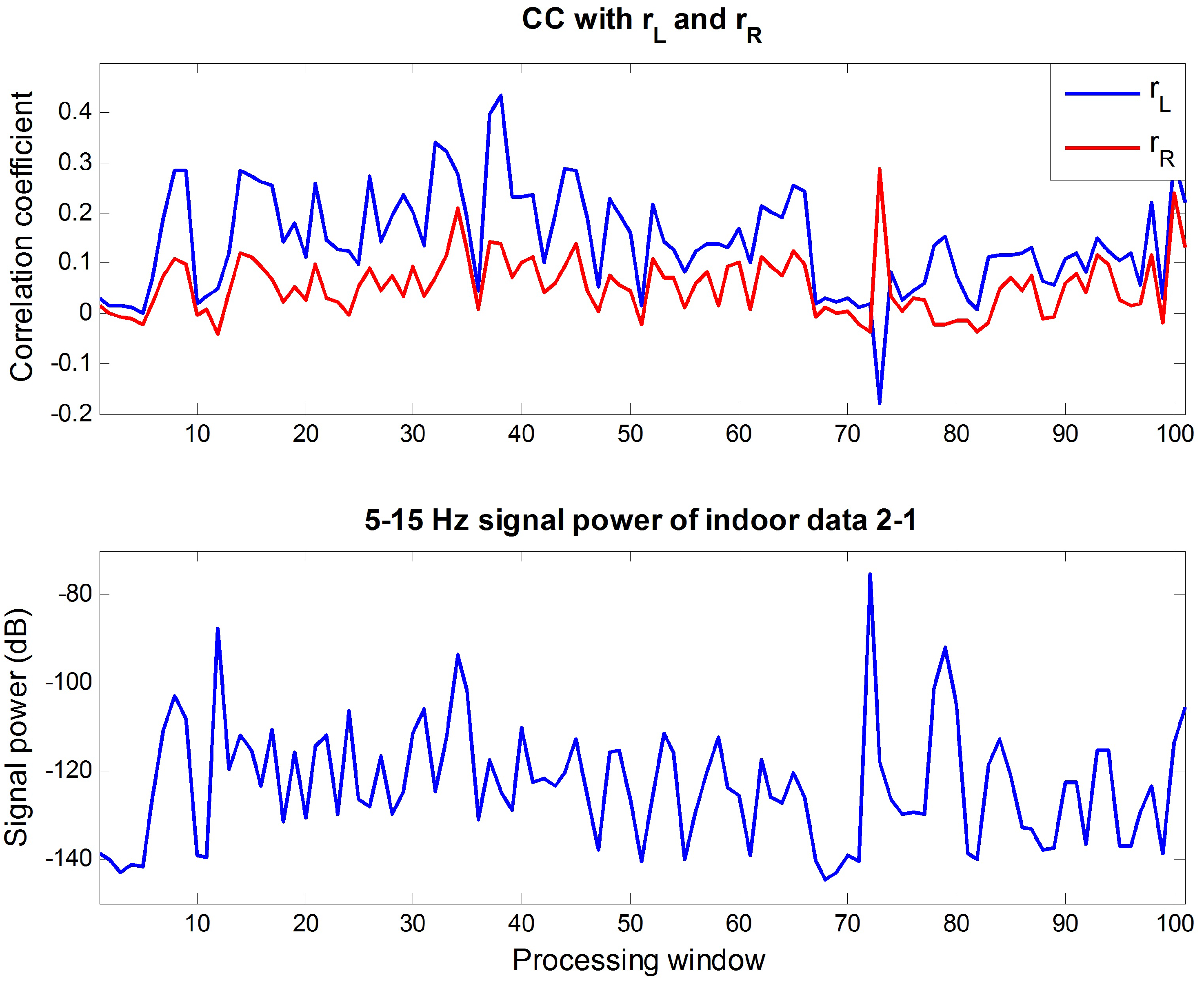
4.1. Analysis on Outdoor Data
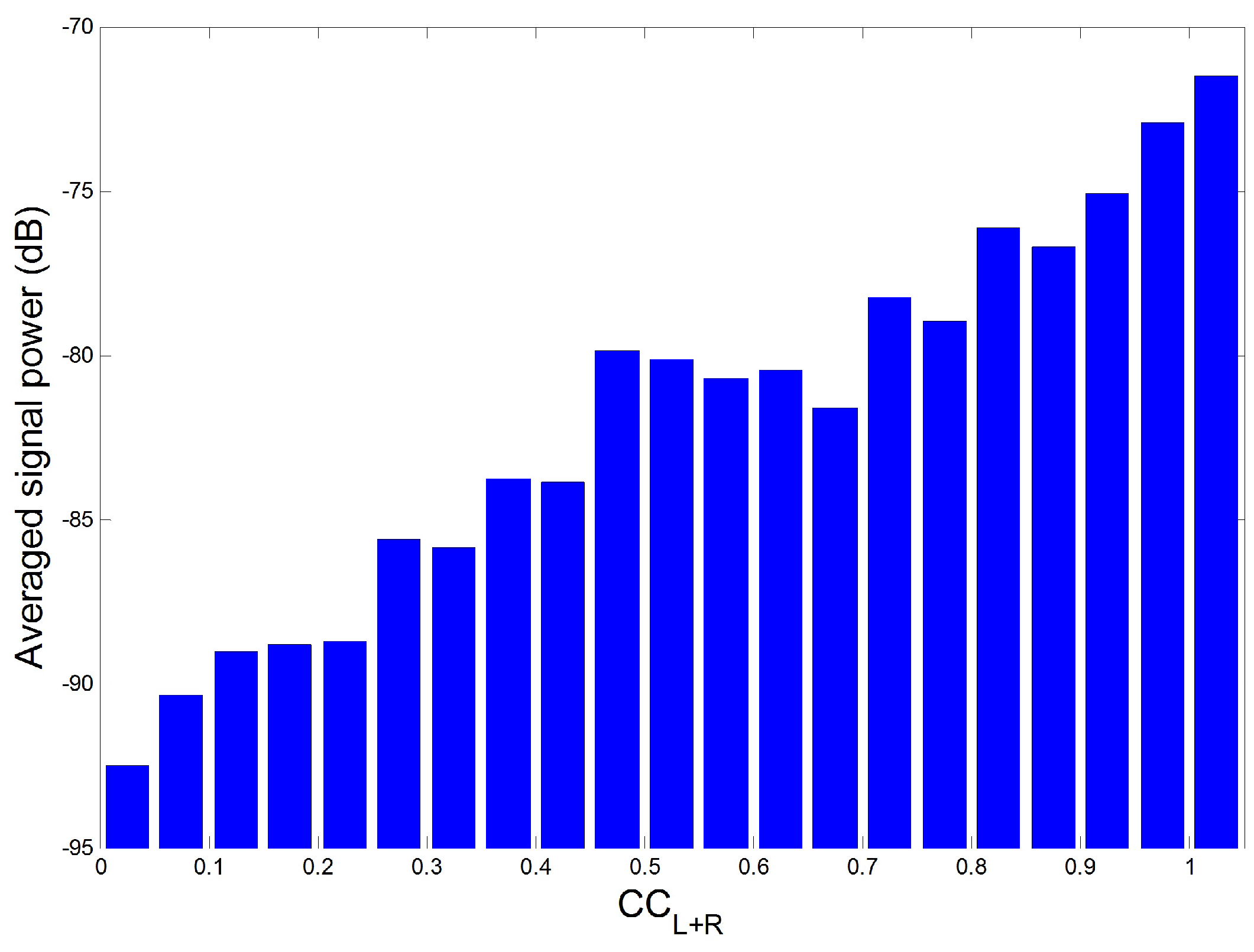
4.2. Appropriateness of Proposed Reference Signal
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Webster, J. Medical Instrumentation: Application and Design, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Kleiger, R.E.; Stein, P.K.; Bigger, J.T. Heart rate variability: Measurement and clinical utility. Ann. Noninvasive Electrocardiol. 2005, 10, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Malik, M. Heart rate variability standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Patel, M.; Lal, S.K.L.; Kavanagh, D.; Rossiter, P. Applying neural network analysis on heart rate variability data to assess driver fatigue. Expert Syst. Appl. 2011, 38, 7235–7242. [Google Scholar] [CrossRef]
- Vicente, J.; Laguna, P.; Bartra, A.; Bailón, R. Drowsiness detection using heart rate variability. Med. Biol. Eng. Comput. 2016, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Heo, J.; Lee, W.K.; Lim, Y.G.; Kim, Y.H.; Park, K.S. Flexible capacitive electrodes for minimizing motion artifacts in ambulatory electrocardiograms. Sensors 2014, 14, 14732–14743. [Google Scholar] [CrossRef] [PubMed]
- Pirbhulal, S.; Zhang, H.; Mukhopadhyay, S.C.; Li, C.; Wang, Y.; Li, G.; Wu, W.; Zhang, Y.-T. An efficient biometric-based algorithm using heart rate variability for securing body sensor networks. Sensors 2015, 15, 15067–15089. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Hwang, S.H.; Yoon, H.N.; Lee, W.K.; Park, K.S. Heart rate variability monitoring during sleep based on capacitively coupled textile electrodes on a bed. Sensors 2015, 15, 11295–11311. [Google Scholar] [CrossRef] [PubMed]
- Fong, E.-M.; Chung, W.-Y. Mobile cloud-computing-based healthcare service by noncontact ecg monitoring. Sensors 2013, 13, 16451–16473. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yu, X.B. An innovative nonintrusive driver assistance system for vital signal monitoring. IEEE J. Biomed. Health Inform. 2014, 18, 1932–1939. [Google Scholar] [CrossRef] [PubMed]
- Wartzek, T.; Eilebrecht, B.; Lem, J.; Lindner, H.-J.; Leonhardt, S.; Walter, M. Ecg on the road: Robust and unobtrusive estimation of heart rate. IEEE Trans. Biomed. Eng. 2011, 58, 3112–3120. [Google Scholar] [CrossRef] [PubMed]
- Nemati, E.; Deen, M.J.; Mondal, T. A wireless wearable ecg sensor for long-term applications. IEEE Commun. Mag. 2012, 50, 36–43. [Google Scholar] [CrossRef]
- Wartzek, T.; Lammersen, T.; Eilebrecht, B.; Walter, M.; Leonhardt, S. Triboelectricity in capacitive biopotential measurements. IEEE Trans. Biomed. Eng. 2011, 58, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Tong, D.A.; Bartels, K.A.; Honeyager, K.S. Adaptive reduction of motion artifact in the electrocardiogram. In Proceedings of the 24th Annual Conference and the Annual Fall Meeting of the Biomedical Engineering Society EMBS/BMES Conference, Houston, TX, USA, 23–26 October 2002; pp. 1403–1404.
- Chen, S.-W.; Chen, Y.-H. Hardware design and implementation of a wavelet de-noising procedure for medical signal preprocessing. Sensors 2015, 15, 26396–26414. [Google Scholar] [CrossRef] [PubMed]
- Wartzek, T.; Bruser, C.; Walter, M.; Leonhardt, S. Robust sensor fusion of unobtrusively measured heart rate. IEEE J. Biomed. Health Inform. 2014, 18, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Romero, I. Pca and ica applied to noise reduction in multi-lead ecg. In Proceedings of the Computing in Cardiology, Hangzhou, China, 18–21 September 2011; pp. 613–616.
- Thakor, N.V.; Zhu, Y.-S. Applications of adaptive filtering to ecg analysis: Noise cancellation and arrhythmia detection. IEEE Trans. Biomed. Eng. 1991, 38, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Yelderman, M.; Widrow, B.; Cioffi, J.M.; Hesler, E.; Leddy, J.A. Ecg enhancement by adaptive cancellation of electrosurgical interference. IEEE Trans. Biomed. Eng. 1983, 7, 392–398. [Google Scholar] [CrossRef]
- Serteyn, A.; Vullings, R.; Meftah, M.; Bergmans, J.W.M. Motion artifacts in capacitive ecg measurements: Reducing the combined effect of dc voltages and capacitance changes using an injection signal. IEEE Trans. Biomed. Eng. 2015, 62, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Sameni, R.; Shamsollahi, M.B.; Jutten, C.; Babaie-Zadeh, M. Filtering noisy ecg signals using the extended kalman filter based on a modified dynamic ecg model. In Proceedings of the Computers in Cardiology, Lyon, France, 25–28 September 2005; pp. 1017–1020.
- Rachim, V.P.; Kang, S.-C.; Chung, W.-Y.; Kwon, T.-H. Implementation of extended kalman filter for real-time noncontact ecg signal acquisition in android-based mobile monitoring system. J. Sens. Sci. Technol. 2014, 23, 7–14. [Google Scholar] [CrossRef]
- Sayed, A.H. Fundamentals of Adaptive Filtering, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Eilebrecht, B.; Wartzek, T.; Willkomm, J.; Schommartz, A.; Walter, M.; Leonhardt, S. Motion artifact removal from capacitive ecg measurements by means of adaptive filtering. In Proceedings of the 5th European Conference of the International Federation for Medical and Biological Engineering, Budapest, Hungary, 14–18 September 2012; pp. 902–905.
- Leonhardt, S.; Aleksandrowicz, A. Non-contact ecg monitoring for automotive application. In Proceedings of the 5th International Summer School and Symposium on Medical Devices and Biosensors, Hong Kong, China, 1–3 June 2008; pp. 183–185.
- Wartzek, T.; Weber, H.; Walter, M.; Eilebrecht, B.; Leonhardt, S. Automatic electrode selection in unobtrusive capacitive ecg measurements. In Proceedings of the 25th International Symposium on Computer-Based Medical Systems (CBMS), Ribeirão Preto, Brazil, 22–25 June 2012; pp. 1–4.
- Winter, B.B.; Webster, J.G. Driven-right-leg circuit design. IEEE Trans. Biomed. Eng. 1983, 1, 62–66. [Google Scholar] [CrossRef]
- Clifford, G.D.; Azuaje, F.; McSharry, P. Advanced Methods and Tools for ECG Data Analysis; Artech House, Inc.: Norwood, MA, USA, 2006. [Google Scholar]
- Pan, J.; Tompkins, W.J. A real-time qrs detection algorithm. IEEE Trans. Biomed. Eng. 1985, 3, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Aquino-Santos, R.; Martinez-Castro, D.; Edwards-Block, A.; Murillo-Piedrahita, A.F. Wireless sensor networks for ambient assisted living. Sensors 2013, 13, 16384–16405. [Google Scholar] [CrossRef] [PubMed]
- Feldhege, F.; Mau-Moeller, A.; Lindner, T.; Hein, A.; Markschies, A.; Zettl, U.K.; Bader, R. Accuracy of a custom physical activity and knee angle measurement sensor system for patients with neuromuscular disorders and gait abnormalities. Sensors 2015, 15, 10734–10752. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.; Hu, S.; Azorin-Peris, V.; Barrett, L.; Esliger, D.; Hayes, M.; Akbare, S.; Achart, J.; Kuoch, S. A multi-channel opto-electronic sensor to accurately monitor heart rate against motion artefact during exercise. Sensors 2015, 15, 25681–25702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Kadi, M.I.; Ibne Reaz, M.B.; Mohd Ali, M.A. Evolution of electroencephalogram signal analysis techniques during anesthesia. Sensors 2013, 13, 6605–6635. [Google Scholar] [CrossRef] [PubMed]
- Widrow, B.; Glover, J.R., Jr.; McCool, J.M.; Kaunitz, J.; Williams, C.S.; Hearn, R.H.; Zeidler, J.R.; Dong, E., Jr.; Goodlin, R.C. Adaptive noise cancelling: Principles and applications. Proc. IEEE 1975, 63, 1692–1716. [Google Scholar] [CrossRef]
- Mateo, J.; Laguna, P. Analysis of heart rate variability in the presence of ectopic beats using the heart timing signal. IEEE Trans. Biomed. Eng. 2003, 50, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Winokur, E.S.; Delano, M.K.; Sodini, C.G. A wearable cardiac monitor for long-term data acquisition and analysis. IEEE Trans. Biomed. Eng. 2013, 60, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Poornachandra, S. Wavelet-based denoising using subband dependent threshold for ecg signals. Digit. Signal Process. 2008, 18, 49–55. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning, 1st ed.; Springer: New York, NY, USA, 2013. [Google Scholar]

| Data | Wavelet | EKF | ANC | After Post-Processing | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-1 | 66.41 | 51.83 | 65.63 | 51.12 | −1.49 | 66.41 | 51.10 | −0.73 | 74.22 | 58.28 | 14.27 | 75.00 | 58.90 | 15.66 | ||||
| 1-2 | 88.51 | 72.84 | 87.53 | 73.36 | −0.45 | 88.26 | 73.08 | 0.00 | 88.26 | 74.43 | 1.35 | 88.75 | 75.00 | 2.41 | ||||
| 1-3 | 84.06 | 72.96 | 83.82 | 73.36 | 0.16 | 85.02 | 74.58 | 2.59 | 84.54 | 75.27 | 2.80 | 85.51 | 75.97 | 4.46 | ||||
| 2-1 | 53.18 | 45.63 | 50.38 | 41.86 | −6.57 | 54.45 | 46.62 | 2.26 | 68.96 | 56.58 | 26.72 | 68.70 | 56.37 | 26.26 | ||||
| 2-2 | 65.59 | 58.89 | 66.09 | 61.81 | 3.41 | 66.09 | 60.68 | 2.29 | 72.52 | 66.44 | 14.48 | 72.77 | 66.37 | 14.66 | ||||
| 2-3 | 65.51 | 58.41 | 64.02 | 56.95 | −2.94 | 67.25 | 58.53 | 1.86 | 69.73 | 60.17 | 5.98 | 69.98 | 61.17 | 7.23 | ||||
| 3-1 | 84.84 | 72.59 | 84.11 | 72.73 | −0.60 | 85.82 | 73.28 | 1.66 | 90.95 | 80.87 | 14.39 | 90.95 | 81.40 | 14.92 | ||||
| 3-2 | 89.39 | 78.32 | 89.39 | 78.49 | 0.17 | 89.14 | 79.15 | 0.58 | 92.17 | 79.52 | 3.98 | 91.67 | 79.61 | 3.56 | ||||
| 3-3 | 93.35 | 85.61 | 93.09 | 84.95 | −0.92 | 94.15 | 86.55 | 1.74 | 92.82 | 85.12 | −1.02 | 93.09 | 86.42 | 0.54 | ||||
| 4-1 | 86.39 | 72.56 | 85.15 | 71.97 | −1.83 | 87.13 | 74.11 | 2.29 | 86.88 | 75.16 | 3.10 | 87.87 | 75.85 | 4.78 | ||||
| 4-2 | 64.57 | 54.33 | 64.32 | 55.05 | 0.47 | 66.33 | 54.32 | 1.75 | 71.61 | 59.25 | 11.95 | 71.61 | 58.88 | 11.59 | ||||
| 4-3 | 92.33 | 81.73 | 92.59 | 82.35 | 0.88 | 93.39 | 82.48 | 1.80 | 91.80 | 82.42 | 0.16 | 92.86 | 82.01 | 0.81 | ||||
| Average | 77.84 | 67.14 | 77.18 | 67.00 | −0.81 | 78.62 | 67.87 | 1.51 | 82.04 | 71.13 | 8.18 | 82.40 | 71.49 | 8.91 | ||||
| Data | Wavelet | EKF | ANC | After Post-Processing | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 94.27 | 87.52 | 94.19 | 88.07 | 0.48 | 94.81 | 87.64 | 0.66 | 92.74 | 85.37 | −3.67 | 94.27 | 88.21 | 0.69 | ||||
| 2 | 90.14 | 88.65 | 90.14 | 88.79 | 0.14 | 91.17 | 89.88 | 2.26 | 89.74 | 87.37 | −1.68 | 91.49 | 89.56 | 2.26 | ||||
| 3 | 96.59 | 92.86 | 96.50 | 92.86 | −0.09 | 96.50 | 92.70 | −0.24 | 96.16 | 92.45 | −0.84 | 96.59 | 93.17 | 0.31 | ||||
| 4 | 92.50 | 77.96 | 91.98 | 78.49 | 0.00 | 93.13 | 79.89 | 2.55 | 94.69 | 86.24 | 10.47 | 95.31 | 85.83 | 10.68 | ||||
| 5 | 78.01 | 65.12 | 77.04 | 65.47 | −0.62 | 79.68 | 64.81 | 1.35 | 87.07 | 73.99 | 17.93 | 87.07 | 74.49 | 18.43 | ||||
| 6 | 84.52 | 78.50 | 84.45 | 78.69 | 0.12 | 85.42 | 78.63 | 1.03 | 90.12 | 81.40 | 8.50 | 90.95 | 83.88 | 11.80 | ||||
| Average | 89.34 | 81.77 | 89.05 | 82.06 | 0.00 | 90.12 | 82.26 | 1.27 | 91.75 | 84.47 | 5.12 | 92.61 | 85.86 | 7.36 | ||||
| Data | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| + | 181.79 | 178.79 | 189.45 | 170.46 | 143.14 | 136.02 |
| 3.25 | 67.93 | 65.48 | 4.35 | 9.26 | 7.41 |
| Data | 1-1 | 1-2 | 1-3 | 2-1 | 2-2 | 2-3 | 3-1 | 3-2 | 3-3 | 4-1 | 4-2 | 4-3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.38 | 0.33 | 0.40 | 0.65 | 0.43 | 0.66 | 0.36 | 0.30 | 0.31 | 0.43 | 0.33 | 0.33 | 0.17 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.; Jeong, J.J.; Kim, S.H.; Kim, S.W. Reduction of Motion Artifacts and Improvement of R Peak Detecting Accuracy Using Adjacent Non-Intrusive ECG Sensors. Sensors 2016, 16, 715. https://doi.org/10.3390/s16050715
Choi M, Jeong JJ, Kim SH, Kim SW. Reduction of Motion Artifacts and Improvement of R Peak Detecting Accuracy Using Adjacent Non-Intrusive ECG Sensors. Sensors. 2016; 16(5):715. https://doi.org/10.3390/s16050715
Chicago/Turabian StyleChoi, Minho, Jae Jin Jeong, Seung Hun Kim, and Sang Woo Kim. 2016. "Reduction of Motion Artifacts and Improvement of R Peak Detecting Accuracy Using Adjacent Non-Intrusive ECG Sensors" Sensors 16, no. 5: 715. https://doi.org/10.3390/s16050715






