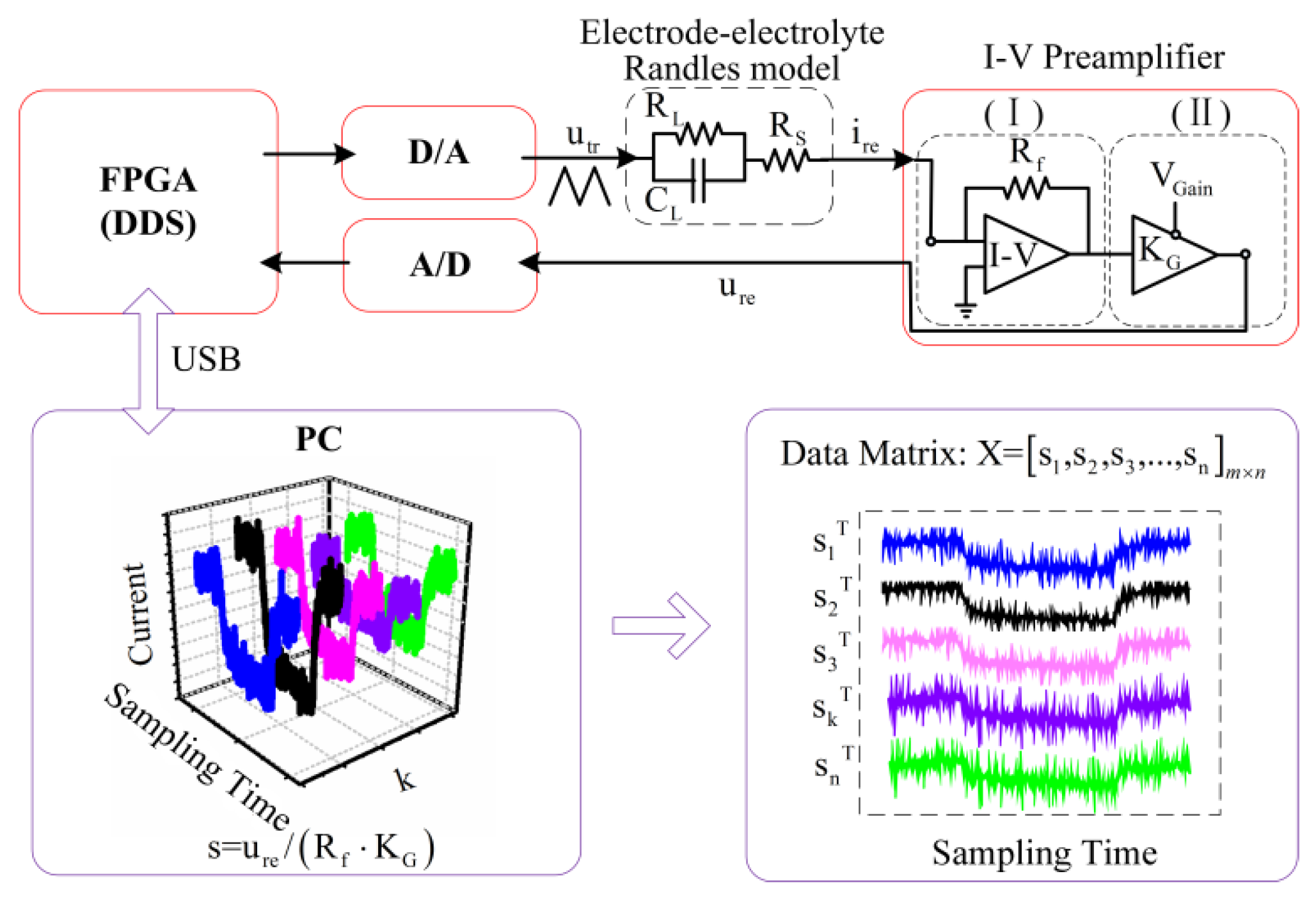
3.1. Experimental Setup
The interdigitated electrodes immersed in the solutions had been tested experimentally by the measurement setup as shown in
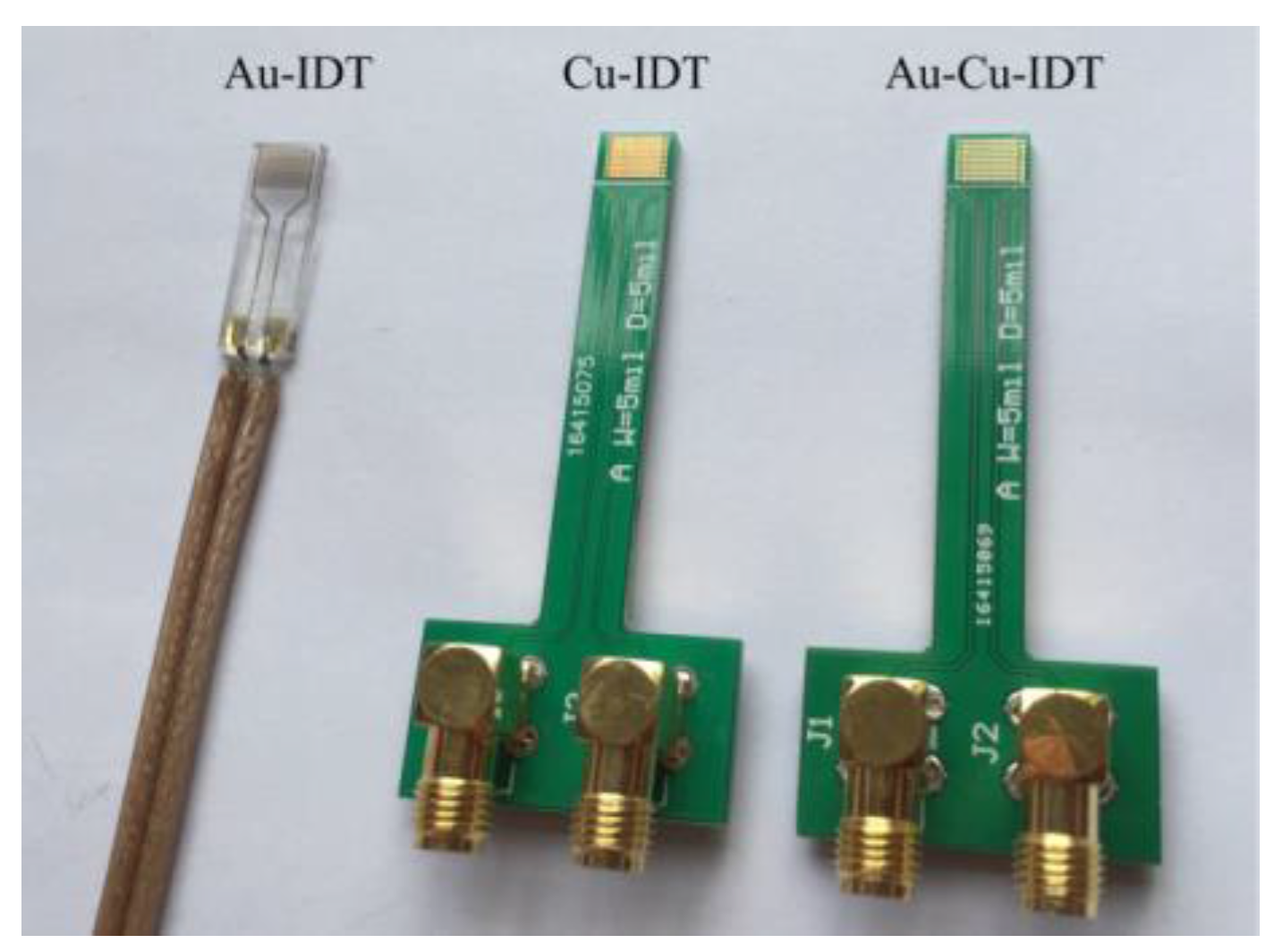
Figure 1. Three kinds of planar-interdigitated electrodes were used in the experiments;
i.e., Au electrode on Si substrate (Au-IDT), Cu electrode on PCB substrate (Cu-IDT) and Au electrode on PCB substrate (Au-Cu-IDT), as shown in
Figure 5. All electrodes consist of eight interdigitated finger-pairs, with 5 mm finger length, 127 μm finger width, and 254 μm space between adjacent groups of interdigitated fingers. Au-IDT was fabricated on a silicon wafer, depositing a 20 nm layer of titanium (for enhancing Au adhesion) followed by a 200 nm layer of gold. Cu-IDT was fabricated on a printed circuit board (PCB) with a 35 μm layer of copper, and Au-Cu-IDT was fabricated by depositing a 30 nm layer of gold on Cu-IDT.
Au-IDT was reused in the experiment. Before each measurement, Au-IDT was cleaned in an ultrasonic bath using alcohol and then using deionized water for 10 min each. In order to avoid the electrode aging effect caused by Faradaic reactions of copper during the experiments, new electrodes were used in the Cu-IDT and Au-Cu-IDT experiments.
The sample solutions used in the experiments were KCl base solution (20 mmol/L), KCl background solution (50 mmol/L), thiol solution (10 mmol/L) and MPA solution (50 mmol/L). The thiol solution was prepared by dissolving 3-mercaptopropionic acid into anhydrous alcohol. The MPA solution was obtained by mixing 3-mercaptopropionic acid and the KCl background solution.
3.2. Experiment Results
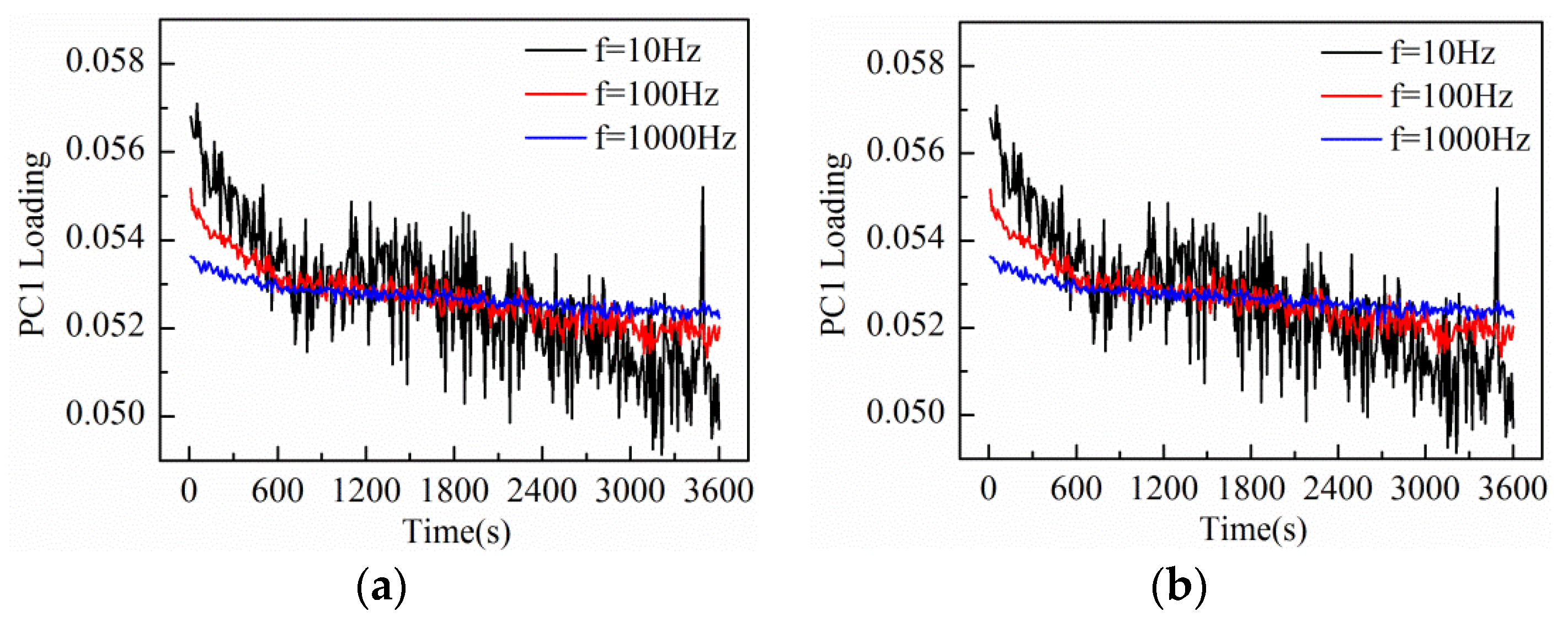
In order to evaluate the influence of the triangular waveform voltage parameters, the impedance of the KCl base solution (20 mmol/L, 20 mL) was monitored using Au-IDT excited by triangular waveform voltage at different frequencies and amplitudes. The measurement peak-to-peak amplitudes chosen are 0.2 and 1 V, while the measurement frequencies are 10 Hz, 100 Hz, and 1 kHz.
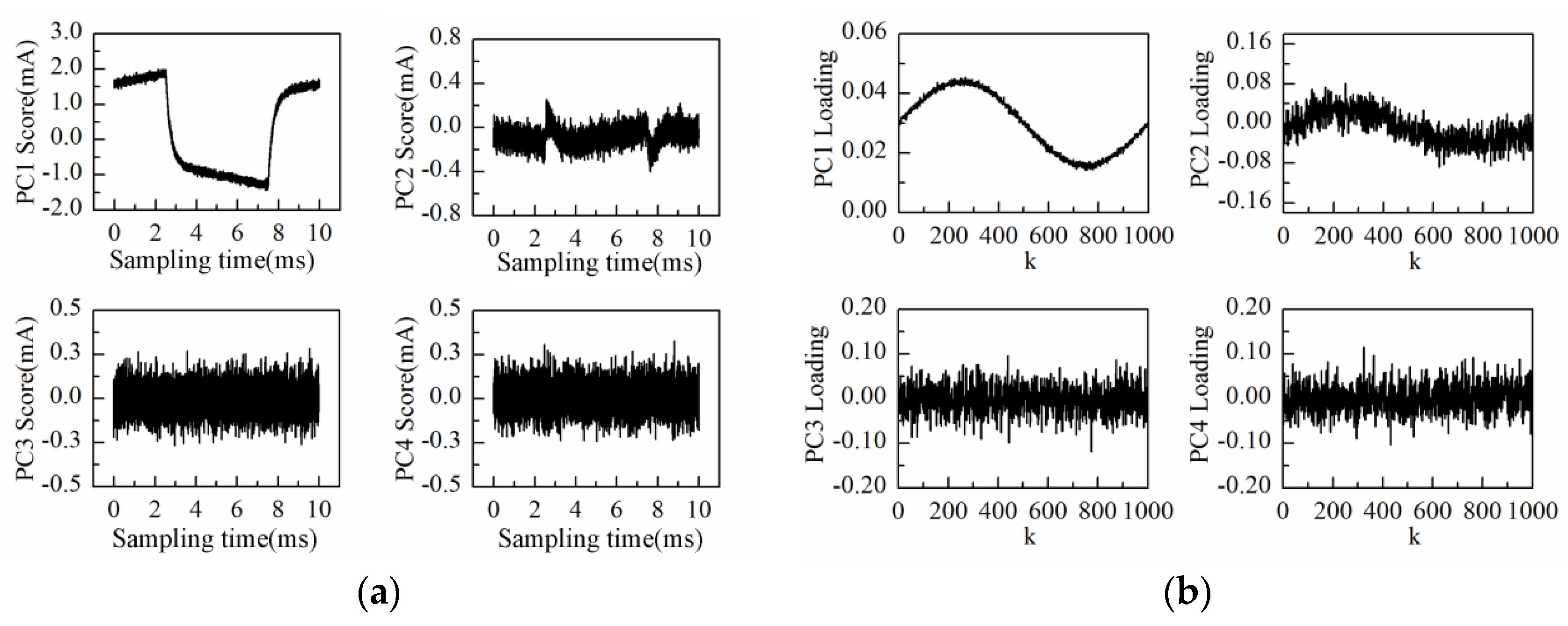
The amplitude and frequency sweep test was conducted by the impedance measurement system at the interval of 10 s. At the same time, the response current during one duty cycle was sampled to construct the measurement matrix. After performing PCA on the data matrix of 1 h measurement results, the loadings plot of the first principal component (PC1) is shown in
Figure 6. When the electrode was immersed in the KCl base solution, the equivalent impedance varied due to the charge distribution variation between electrode and electrolyte interface under external voltage excitation [
24]. So, all the PC1 loading curves in
Figure 6 show a general decrease trend in the initial period and then come to a steady state.
The experimental results indicate that the frequency and amplitude of the triangular waveform voltage both influence the measurement results. It can be seen that PC1 loading curves at higher frequencies are more relatively insensitive to the charge distribution variation of electrode interface, while PC1 loading curves at lower frequencies are more sensitive to the electrode interface variation. However, in the case of lower measurement frequencies, the response current signals present the characteristic of small amplitude with high noise level, thus leading to a poorer SNR. On the other hand, under the triangular waveform voltage excitation of larger amplitude, the SNR of the response current signal becomes better, so the measurement curves excited by 1 V amplitude is smoother than that excited by 0.2 V amplitude. In addition, we can see that PC1 loading curves excited by larger amplitude are more sensitive to the electrode interface variation. However, a voltage excitation amplitude that is too large will cause thermal effects at the electrode interface, even destroy the electrode. Consequently, considering the influencing factors of amplitude and frequency comprehensively, the triangular waveform voltage of 100 Hz frequency and 1 V peak-to-peak amplitude was chosen for the following measurement of solution impedance variation and real-time noise variation in electrode-electrolyte interface.
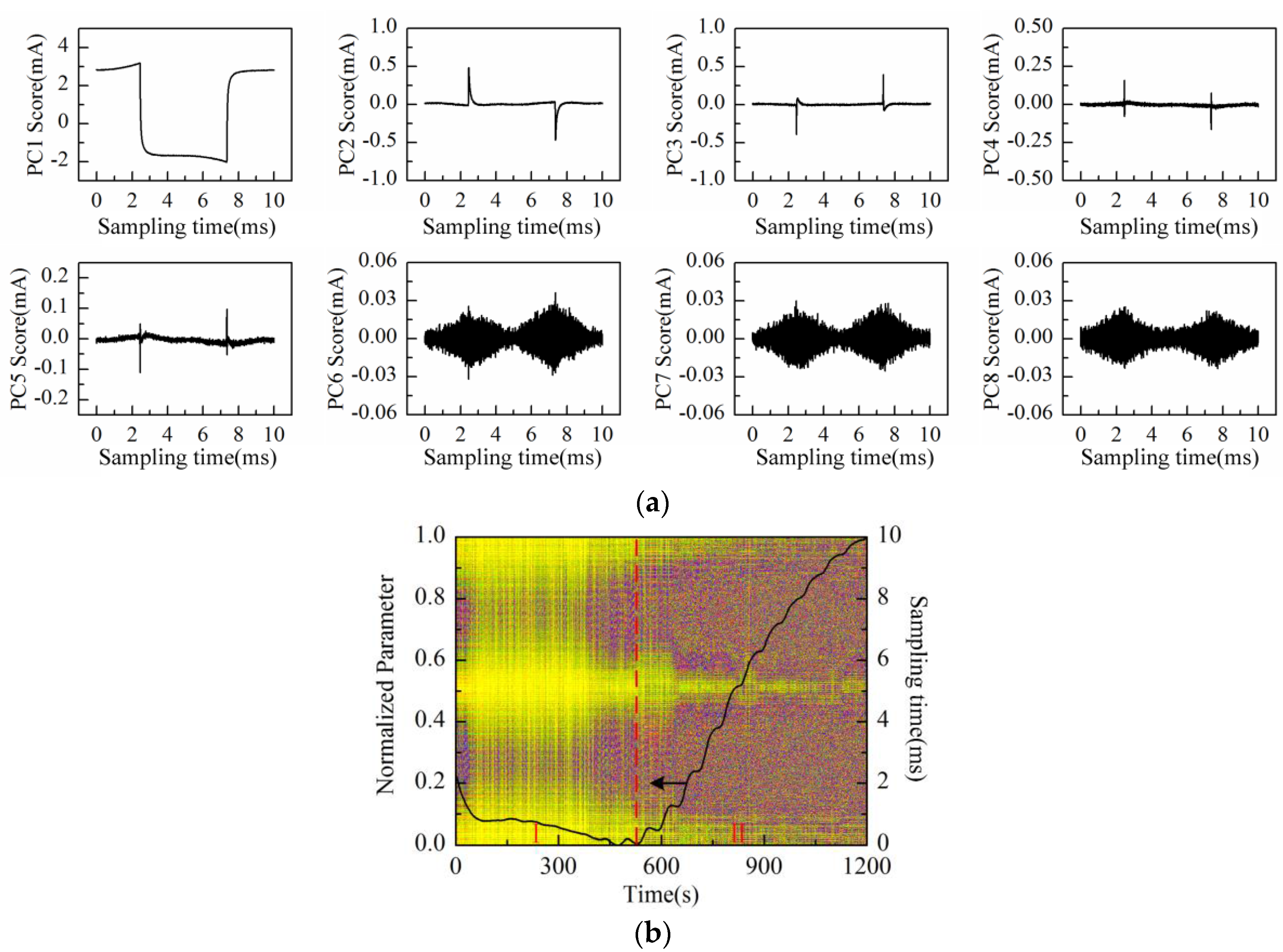
Then, the process of successive increase in solution concentration was monitored using Au-IDT. Low-concentration KCl solution, which is commonly used in biological impedance measurement, was adopted as the measuring object. By adding 1 mol/L KCl solution into the KCl base solution (20 mmol/L, 20 mL) every 1 min, the solution concentration was therefore increased linearly by 5 mmol/L after every addition. The response current was sampled by impedance measurement system at the interval of 1 s. Then, the data matrix was constructed with the obtained response current signals of 20 min measurement time. After performing PCA on the data matrix of measurement results, the scores plot of the first eight principal components (PC1–PC8) is shown in
Figure 7a.
As can be observed in
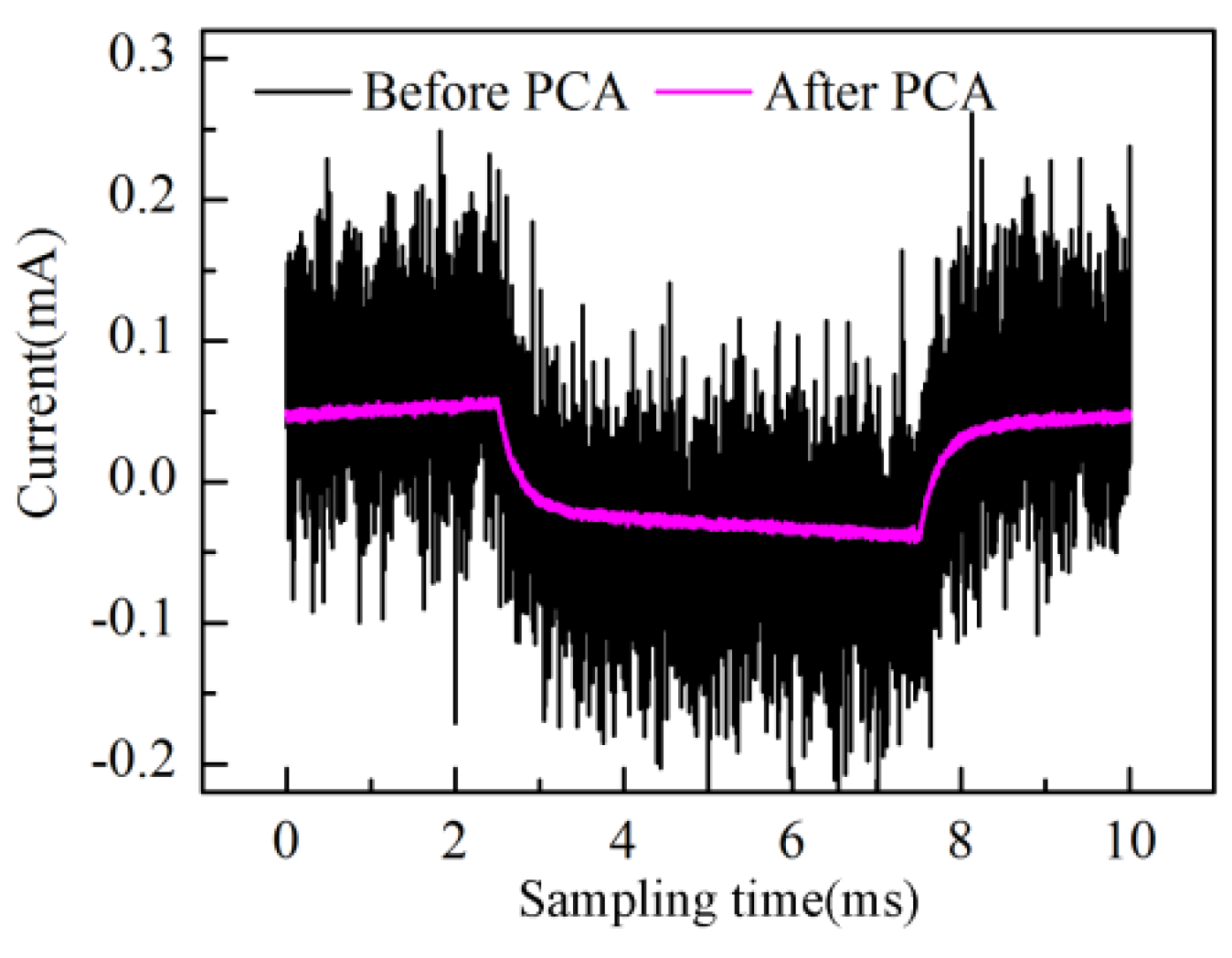
Figure 7a, the time-domain waveform information of response current in one sampling period is mostly contained in PC1 to PC4 scores; especially the PC1 score contains the main information of the response current waveform. On the other hand, the high frequency fluctuation information appears in the PC5 score and so on. It is notable that PC5 and PC6 scores present the “rhombus noise” related to voltage amplitude. In contrast, the noise in PC7 and PC8 scores is unrelated to voltage amplitude. In order to analyze the changing process of the “rhombus noise” with the concentration increase, the “rhombus noise” signals are reconstructed by the principal components of PC5 and PC6, as observed in
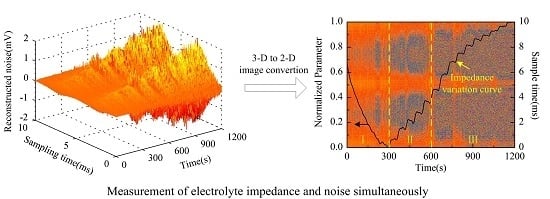
Figure 7b (3-D image). It can be seen in
Figure 7b that the noise amplitude shows a general trend of increase. The 3-D image is then converted to a 2-D image as shown in
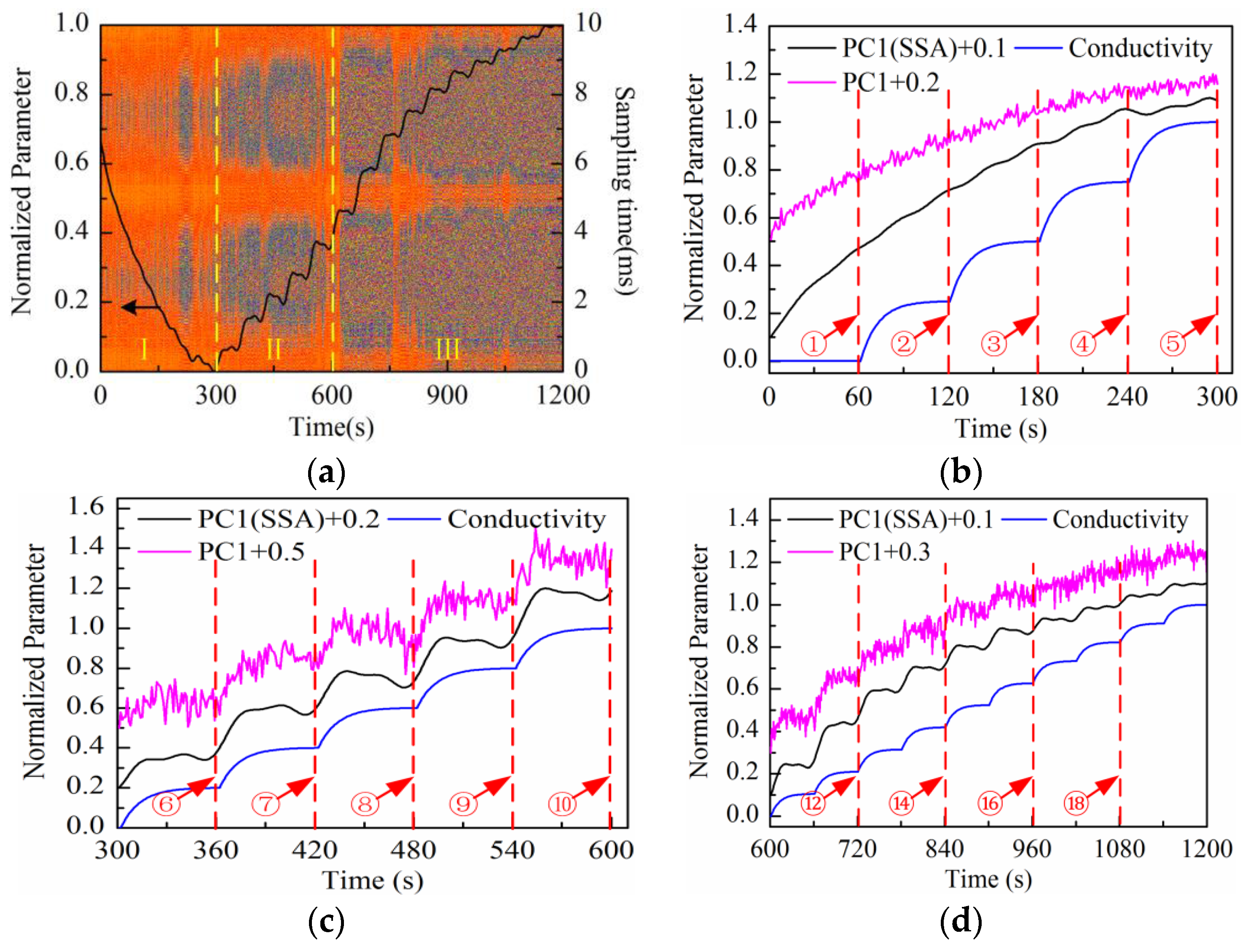
Figure 8a, in which the color variation of pixels stands for the distribution of noise amplitude.
The solid line in
Figure 8a refers to the changing curve of PC1 loading after SSA and normalization processing. It is demonstrated that the PC1 loading curve presents different characteristics at different stages (І–II). In order to analyze the characteristics in more detail, the enlarged sectional curves at each stage are shown in
Figure 8b–d. For comparison, the theoretical curve of solution conductivity variation caused by the addition of highly-concentrated solution and the PC1 loading curve before SSA are also presented in the same figure, with the arrows indicating the time of addition. Considering the diffusion process after every addition, the theoretical curve of solution conductivity variation is fitted by exponent rule. For convenient observation, the direction of the PC1 loading curve before and after SSA is reversed at the first stage (І). A certain constant bias value is superimposed to PC1 loading curves before and after SSA at each stage (see
Figure 8b–d). It can be seen that the changing trend of solution conductivity can be extracted effectively by SSA processing. Especially during the measurement period after 720 s (adding at ⑫ in
Figure 8d), the PC1 loading curve after SSA shows a more obvious stair-step increase feature compared to the curve before SSA.
At the first stage (see
Figure 8b), the PC1 loading curve increases gradually so the solution impedance increases correspondingly. This is because the equivalent impedance varies due to the charge distribution variation of the electrode-electrolyte interface under the excitation of triangular waveform voltage at low frequencies. Therefore, it is hard to observe the impedance variation process caused by the addition of highly-concentrated solution. At the second stage (see
Figure 8c), the PC1 loading curve shows the stair-step increase feature gradually, but is not stable because the curve maintains the descending trend as mentioned above. Compared with the first two stages, the curve shows a remarkable stair-step increase feature at the third stage (see
Figure 8d). On the other hand, the amplitude distribution of “rhombus noise” varies regularly with the solution concentration increase. At the first stage, the noise level is low and unstable; meanwhile, the PC1 loading curve cannot effectively reflect the changing process of solution conductivity. At the second stage, the noise level becomes higher and the PC1 loading curve shows a gradual stair-step increase feature. At the third stage, the noise level remains stable and the PC1 loading curve shows a remarkable stair-step increase feature consistent with the theoretical conductivity variation curve.
Finally, cleaned Au-IDT was immersed in the thiol solution (10 mmol/L) for 4 h and then rinsed by deionized water. So, we obtained the modified Au-IDT covered with self-assembled monolayers (SAM-Au-IDT). The changing process of KCl solution conductivity had been monitored again using SAM-Au-IDT. The measurement process was the same as the experiment with Au-IDT shown in
Figure 8.
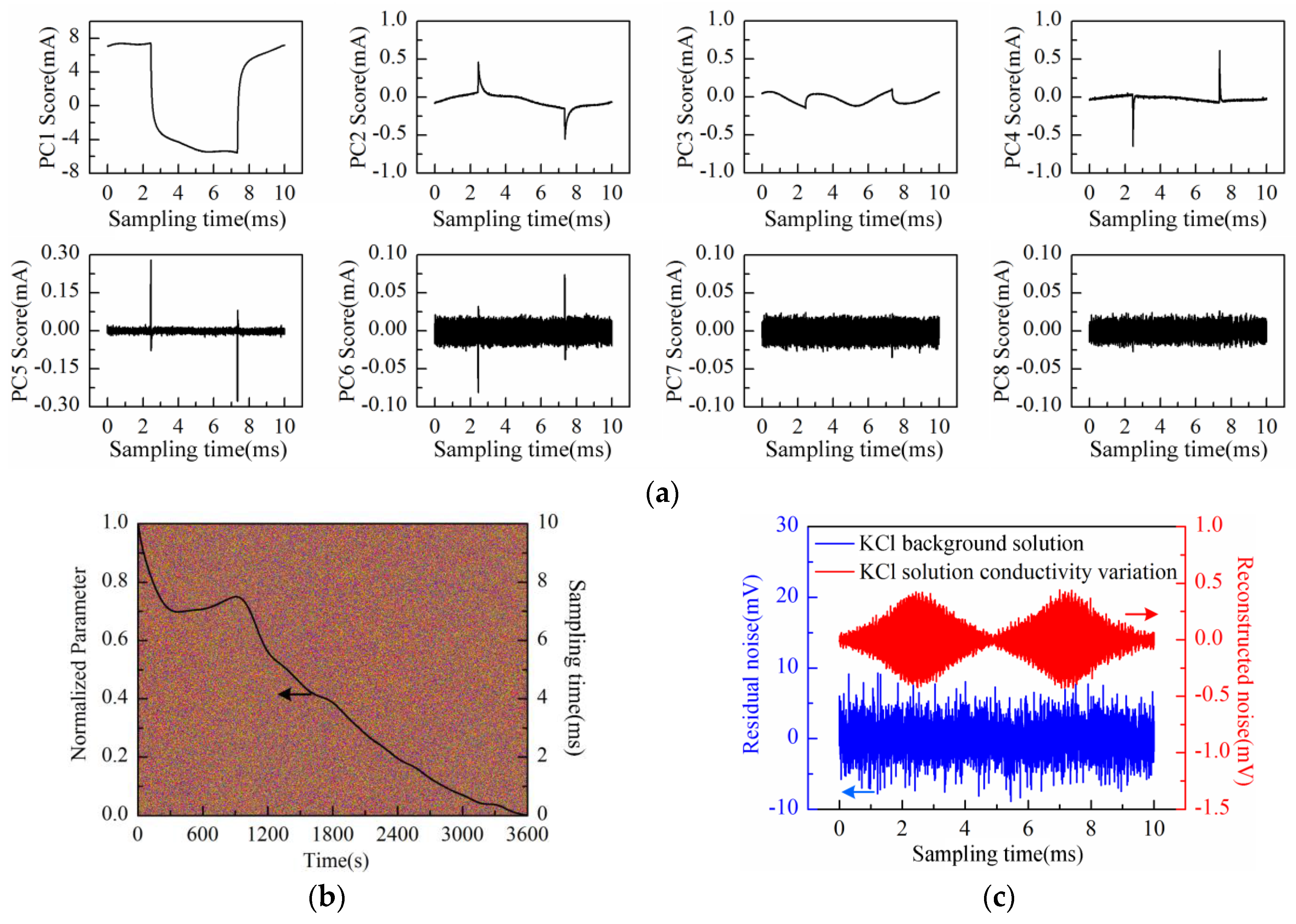
The PC1 to PC8 scores plot after being processed by PCA is shown in
Figure 9a. Obviously, the “rhombus noise” appears from PC6 to PC8. The noise image reconstructed by the above three principal components and the PC1 loading curve are shown in
Figure 9b at the same time. It can be seen in
Figure 9b that at the first stage (І), the PC1 loading curve of SAM-Au-IDT still shows a descending trend with significant fluctuations. However, the curve shows a remarkable stair-step increase feature at the second stage (II). Correspondingly, the noise amplitude in the first stage (I) is relatively unstable while it is increased gradually to a stable status at the second stage (II). Compared with Au-IDT, the SAM-Au-IDT curve has a relatively flatter slope but a longer descending time in the initial period. In addition, the smoothness of the SAM-Au-IDT curve is better in the stair-step increase period.
3.3. Discussion
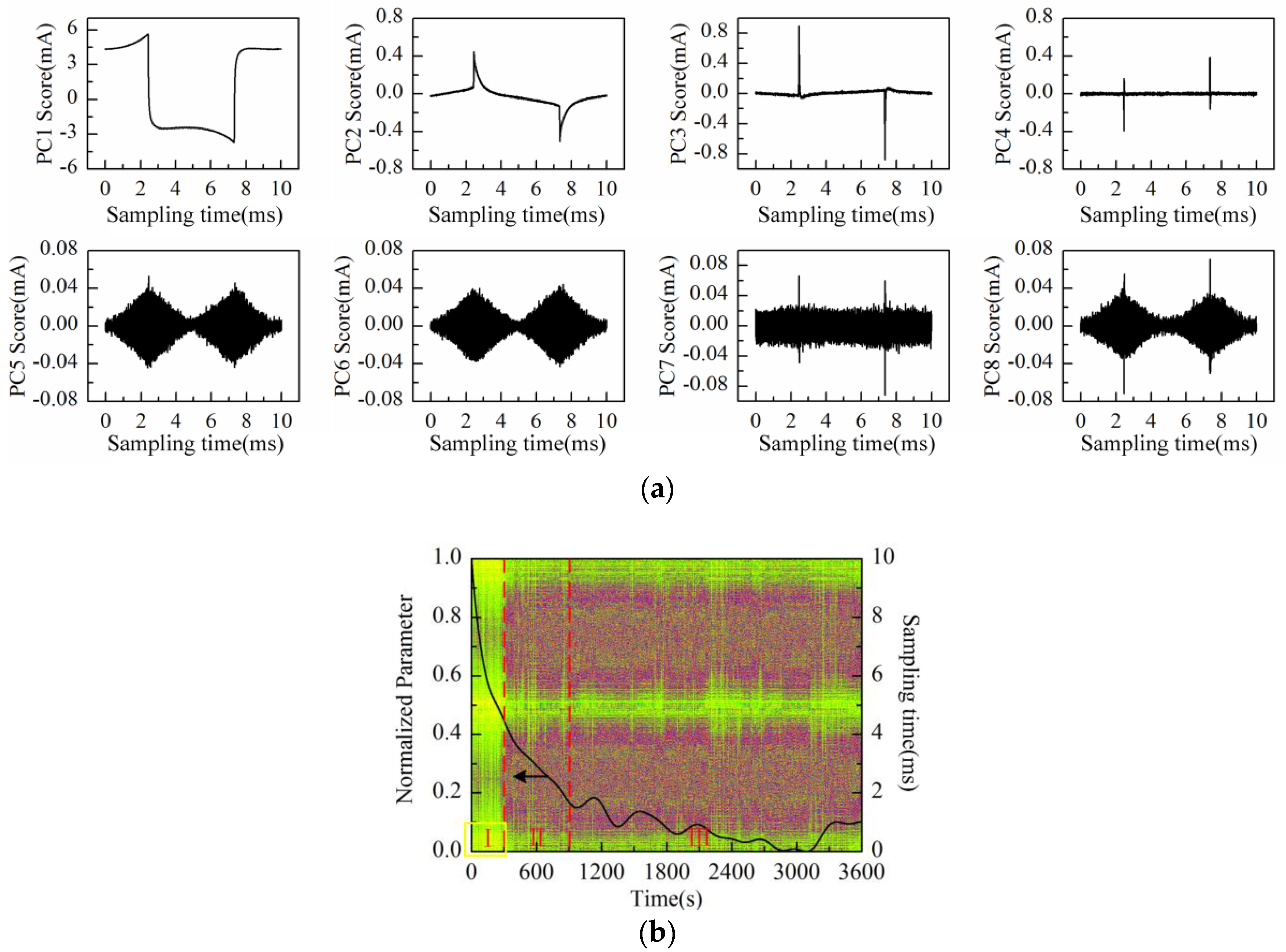
In order to analyze the relationship between the “rhombus noise” and solution concentration, the KCl solution concentration of 50 mmol/L was selected as the measurement object corresponding to the appearance of obvious “rhombus noise” (360 s, adding at ⑥ in
Figure 8a) during the measurement process. The experiment was then conducted using Au-IDT to investigate the noise characteristics of the selected KCl background solution (50 mmol/L, 20 mL). The measurement time was 1 h.
The scores plot of the first eight principal components (PC1–PC8) is illustrated in
Figure 10a after performing PCA on the data matrix of measurement results. Obviously, PC1 to PC8 scores do not represent the “rhombus noise” related to voltage amplitude. PC1 to PC6 scores all contain partial information of the response current waveform. The residual noise, except the first six principal components, is therefore obtained as shown in
Figure 10b (2-D image) together with the PC1 loading curve (after SSA and normalization processing).
As can be observed in
Figure 10b, similar to the above experiment results, the PC1 loading curve appears in a general descending trend after immersing Au-IDT in the KCl background solution. However, the curve presents a slight upward fluctuation between 300 s and 1200 s. This phenomenon might be caused by the charge distribution variation of the electrode interface under the voltage excitation in highly-concentrated electrolyte solutions. According to the noise image, noise level remains relatively constant in the measurement process. The residual noise signal at 360 s is shown in
Figure 10c. For comparison, the reconstructed noise signal at the same time in the experiment of
Figure 8a is also illustrated in
Figure 10c. From the above experimental results, it can be deduced that the “rhombus noise” has no direct connection with the absolute value of solution concentration.
According to the changing process of
Figure 8a, another reason for the occurrence of the “rhombus noise” might be the dynamic process of solution concentration variation, thus causing the variation of contact conditions in the electrode-electrolyte interface. In order to verify this assumption, Au-IDT was soaked in aqueous MPA solution (50 mmol/L, 20 mL) for the impedance experiment. Since thiols bind to the gold surface rapidly to form self-assembled monolayers on the electrode interface [
25], the contact status in the electrode-electrolyte interface will definitely be varied. The 50 mmol/L KCl background solution was used to prepare MPA solution, and the test process was the same as the above experiment (shown in
Figure 10).
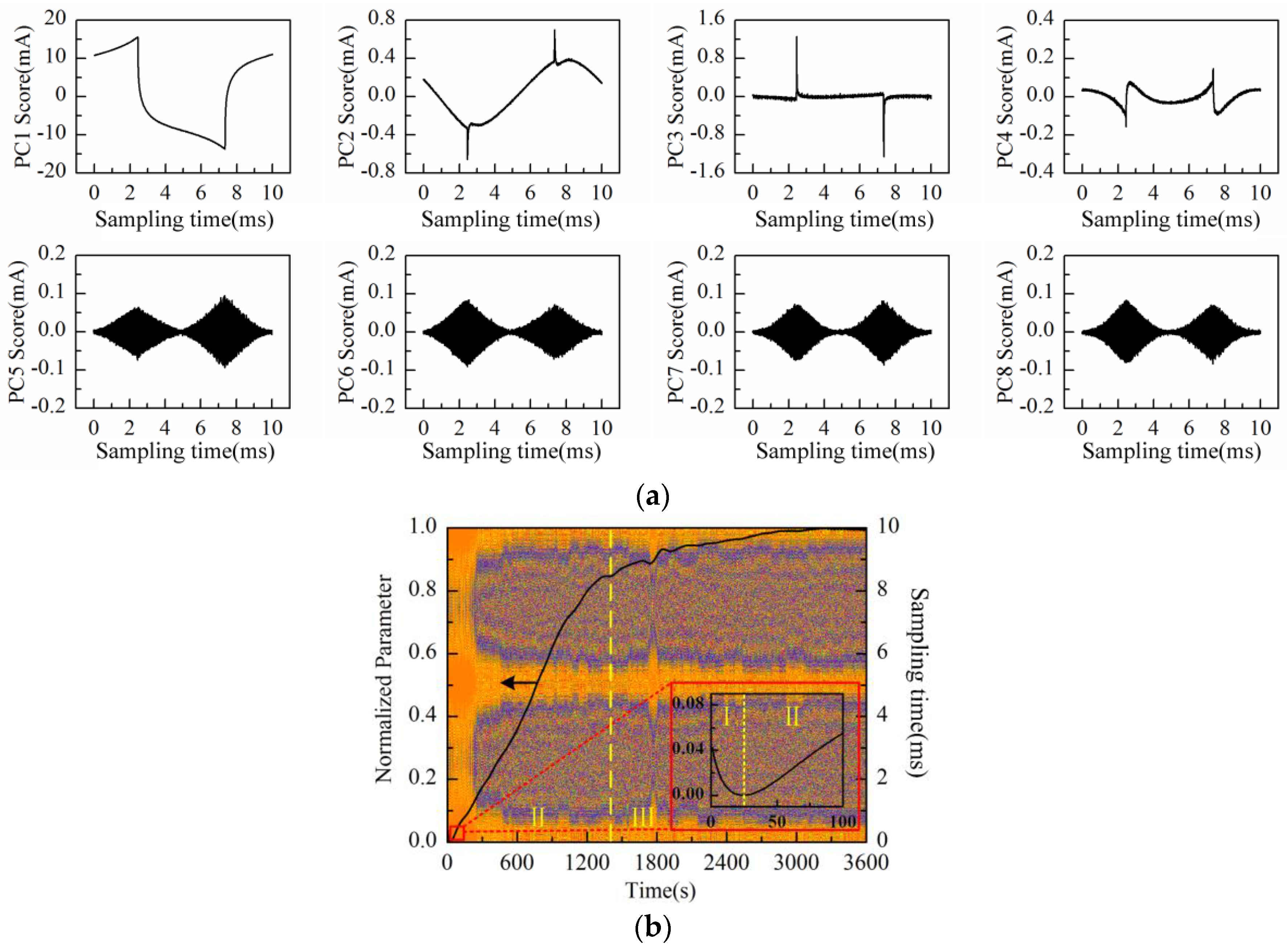
The PC1–PC8 scores plot after being processed by PCA is shown in
Figure 11a. Obviously, PC5, PC6, and PC8 represent the “rhombus noise” related to voltage amplitude. To extract the changing process of “rhombus noise”, the “rhombus noise” signals are reconstructed by the above-mentioned three principal components. The noise image is then obtained in
Figure 11b, and the PC1 loading curve (after SSA and normalization processing) is also presented in the same figure. It can be seen in
Figure 11b that the PC1 loading curve decreases gradually at the first stage (І) after soaking Au-IDT in MPA solution. With the formation of thiol monolayers on electrodes, the descending rate of the curve is slowed down at the second stage (II). After about 15 min (III), the descending trend stops and the curve shows the characteristic fluctuations. On the other hand, it can be seen from the noise image that the amplitude of “rhombus noise” is relatively small in the first stage (I). Then, the noise amplitude increases rapidly and comes to a steady state after about 5 min (II–III). Therefore, it could be inferred that the occurrence of the “rhombus noise” is related to the dynamic variation process of the electrode surface.
To further verify the above-mentioned assumption, Au-Cu-IDT was tested experimentally in the KCl background solution (50 mmol/L, 20 mL) instead. Since there was copper composition in Au-Cu-IDT, a Faradaic process caused by the dissolution of a small amount of copper during the measurement would also change the contact status of the electrode surface. The measurement time was also 1 h.
The scores plot of the first eight principal components (PC1–PC8) is shown in
Figure 12a. The “rhombus noise” appears from PC5 to PC8, in accordance with expectations. Reconstruction is performed by the above-mentioned four principal components.
Figure 12b shows the noise image together with the PC1 loading curve (after SSA and normalization processing). The enlarged sectional view of the curve in the first 100 s is embedded in
Figure 12b. As can be observed in
Figure 12b, the noise level increases rapidly after about 300 s, which might be caused by the Faradaic reactions of the adhesive copper foil on the electrode surface. Similar to the measurement results of Au-IDT, the Au-Cu-IDT curve still shows a monotonic descending trend at the first stage (I). After about 20 s (II), the curve shows approximately the trend of linear increase. With the reaction process becoming stable gradually, the ascending rate of the curve is therefore slowed down after about 1400 s (Ш) and comes to a steady state gradually.
In order to further verify whether the occurrence of the “rhombus noise” was caused by Faradaic reactions on the electrode surface, Cu-IDT had been tested experimentally in the KCl background solution (50 mmol/L, 20 mL). The measurement time was also 1 h.
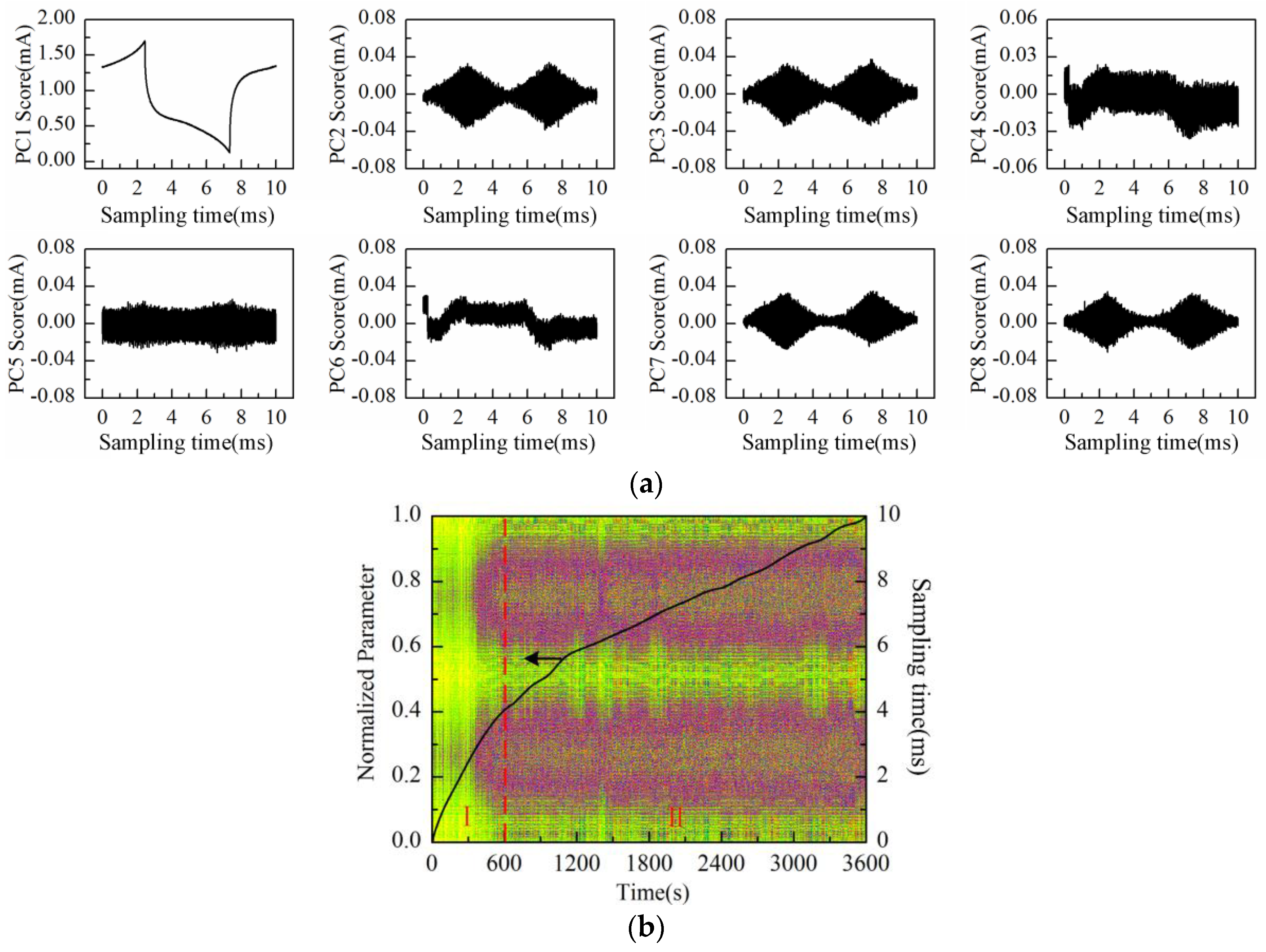
The PC1 to PC8 scores plot after being processed by PCA is shown in
Figure 13a. Obviously, PC2, PC3, PC7, and PC8 scores represent the “rhombus noise”. Reconstruction is then performed by the above-mentioned four principal components.
Figure 13b shows the noise image and PC1 loading curve (after SSA and normalization processing) simultaneously. After immersion of Cu-IDT in the KCl background solution (50 mmol/L, 20 mL), the Faradaic reactions are gradually enhanced. Therefore, the curve still approximately demonstrates the trend of linear increase at the first stage (I). Then, the Faradaic reactions are gradually weakened and stabilized at the second stage (II), and the ascending rate of the curve is gradually decreased. As can be observed from the noise image in
Figure 13b, similar to the measurement results of Au-Cu-IDT, the noise level exhibits a relatively rapid rise after about 300 s, caused by the Faradaic reactions, which is consistent with the experimental results in [
26,
27].
The above-mentioned experimental verification concerning the occurrence of the “rhombus noise” is summarized in
Table 1 below. Combined with all the measurement results, we can conclude that the changing process of the electrode–electrolyte contact interface will lead to the occurrence of such “rhombus noise”.