Electrochemical Detection of Hydrazine Using Poly(dopamine)-Modified Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Electrochemical Measurement
3. Results and Discussion
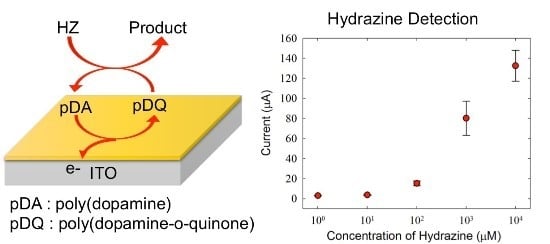
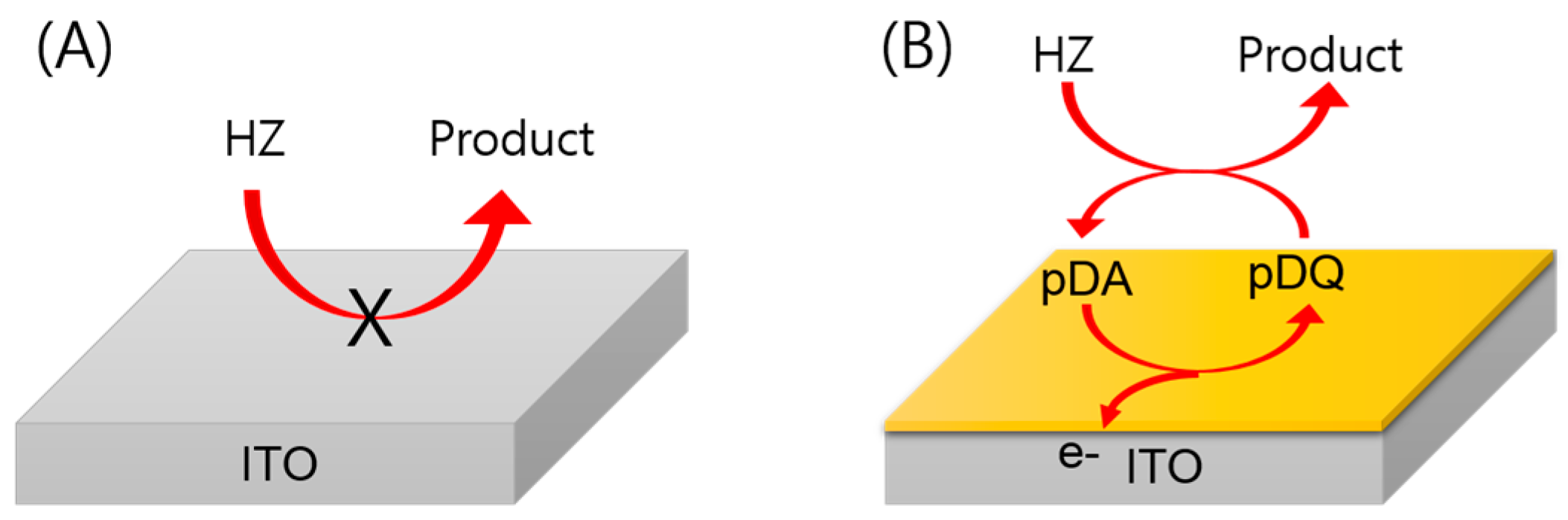
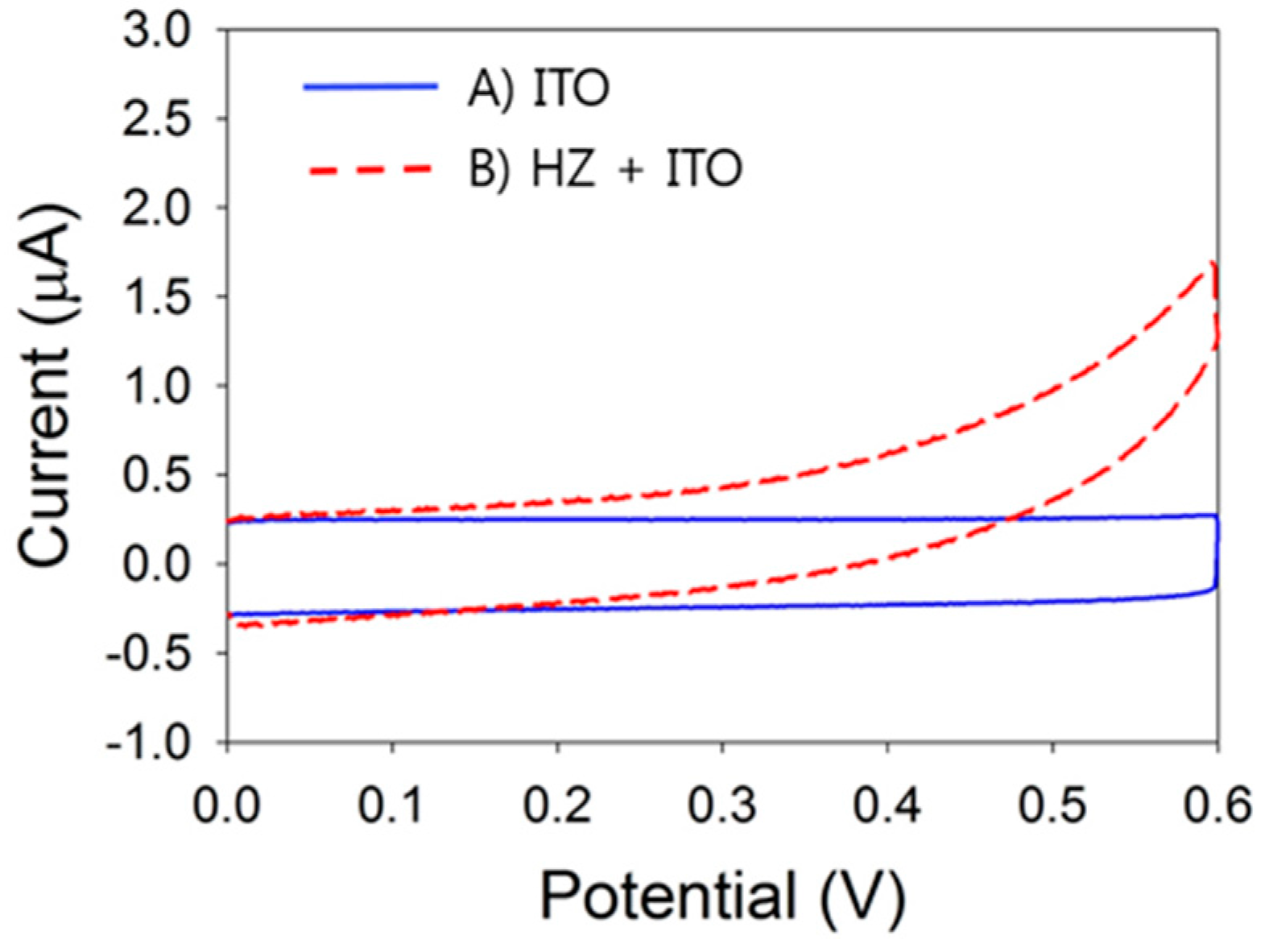
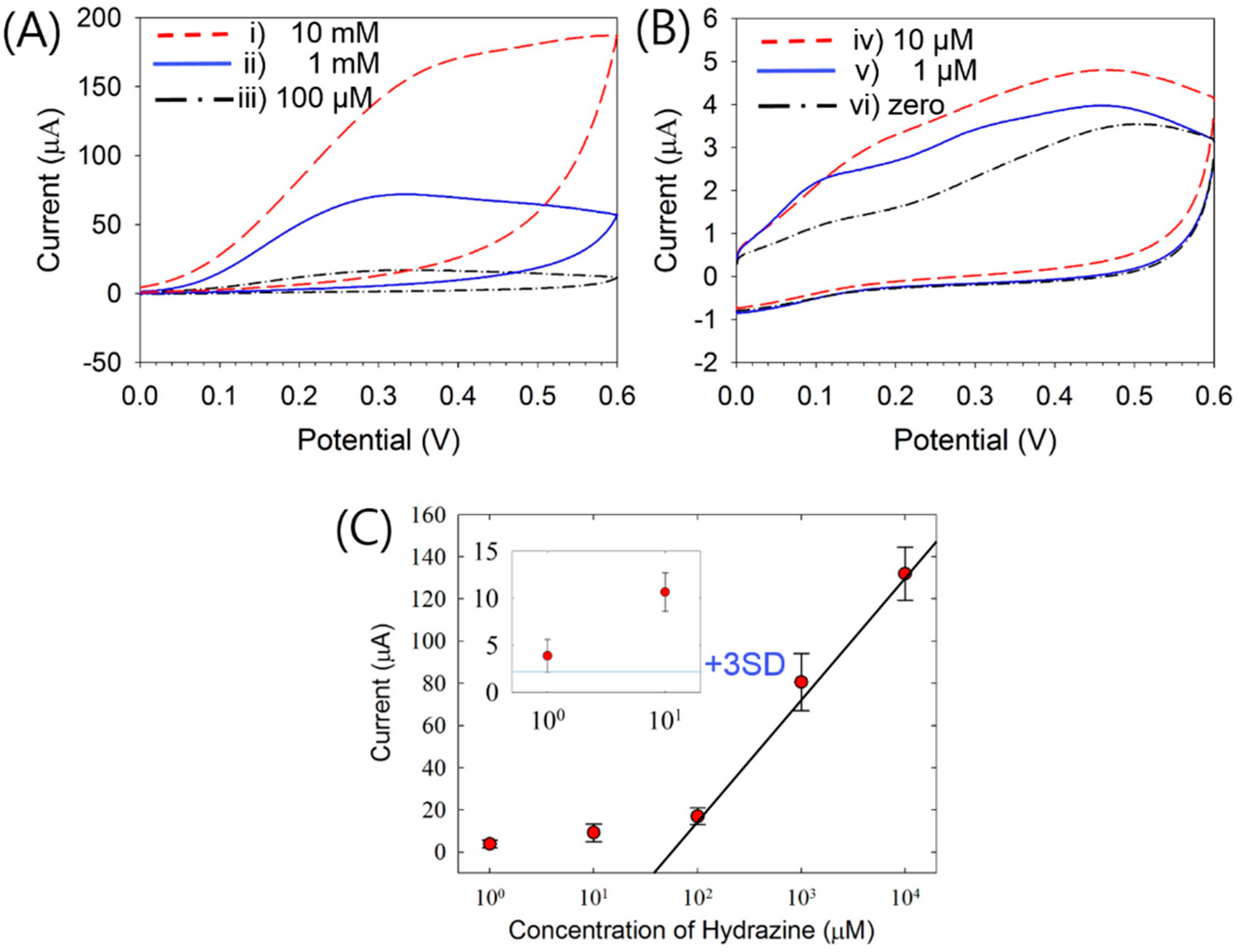
3.1. Electrochemical Detection of HZ
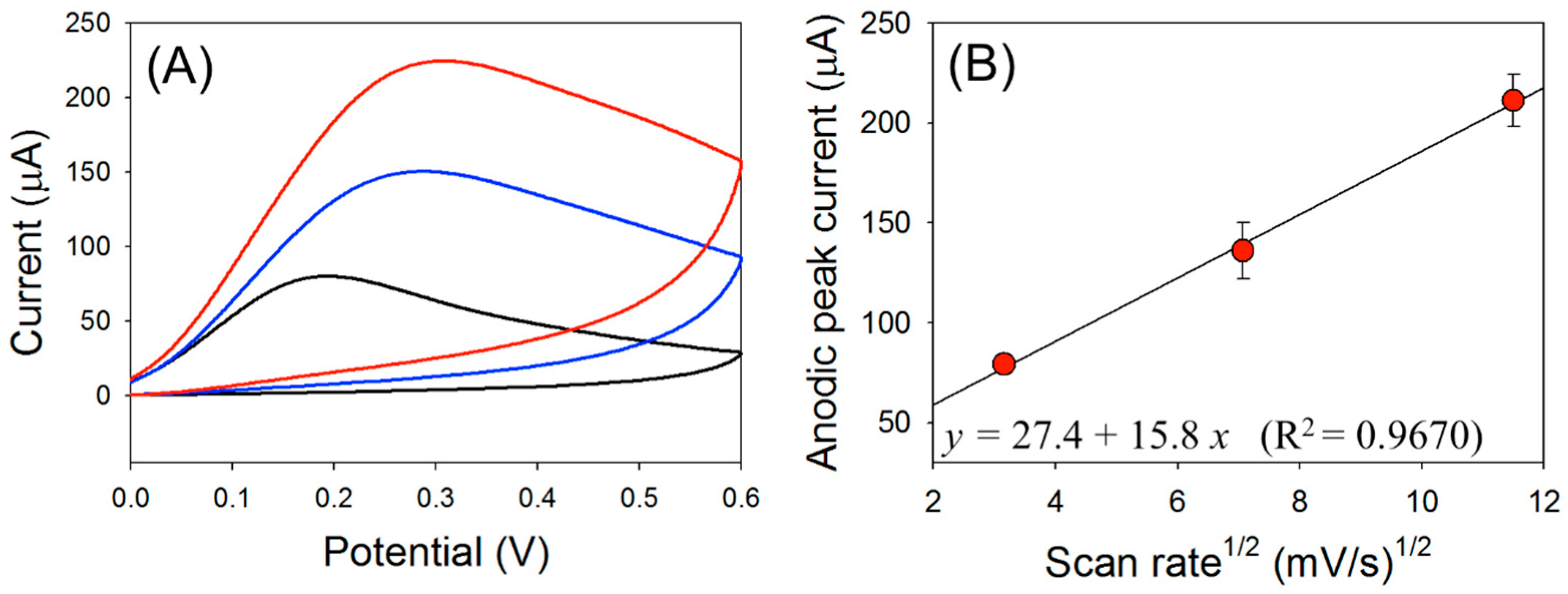
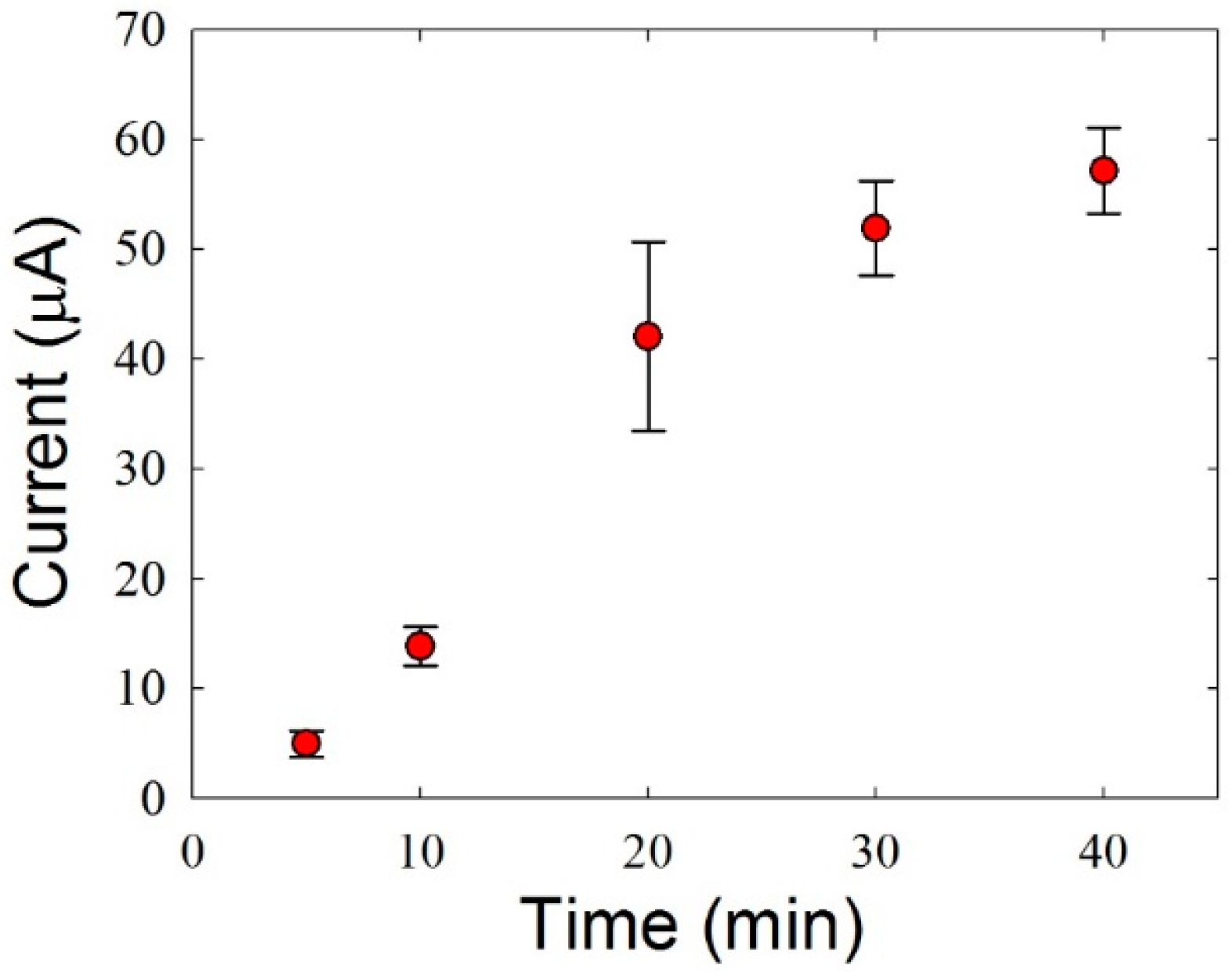
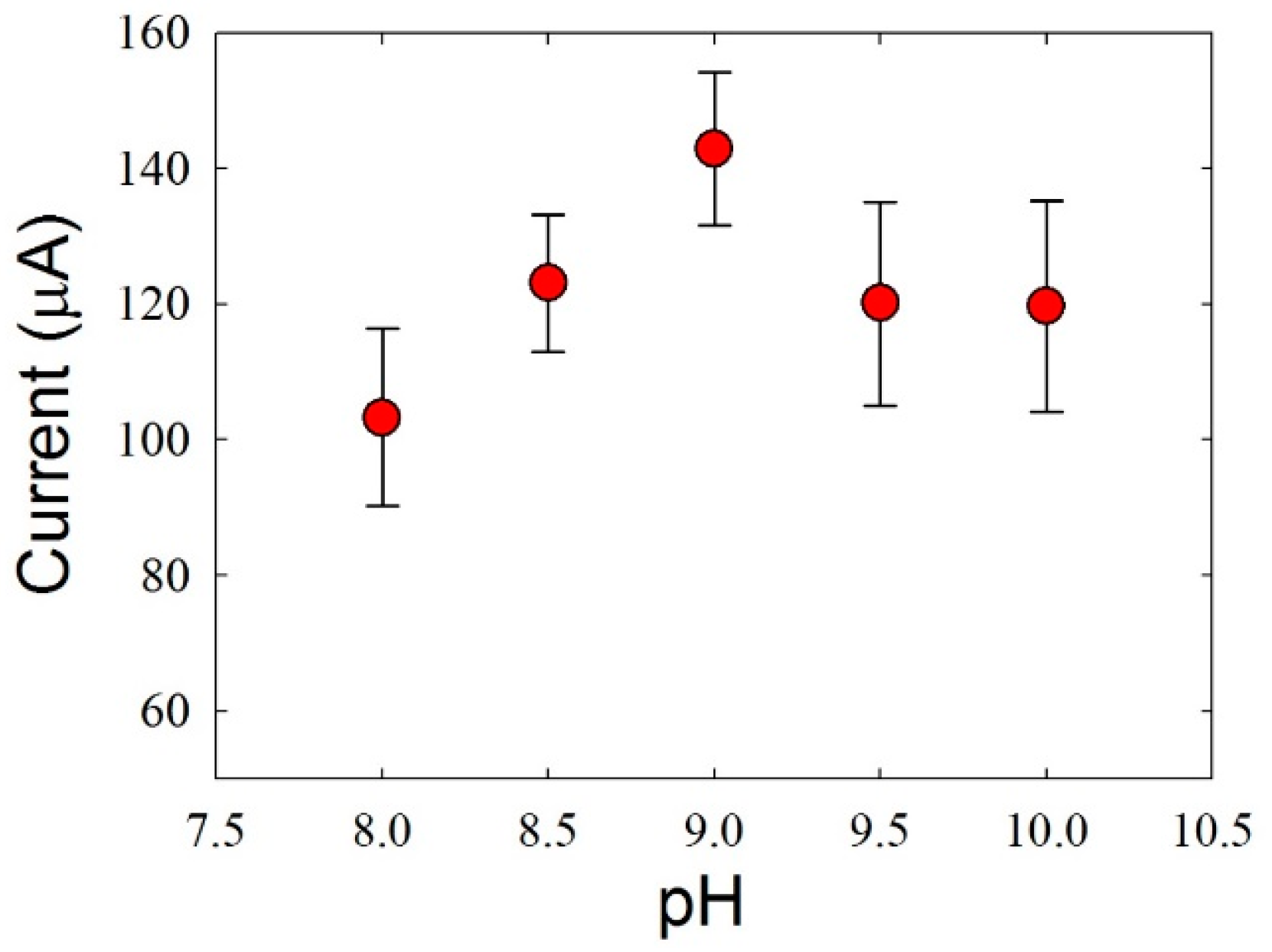
3.2. Optimization of the Experimental Conditions for HZ Detection
3.3. HZ-Sensing Performances
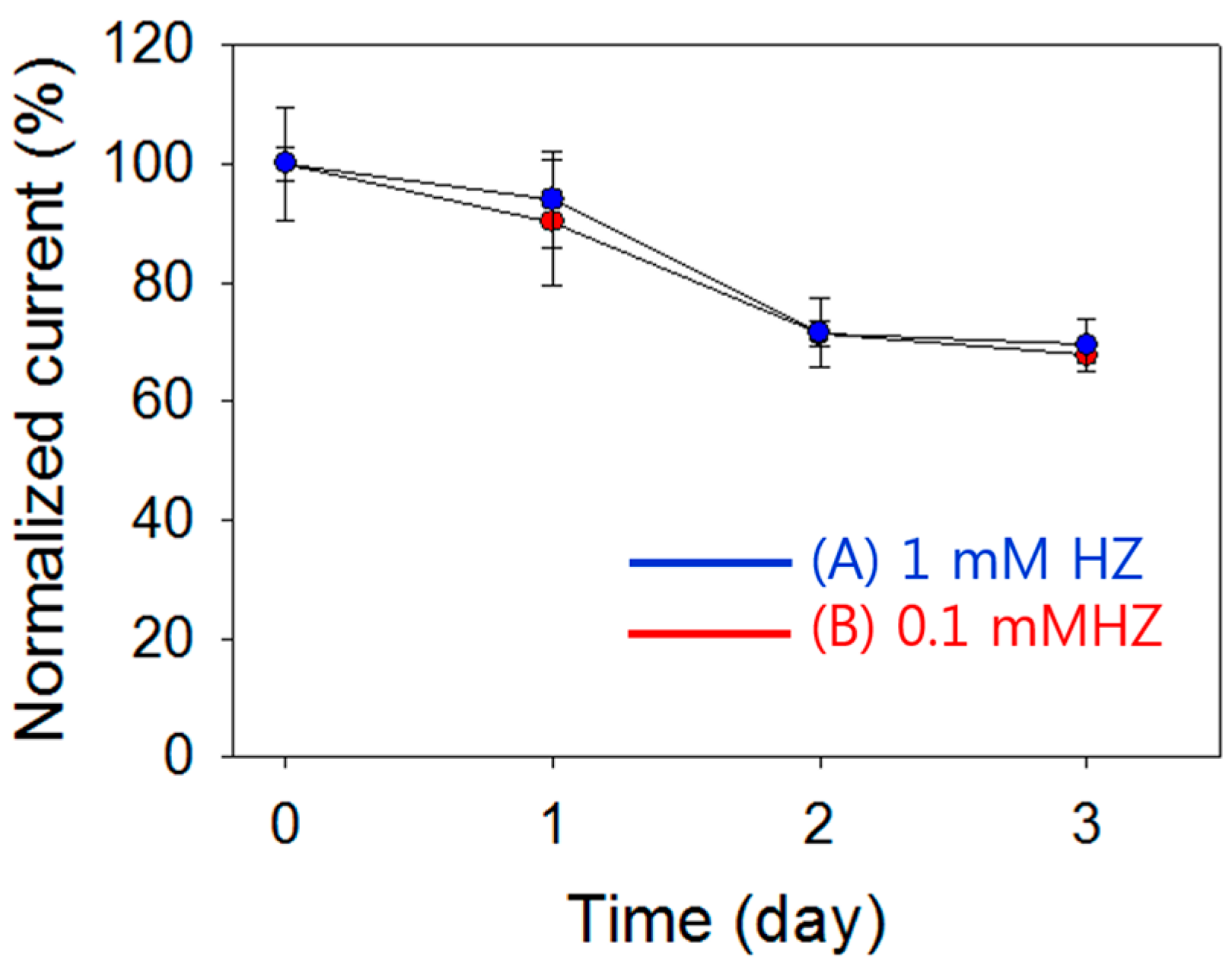
3.4. Influence of Interference Molecules for Detecting HZ and the Stability of pDA-ITO Electrodes
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| EPA | Environmental Protection Agency |
| HZ | Hydrazine |
| ITO | Indium tin oxide |
| pDA | Poly(dopamine) |
| pDQ | Poly(dopamine-o-quinone) |
References
- Durga, S.; Ponmani, K.; Kiruthika, S.; Muthukumaran, B. Electrochemical oxidation of hydrazine in membraneless fuel cells. J. Electrochem. Sci. Technol. 2014, 5, 73–81. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Technology Transfer Network‒Air Toxics Web Site. Available online: http://www.epa.gov/ttn/atw/hlthef/hydrazin.html (accessed on 2 March 2008).
- Budkuley, J.S. Determination of hydrazine and sulfite in the presence of one another. Microchim. Acta 1992, 108, 103–105. [Google Scholar] [CrossRef]
- Mori, M.; Tanaka, K.; Xu, Q.; Ikedo, M.; Taoda, H.; Hu, W.Z. Highly sensitive determination of hydrazine ion by ion-exclusion chromatography with ion-exchange enhancement of conductivity detection. J. Chromatogr. A 2004, 1039, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Arulraj, A.D.; Vijayan, M.; Vasantha, V.S. Spectrophotometric determination of pico-molar level of hydrazine by using Alizarin red in water and urine samples. Spectrochim. Acta A 2015, 148, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, Z.; Guo, Z.; Wang, Q. Flow-injection electrogenerated chemiluminescence detection of hydrazine based on its in-situ electrochemical modification at a pre-anodized platinum electrode. Analyst 2002, 127, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.A.; Mashhadizadeh, M.H.; Behjatmanesh-Ardakani, R.; Sahraie, N. A kinetic-potentiometric method for the simultaneous determination of hydrazine and acetylhydrazine. Asian J. Chem. 2009, 21, 3726–3740. [Google Scholar]
- Guerra, S.V.; Kubota, L.T.; Xavier, C.R.; Nakagaki, S. Experimental optimization of selective hydrazine detection in flow injection analysis using a carbon paste electrode modified with copper porphyrin occluded into zeolite cavity. Anal. Sci. 1999, 15, 1231–1234. [Google Scholar] [CrossRef]
- Jayasri, D.; Narayanan, S.S. Amperometric determination of hydrazine at manganese hexacyanoferrate modified graphite-wax composite electrode. J. Hazard Mater. 2007, 144, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Gu, A.; Wang, W.; Wei, Y.; Wu, J.; Wang, G.; Zhang, X.; Fang, B. Copper oxide nanoarray based on the substrate of Cu applied for the chemical sensor of hydrazine detection. Electrochem. Commun. 2009, 11, 631–634. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Guo, Z.; Xu, J.; Wang, H.; Zhai, K.; Zhuo, X. A novel hydrazine electrochemical sensor based on the high specific surface area grapheme. Microchim. Acta 2010, 169, 1–6. [Google Scholar] [CrossRef]
- Lee, K.K.; Loh, P.Y.; Sow, C.H.; Chin, W.S. CoOOH nanosheet electrodes: Simple fabrication for sensitive electrochemical sensing of hydrogen peroxide and hydrazine. Biosens. Bioelectron. 2013, 39, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Yu, W. Nanoporous gold particles modified titanium electrode for hydrazine oxidation. J. Electroanal. Chem. 2009, 633, 159–164. [Google Scholar] [CrossRef]
- Li, J.; Xie, H.; Chen, L. A sensitive hydrazine electrochemical sensor based on electrodeposition of gold nanoparticles on choline film modified glassy carbon electrode. Sens. Actuators. B: Chem. 2011, 153, 239–245. [Google Scholar] [CrossRef]
- Kim, S.K.; Jeong, Y.N.; Ahmed, M.S.; You, J.-M.; Choi, H.C.; Jeon, S. Electrocatalytic determination of hydrazine by a glassy carbon electrode modified with PEDOP/MWCNTs–Pd nanoparticles. Sens. Actuators B 2011, 153, 246–251. [Google Scholar] [CrossRef]
- Mehtaa, S.K.; Singha, K.; Umar, A.; Chaudharya, G.R.; Singha, S. Ultra-high sensitive hydrazine chemical sensor based on low-temperature grown ZnO nanoparticles. Electrochim. Acta 2012, 69, 128–133. [Google Scholar] [CrossRef]
- Aziz, M.A.; Kawde, A.N. Gold nanoparticle-modified graphite pencil electrode for the high-sensitivity detection of hydrazine. Talanta 2013, 115, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Krittayavathananon, A.; Srimuk, P.; Luanwuthi, S.; Sawangphruk, M. Palladium nanoparticles decorated on reduced graphene oxide rotating disk electrodes toward ultrasensitive hydrazine detection: Effects of particle size and hydrodynamic diffusion. Anal. Chem. 2014, 86, 12272–12278. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Bo, X.; Zhu, D.; Guo, L. Well-dispersed Pt nanoparticles on polydopamine-coated ordered mesoporous carbons and their electrocatalytic application. Talanta 2014, 120, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Devasenathipathy, R.; Mani, V.; Chen, S.M. Highly selective amperometric sensor for the trace level detection of hydrazine at bismuth nanoparticles decorated graphene nanosheets modified electrode. Talanta 2014, 124, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.Z.; Beitollahi, H.; Asadi, E.B. Electrochemical determination of hydrazine using a ZrO2 nanoparticles-modified carbon paste electrode. Environ. Monit. Assess 2015, 187, 122. [Google Scholar] [CrossRef] [PubMed]
- Ozoemena, K.I. Anodic oxidation and amperometric sensing of hydrazine at a glassy carbon electrode modified with cobalt (II) Phthalocyanine–cobalt (II) Tetraphenylporphyrin(CoPc-(CoTPP)4) supramolecular complex. Sensors 2006, 6, 874–891. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Li, P.; Sang, S.; Zhang, W.; Hu, J.; Lian, K. A sensitive hydrazine electrochemical sensor based on zinc oxide nano-wires. J. Electrochem. Soc. 2014, 161, B157–B162. [Google Scholar] [CrossRef]
- You, T.; Niu, L.; Gui, J.Y.; Dong, S.; Wang, E. Detection of hydrazine, methylhydrazine and isoniazid by capillary electrophoresis with a 4-pyridyl hydroquinone self-assembled microdisk platinum electrode. J. Pharm. Biomed. Anal. 1999, 19, 231–237. [Google Scholar] [CrossRef]
- Wu, M.; Ding, W.; Meng, J.; Ni, H.; Li, Y.; Ma, Q. Electrocatalytic behavior of hemoglobin oxidation of hydrazine based on ZnO nano-rods with carbon nanofiber modified electrode. Anal. Sci. 2015, 31, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhao, Z.; Sun, Y.; Wang, Y.; Li, P.; Zhang, W.; Lian, K. Controllable synthesis of branched hierarchical ZnO nanorod arrays for highly sensitive hydrazine detection. Appl. Sur. Sci. 2016, 364, 434–441. [Google Scholar] [CrossRef]
- Ye, W.C.; Wang, D.A.; Zhang, H.; Zhou, F.; Liu, W.M. Electrochemical growth of flowerlike gold nanoparticles on polydopamine modified ITO glass for SERS application. Electrochim. Acta 2010, 55, 2004–2009. [Google Scholar] [CrossRef]
- Amiri, M.; Amali, E.; Nematollahzadeh, A. Poly-dopamine thin film for voltammetric sensing of atenolol. Sens. Actuators B: Chem. 2015, 216, 551–557. [Google Scholar] [CrossRef]
- Fang, C.C.; Deng, Y.F.; Xie, Y.; Su, J.Y.; Chen, G.H. Improving the electrochemical performance of Si nanoparticle anode material by synergistic strategies of polydopamine and graphene oxide coatings. J. Phys. Chem. C 2015, 119, 1720–1728. [Google Scholar] [CrossRef]
- Fu, L.; Lai, G.S.; Jia, B.H.; Yu, A.M. Preparation and electrocatalytic properties of polydopamine functionalized reduced graphene oxide-silver nanocomposites. Electrocatalysis-Us 2015, 6, 72–76. [Google Scholar] [CrossRef]
- Zhang, N.; Ma, W.; He, P.G.; Long, Y.T. A polydopamine derivative monolayer on gold electrode for electrochemical catalysis of H2O2. J. Electroanal. Chem. 2015, 739, 197–201. [Google Scholar] [CrossRef]
- Yang, J.; Niu, L.H.; Zhang, Z.J.; Zhao, J.; Chou, L.J. Electrochemical behavior of a polydopamine nanofilm. Anal. Lett. 2015, 48, 2031–2039. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Son, S.; Lee, K.; Yang, H.; Kwak, J. Dopamine detection using the selective and spontaneous formation of electrocatalytic poly(dopamine) films on indium-tin oxide electrodes. Electroanalysis 2012, 24, 993–996. [Google Scholar] [CrossRef]
- Kim, B.K.; Lee, J.Y.; Park, J.H.; Kwak, J. Electrochemical detection of dopamine using a bare indium-tin oxide electrode and scan rate control. J. Electroanal. Chem. 2013, 708, 7–12. [Google Scholar] [CrossRef]
- Kim, B.K.; Yang, S.Y.; Aziz, M.A.; Jo, K.; Sung, D.; Jon, S.; Woo, H.Y.; Yang, H. Electrochemical immunosensing chip using selective surface modification, capillary-driven microfluidic control, and signal amplification by redox cycling. Electroanalysis 2010, 22, 2235–2244. [Google Scholar] [CrossRef]
- Park, S.; Hwang, S.; Takenaka, S.; Kim, K. Electrochemical sensing performances for uric acid detection on various amine adlayers used in immobilizing reduced graphene oxide. Electroanalysis 2015, 27, 1159–1165. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, F.; Li, J.; Li, B.; Zhao, C. Oxidant-induced dopamine polymerization for multifunctional coatings. Polym. Chem. 2010, 1, 1430–1433. [Google Scholar] [CrossRef]
- Armstrong, J.; Barlow, R.B. The ionization of phenolic amines, including apomorphine, dopamine and catecholamines and an assessment of zwitterion constants. Br. J. Pharmac. 1976, 57, 501–516. [Google Scholar] [CrossRef]
- Malik, P.; Srivastava, M.; Verma, R.; Kumar, M.; Kumar, D.; Singh, J. Nanostructured SnO2 encapsulated guar-gum hybrid nanocomposites for electrocatalytic determination of hydrazine. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Ameen, S.; Akhtar, M.S.; Shin, H.S. Highly sensitive hydrazine chemical sensor fabricated by modified electrode of vertically aligned zinc oxide nanorods. Talanta 2012, 100, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Majidi, M.R.; Jouyban, A.; Asadpour-Zeynali, K. Electrocatalytic oxidation of hydrazine at overoxidized polypyrrole film modified glassy carbon electrode. Electrochim. Acta 2007, 52, 6248–6253. [Google Scholar] [CrossRef]
- Ding, J.; Zhu, S.; Zhu, T.; Sun, W.; Li, Q.; Wei, G.; Su, Z. Hydrothermal synthesis of zinc oxide-reduced graphene oxide nanocomposites for an electrochemical hydrazine sensor. RSC Adv. 2015, 5, 22935–22942. [Google Scholar] [CrossRef]
- Andre, R.S.; Pavinatto, A.; Mercante, L.A.; Paris, E.C.; Mattoso, L.H.C.; Correa, D.S. Improving the electrochemical properties of polyamide 6/polyaniline electrospun nanofibers by surface modification with ZnO nanoparticles. RSC Adv. 2015, 5, 73875–73881. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Xia, X.H.; Liu, S.Q. Direct electrochemistry of cytochrome c immobilized on a novel macroporous gold film coated with a self-assembled 11-mercaptoundecanoic acid monolayer. Talanta 2010, 82, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, W.; Nan, H.; Wang, G. Ultrathin zinc oxide nanofilm on zinc substrate for high performance electrochemical sensors. Electrochim. Acta 2014, 144, 186–193. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, J.; Jiang, X. Porous Mn2O3 nanorods synthesized from thermal decomposition of coordination polymer and used in hydrazine electrochemical sensing. Mater. Lett. 2015, 159, 362–365. [Google Scholar] [CrossRef]
- Zhang, O.; Yu, H.-M.; Lu, L.-M.; Wen, Y.-P.; Duan, X.-M.; Xu, J.-K. Poly(thiophene-3-acetic acid)-palladium nanoparticle composite modified electrodes for supersensitive determination of hydrazine. Chin. J. Polym. Sci. 2013, 31, 419–426. [Google Scholar] [CrossRef]
- Tolstopjatova, E.G.; Kondratiev, V.V.; Eliseeva, S.N. Multi-layer PEDOT:PSS/Pd composite electrodes for hydrazine oxidation. J. Solid State Electrochem. 2015, 19, 2951–2959. [Google Scholar] [CrossRef]
- Rastogi, P.K.; Ganesan, V.; Krishnamoorthi, S. Palladium nanoparticles decorated gaur gum based hybrid material for electrocatalytic hydrazine determination. Electrochim. Acta 2014, 125, 593–600. [Google Scholar] [CrossRef]
- Kocak, S.; Aslısen, B. Hydrazine oxidation at gold nanoparticles and poly(bromocresol purple) carbon nanotube modified glassy carbon electrode. Sens. Actuators B: Chem. 2014, 196, 610–618. [Google Scholar] [CrossRef]
- Kazemi, S.H.; Hosseinzadeh, B.; Zakavi, S. Electrochemical fabrication of conducting polymer of Ni-porphyrin as nano-structured electrocatalyst for hydrazine oxidation. Sens. Actuators B: Chem. 2015, 210, 343–348. [Google Scholar] [CrossRef]
- Foster, C.W.; Pillay, J.; Metters, J.P.; Banks, C.E. Cobalt phthalocyanine modified electrodes utilised in electroanalysis: Nano-structured modified electrodes vs. bulk modified screen-printed electrodes. Sensors 2014, 14, 21905–21922. [Google Scholar] [CrossRef] [PubMed]

| Type of Electrode | Detection Limit (μM) | Linear Range (μM) | Sample Condition | Reference |
|---|---|---|---|---|
| a MnHCF-modified graphite-wax composite | 6.65 | 33.3–8180 | Fe(CN)64−/Fe(CN)63 | [9] |
| CoOOH nanosheets | 20 | 0–1000 | 0.1 M NaOH | [12] |
| b CoPc-(CoTPP)4/c GCE | 230 | _ | 0.2 M NaOH | [22] |
| d Hb/ZnO/e CNF/GCE | 6.60 | 19.8–1710 | 0.1 M f PBS (pH 7) | [25] |
| SnO2/guar-gum hybrid nanocomposite | 2760 | 2000–22,000 | Fe(CN)64−/Fe(CN)63 | [40] |
| Zinc oxide nanorods | ~515.7 | 0.3–300 | 0.1 M PBS | [41] |
| Overoxidized polypyrrole | 3.7 | 1.3–2000 | 0.1 M ammonium buffer (pH 9) | [42] |
| ZnO-g RGO | 0.8 | 1–33,500 | 0.1 M NaOH | [43] |
| ZnO nanoparticles | 0.35 | 0.5–5000 | 0.01 M PBS (pH 7.5) | [44] |
| ZnO nanoparticles II | 0.147 | _ | 0.01 M PBS (pH 7.0) | [45] |
| ZnO nanofilm | 0.5 | 0.5–14,200 | 0.1 M NaOH | [46] |
| Mn2O3 nanorods | 1.1 | 2–1300 | 0.01 M PBS (pH 7.0) | [47] |
| h PdNPs/I PTAA/GCE | 0.00267 | 0.008–10 | 0.01 M PBS (pH 7.0) | [48] |
| j PEDOT:k PSS/Pd | 0.12 | 0.4–100 | 0.2 M PBS (pH 6.86) | [49] |
| Pd-lGG-g-PAM-silica | 4.1 | 50–180,000 | PBS (pH 7.0) | [50] |
| m AuNPs/n poly(BCP)/° CNT/GCE | 0.1 | 0.5–1000 | 0.1 M PBS (pH 10.0) | [51] |
| p PNi-TPPS4-NPs | 0.11 | _ | 0.1 M NaOH | [52] |
| q nano-CoTAPC SPEs | 30 | 10–100 | PBS (pH 7.4) | [53] |
| PdNPs/RGO r RDEs | 0.007 | 0.1–1000 | 0.2 M PBS (pH 7.4) | [18] |
| pDA/ITO | 1 | 100–10,000 | 0.05 M Tris buffer | This work |
| Added (μM) | Sample | Current at 0.3 V (μA) | Found (μM) | Recovery (%) a |
|---|---|---|---|---|
| 100 | 1 | 21.5 | 105 | 105 |
| 2 | 20.1 | 100 | 100 | |
| 3 | 23.2 | 112 | 112 | |
| 4 | 21.1 | 104 | 104 | |
| 1000 | 1 | 78.9 | 940 | 94 |
| 2 | 81.3 | 1030 | 103 | |
| 3 | 79.6 | 966 | 96.6 | |
| 4 | 84.2 | 1151 | 115 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.Y.; Nguyen, T.L.; Park, J.H.; Kim, B.-K. Electrochemical Detection of Hydrazine Using Poly(dopamine)-Modified Electrodes. Sensors 2016, 16, 647. https://doi.org/10.3390/s16050647
Lee JY, Nguyen TL, Park JH, Kim B-K. Electrochemical Detection of Hydrazine Using Poly(dopamine)-Modified Electrodes. Sensors. 2016; 16(5):647. https://doi.org/10.3390/s16050647
Chicago/Turabian StyleLee, Ji Young, Truc Ly Nguyen, Jun Hui Park, and Byung-Kwon Kim. 2016. "Electrochemical Detection of Hydrazine Using Poly(dopamine)-Modified Electrodes" Sensors 16, no. 5: 647. https://doi.org/10.3390/s16050647