Development of a Microforce Sensor and Its Array Platform for Robotic Cell Microinjection Force Measurement
Abstract
:1. Introduction
2. Sensor Design
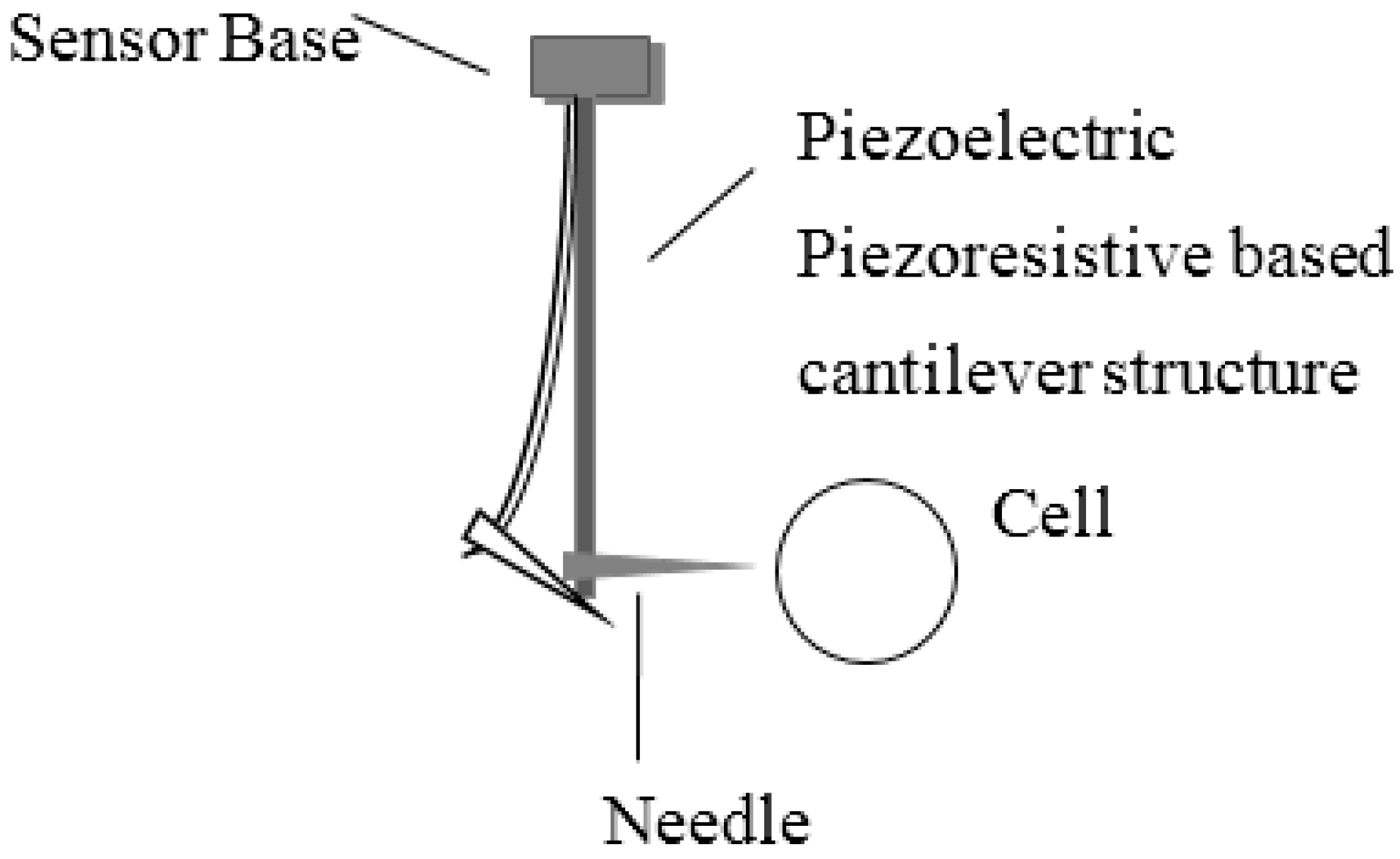
2.1. Sensor Construction
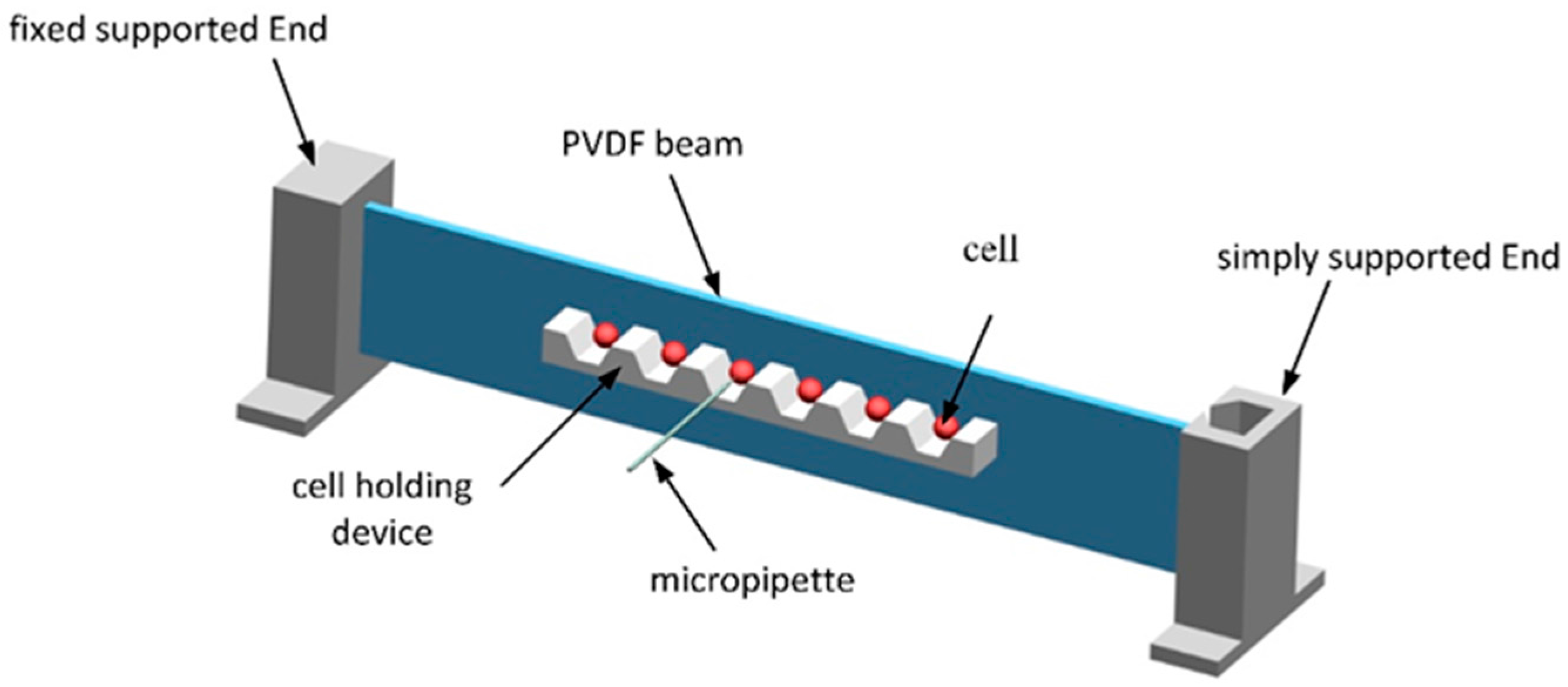
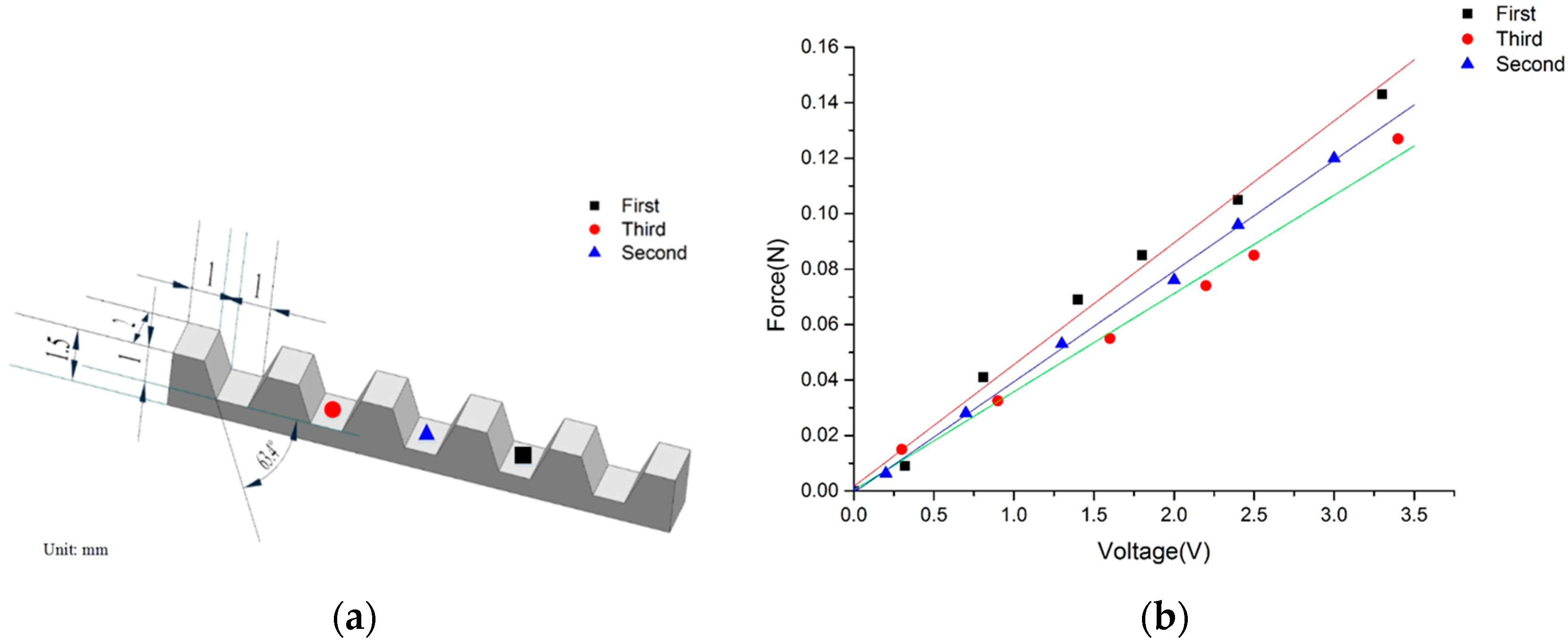
2.2. Microfabrication of Cell-Holding Device
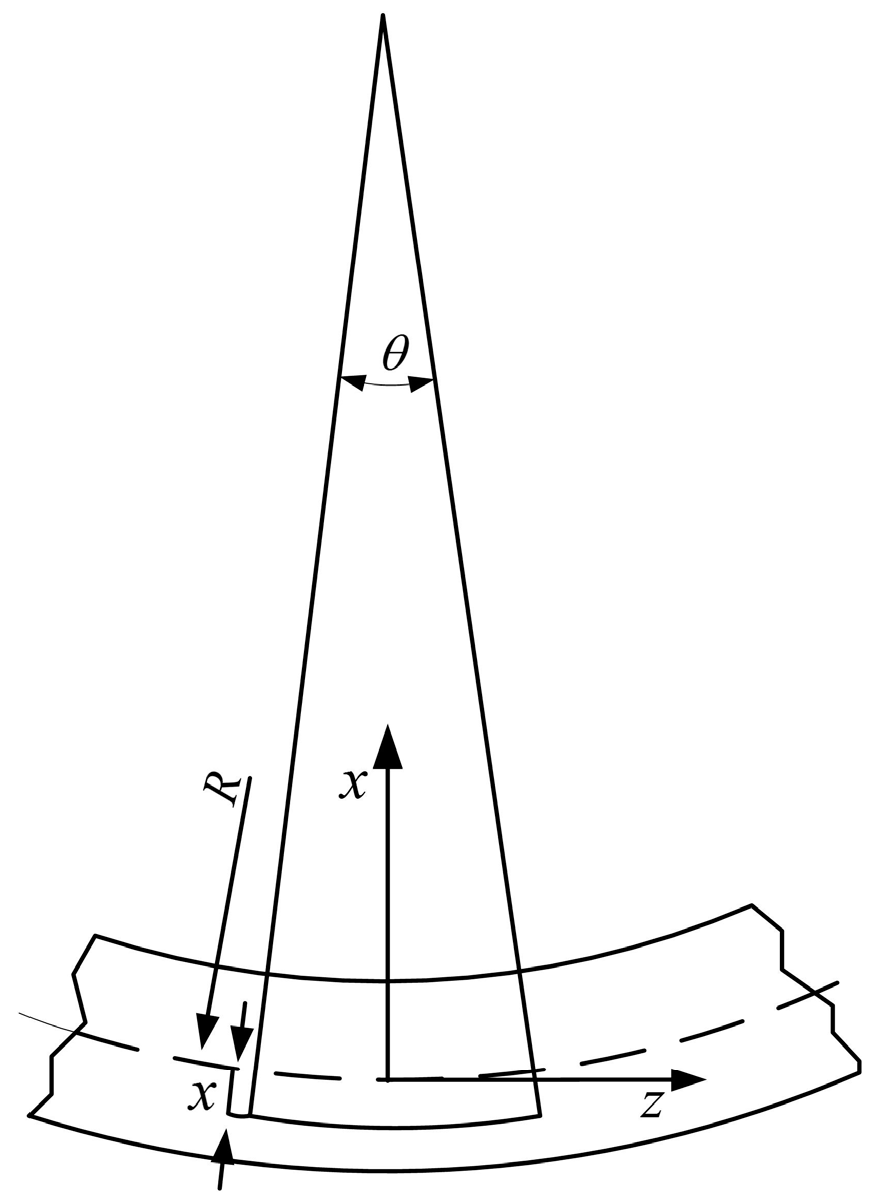
2.3. Sensor Mechanical Model
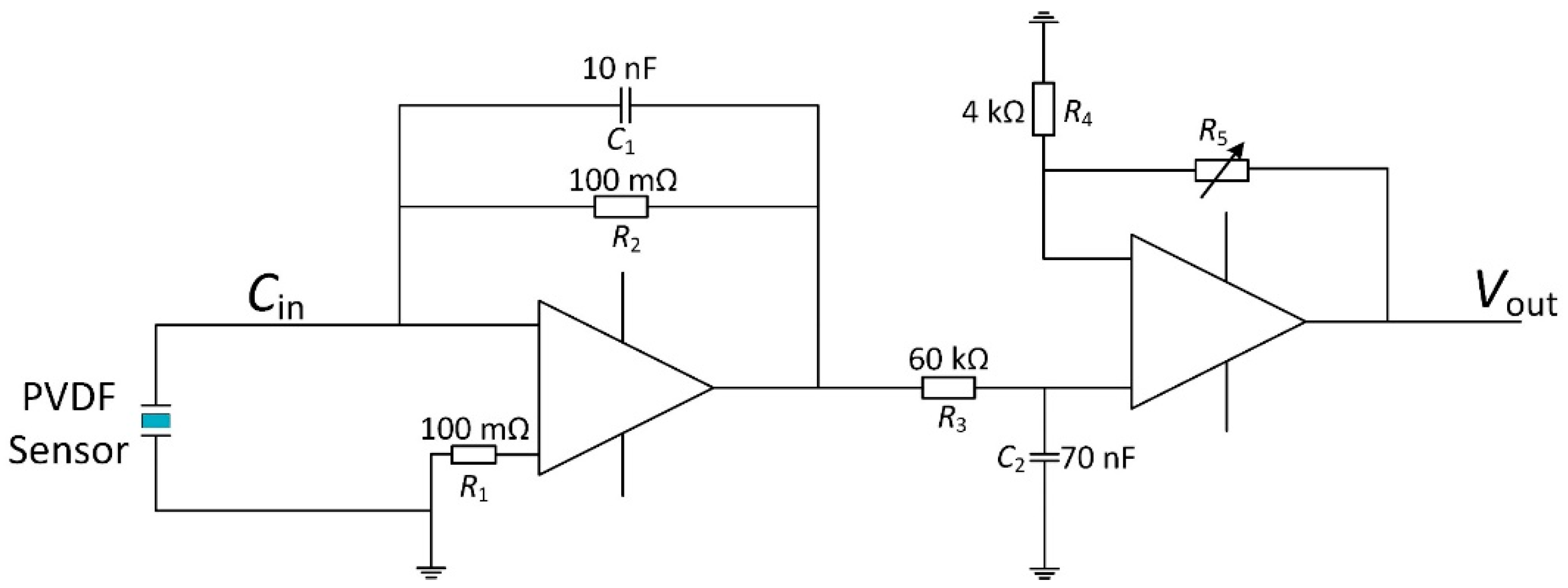
2.4. Sensor Electrical Model
2.5. Analysis of Force Sensor Configuration
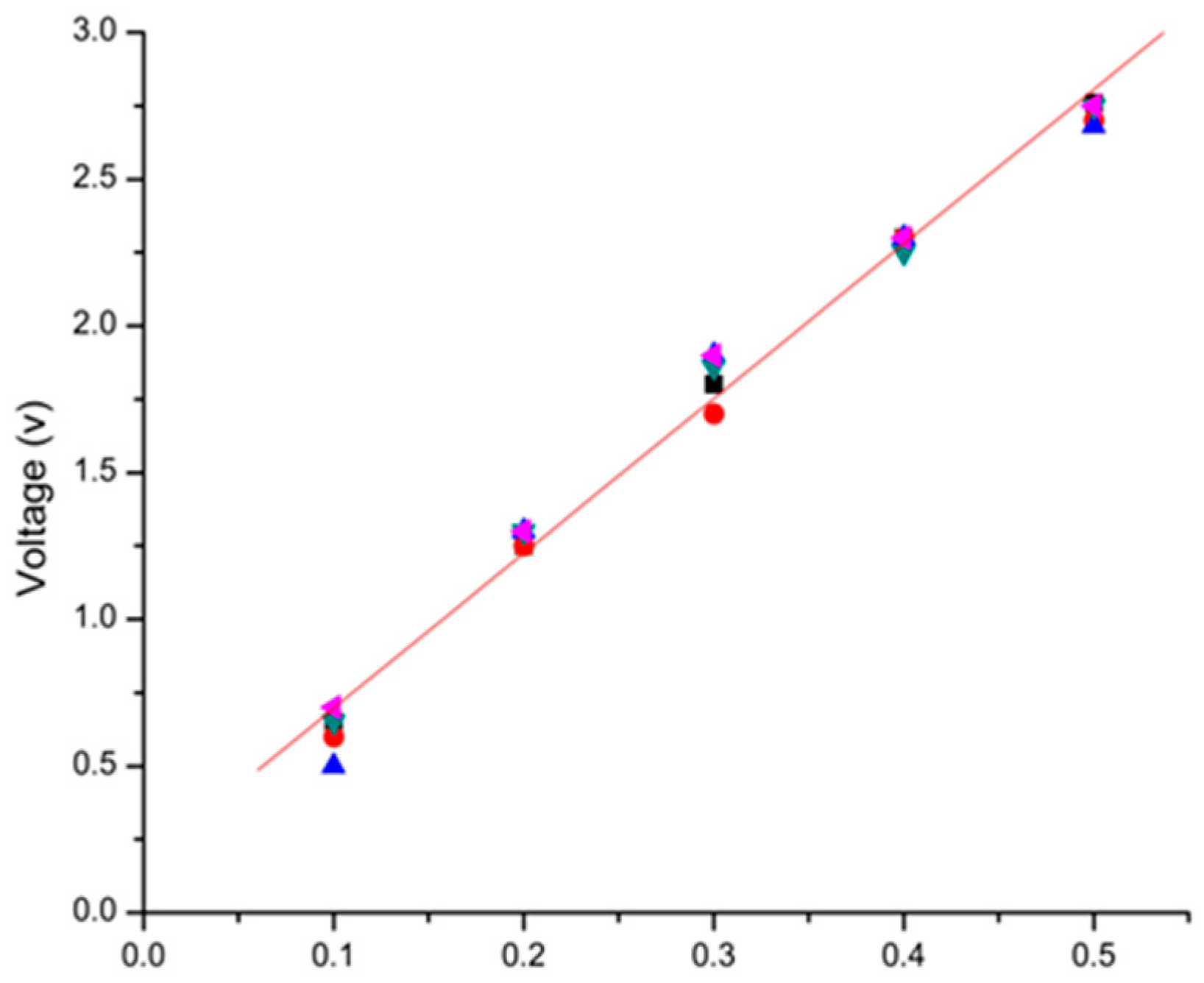
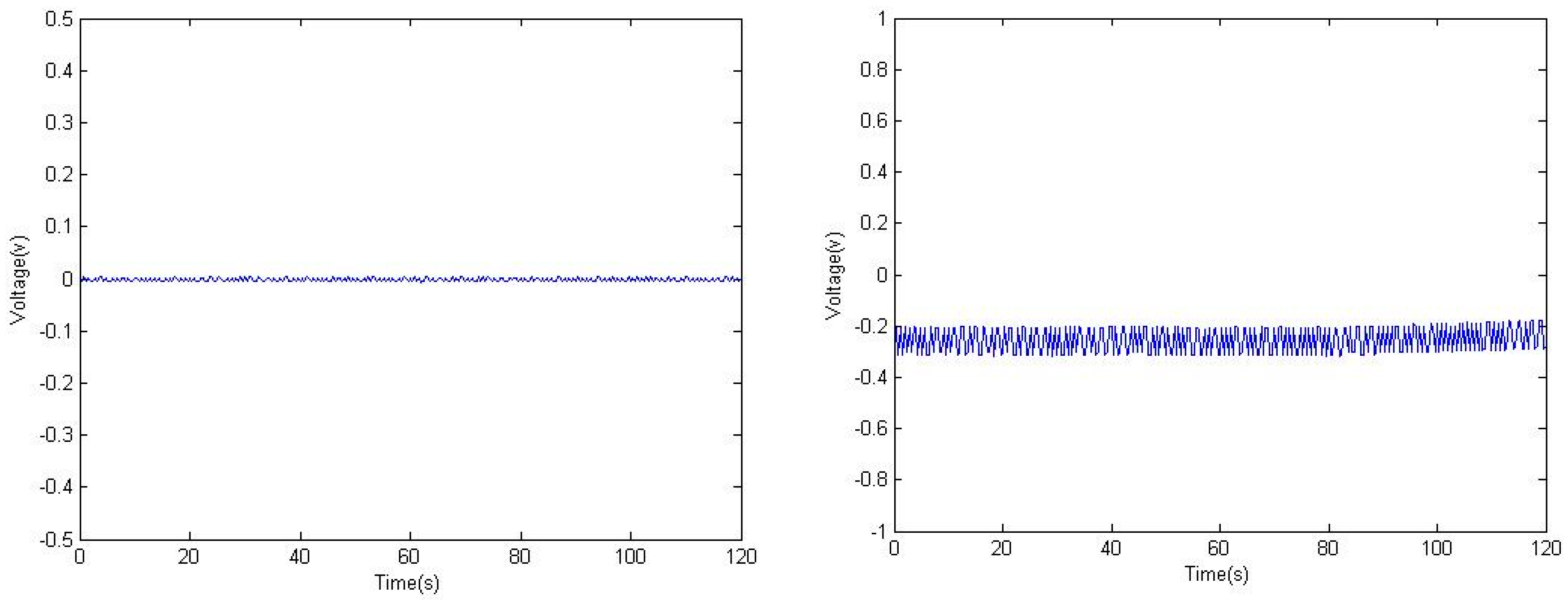
3. Calibration Experiments
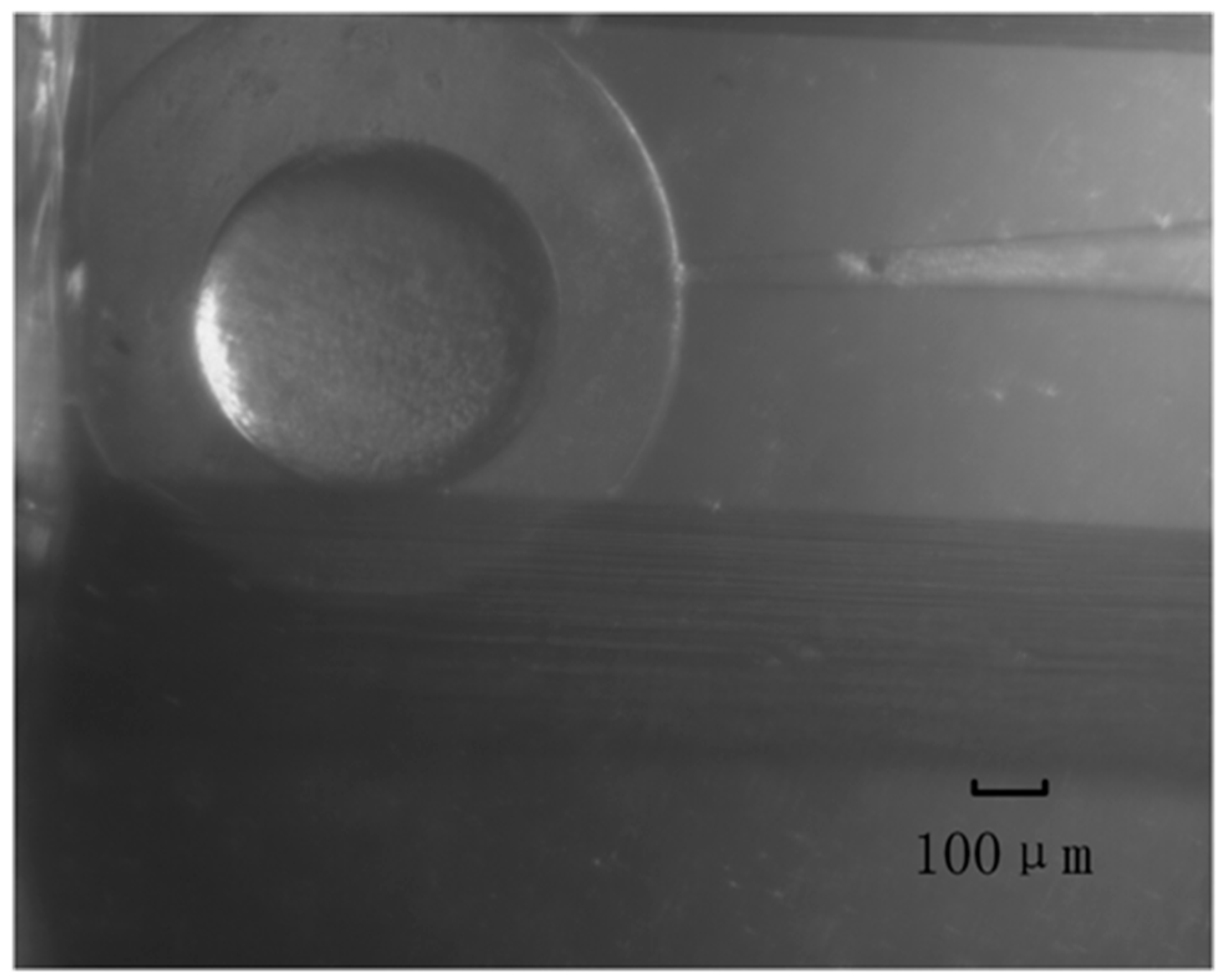
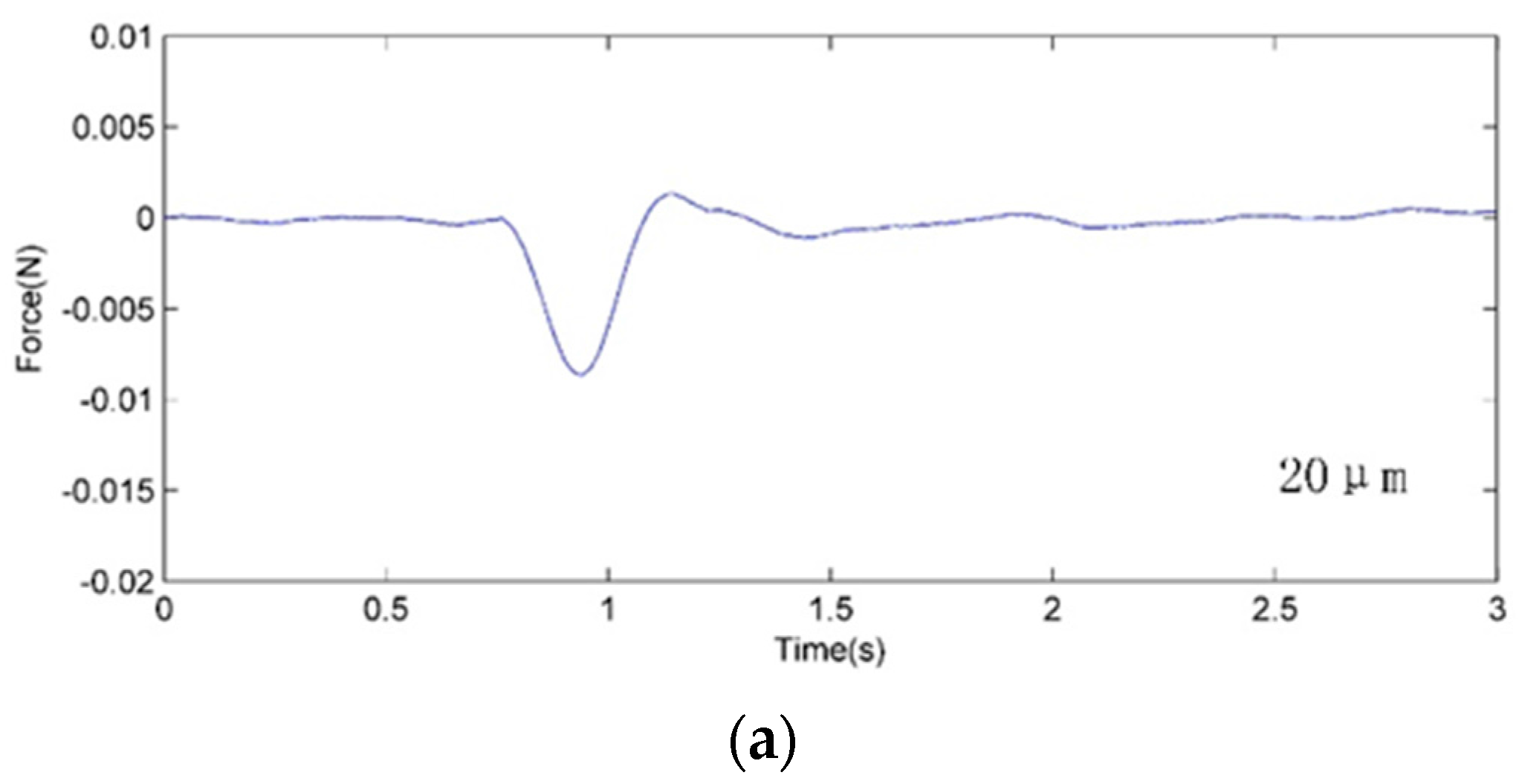
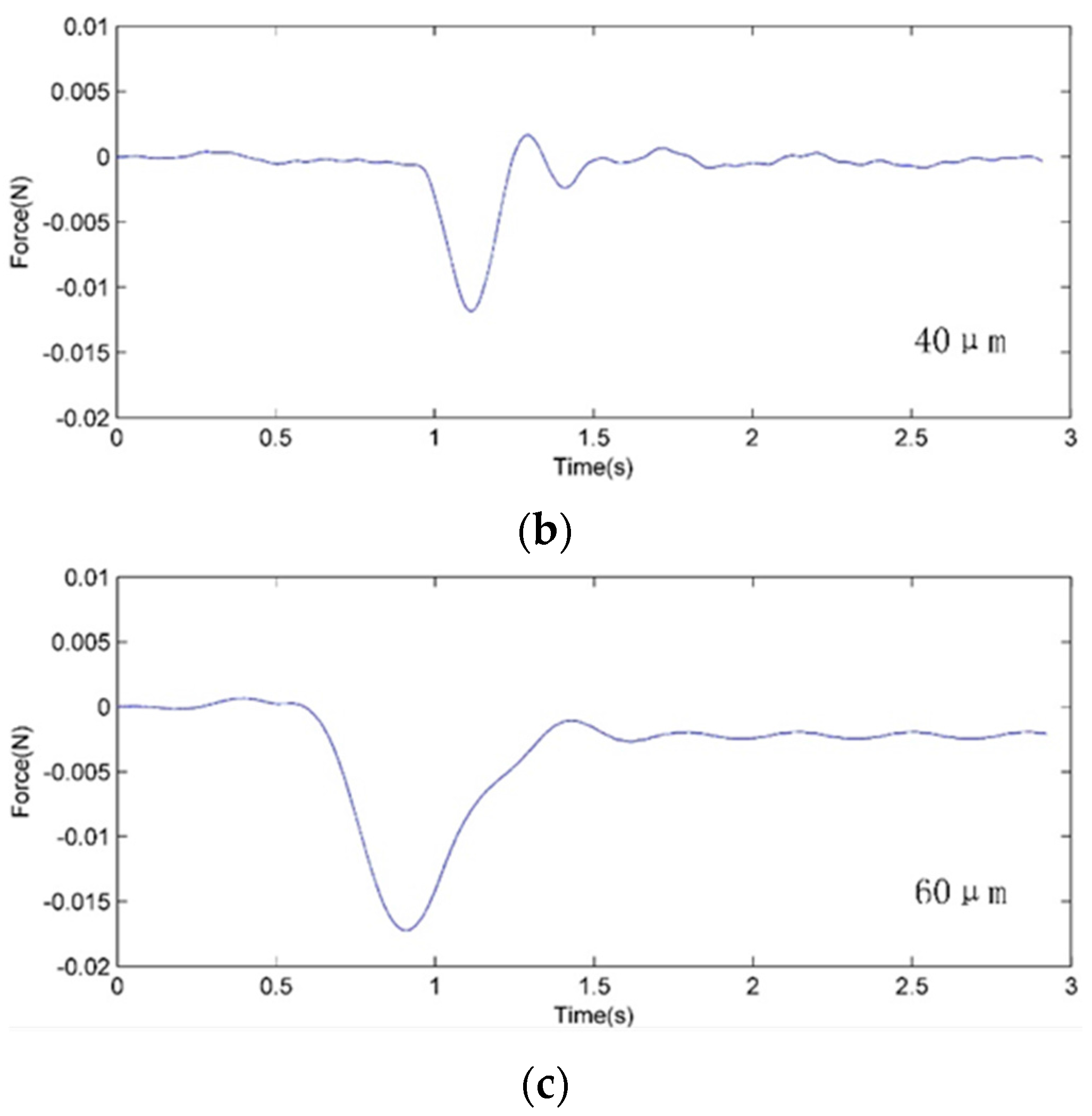
4. Cell Injection Experiment on Zebrafish Embryo
4.1. Experiment Materials and Setup
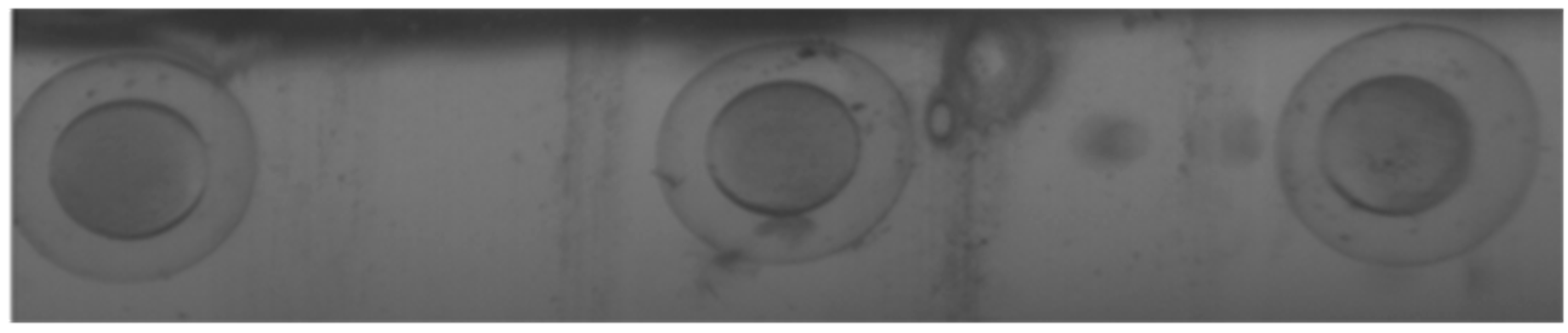
4.2. Experiment Method and Results
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Matsuoka, H.; Shimoda, S.; Miwa, Y.; Saito, M. Automatic positioning of a microinjector in mouse ES cells and rice protoplasts. Bioelectrochemistry 2006, 69, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Sun, D.; Mills, J.K.; Li, W.J. A visual impedance force control of a robotic cell injection system. In Proceedings of the IEEE International Conference on Robotics and Biomimetics, Kunming, China, 17–20 December 2006; pp. 233–238.
- Campbell, G.A.; Mutharasan, R.A.J. Escherichia coli O157: H7 detection limit of millimeter-sized PZT cantilever sensors is 700 cells/mL. Anal. Sci. 2005, 21, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Liu, X.Y.; Sun, Y. Autonomous zebrafish embryo injection using a microrobotic system. In Proceedings of the IEEE International Conference on Automation Science and Engineering, Scottsdale, AZ, USA, 22–25 September 2007; pp. 363–368.
- Kallio, P.; Ritala, T.; Lukkari, M.; Kuikka, S. Injection guidance system for cellular microinjections. Int. J. Robot. Res. 2007, 26, 1303–1313. [Google Scholar] [CrossRef]
- Liu, X.; Kim, K.; Zhang, Y.; Sun, Y. Nanonewton force sensing and control in microrobotic cell manipulation. Int. J. Robot. Res. 2009. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, P.C.Y.; Nam, J.; Ge, R.; Lin, W. A micromanipulation system with dynamic force-feedback for automatic batch microinjection. J. Micromech. Microeng. 2007, 17, 314. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Gelinas, D.; Ciruna, B.; Sun, Y. A fully automated robotic system for microinjection of zebrafish embryos. PLoS ONE 2007, 2, e862. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, R.; Alireza, T.M.; Taher, A.S. Drosophila Neurons Actively Regulate Axonal Tension in Vivo. Biophys. J. 2010, 99, 3208–3215. [Google Scholar]
- DiMaio, S.P.; Salcudean, S.E. Needle insertion modeling and simulation. IEEE Trans. Robot. Autom. 2003, 19, 864–875. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Y.; Wang, W.; Lansdorp, B.M. Vision-based cellular force measurement using an elastic microfabricated device. J. Micromech. Microeng. 2007, 17, 1281. [Google Scholar] [CrossRef]
- Ohara, K.; Kawakami, D.; Takubo, T.; Mae, Y.; Tanikawa, T.; Honda, A.; Arai, T. Dextrous cell diagnosis using two-fingered microhand with micro force sensor. J. Micro Nano Mech. 2012, 7, 13–20. [Google Scholar] [CrossRef]
- Boresi, A.P.; Schmidt, R.J.; Sidebottom, O.M. Advanced Mechanics of Materials; Wiley: New York, NY, USA, 1993. [Google Scholar]
- Xie, Y.; Sun, D.; Tse, H.Y.; Liu, C.; Cheng, S.H. Force Characteristics in Robot-Assisted Microinjections on Zebrafish Embryos. IEEE Trans. Mech. 2011, 16, 1002–1010. [Google Scholar] [CrossRef]
- Verbraecken, J. Applications of evolving technologies in sleep medicine. Breathe 2013, 9, 442–455. [Google Scholar] [CrossRef]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Zhou, Y.; Lin, Y.; Wang, L.; Xi, W. Development of a Microforce Sensor and Its Array Platform for Robotic Cell Microinjection Force Measurement. Sensors 2016, 16, 483. https://doi.org/10.3390/s16040483
Xie Y, Zhou Y, Lin Y, Wang L, Xi W. Development of a Microforce Sensor and Its Array Platform for Robotic Cell Microinjection Force Measurement. Sensors. 2016; 16(4):483. https://doi.org/10.3390/s16040483
Chicago/Turabian StyleXie, Yu, Yunlei Zhou, Yuzi Lin, Lingyun Wang, and Wenming Xi. 2016. "Development of a Microforce Sensor and Its Array Platform for Robotic Cell Microinjection Force Measurement" Sensors 16, no. 4: 483. https://doi.org/10.3390/s16040483




