Spectroscopic Terahertz Imaging at Room Temperature Employing Microbolometer Terahertz Sensors and Its Application to the Study of Carcinoma Tissues
Abstract
:1. Introduction
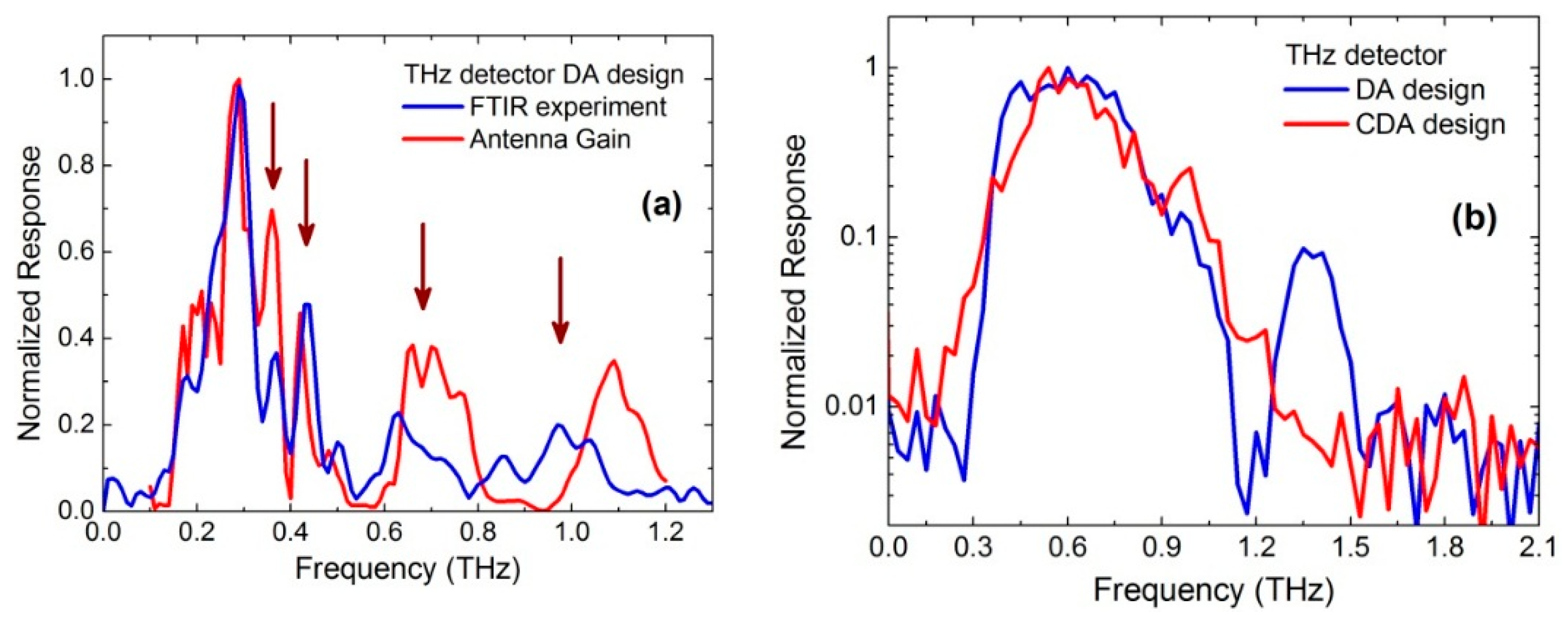
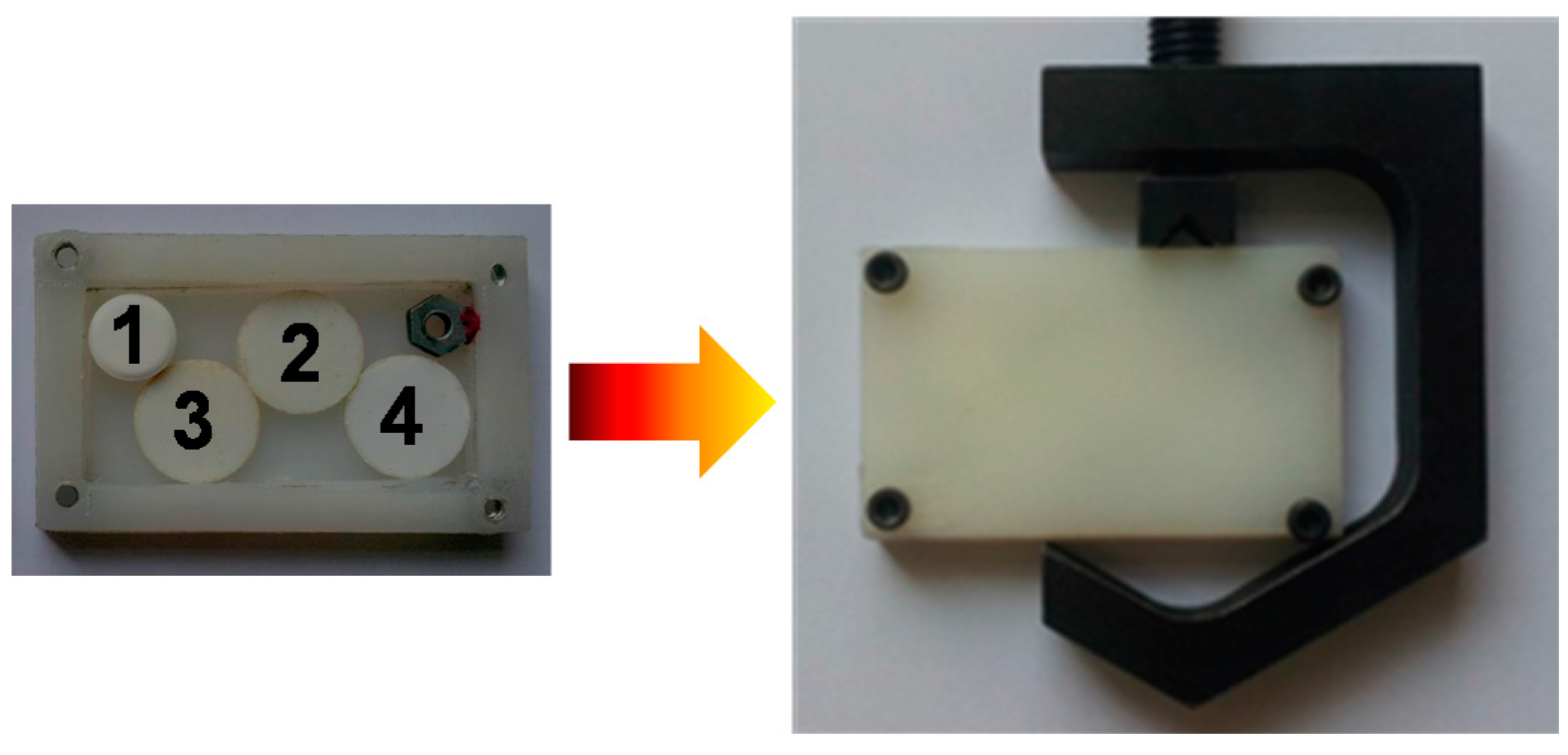
2. Antenna Coupled Titanium Microbolometer Sensors
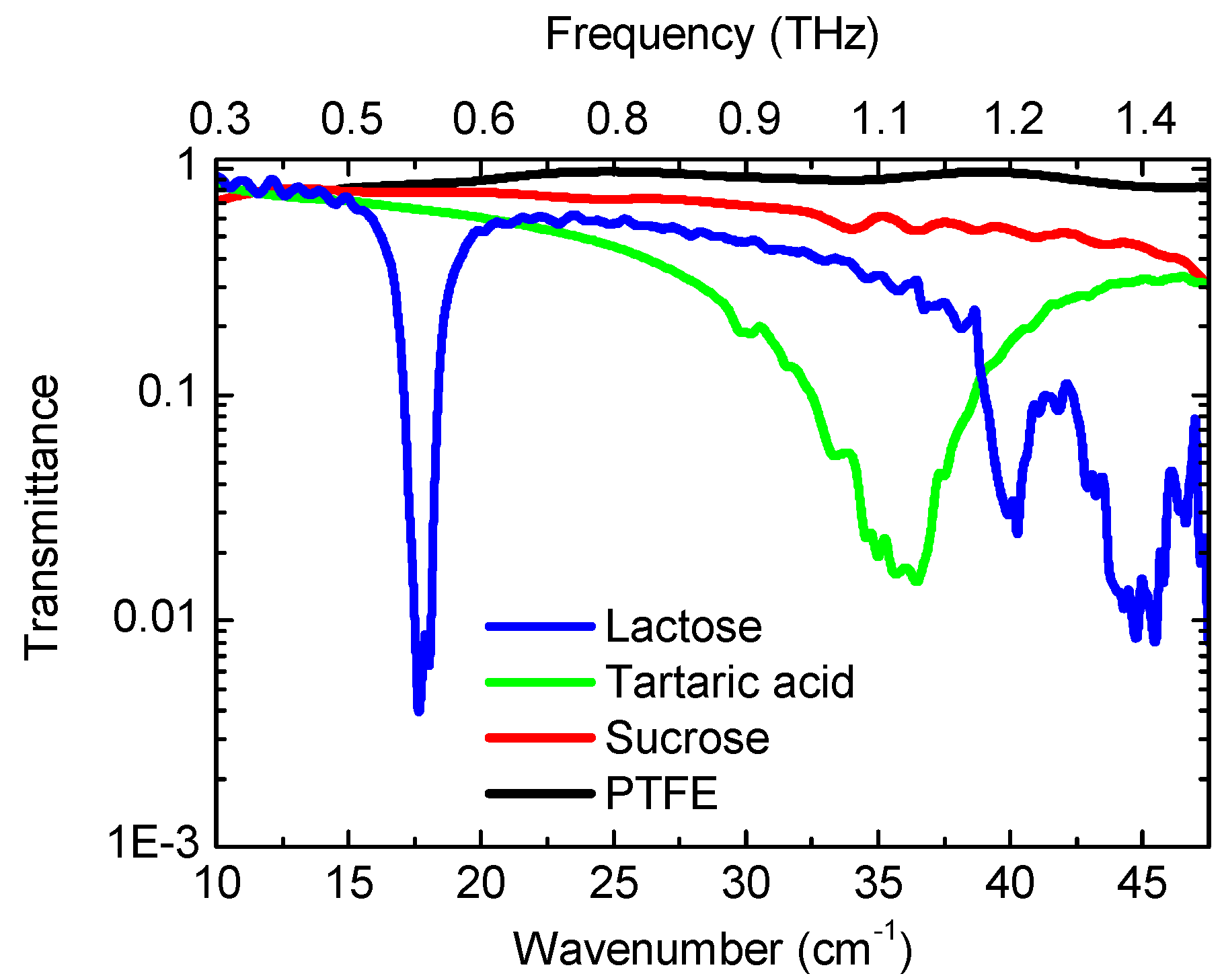
3. Multispectral THz Imaging
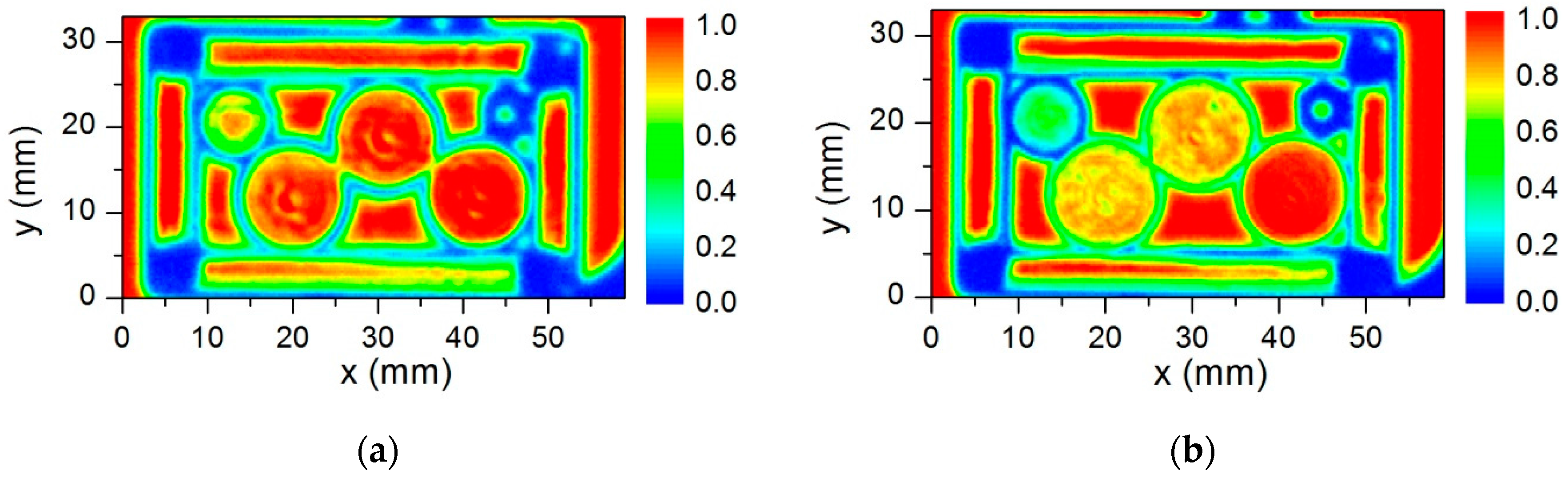
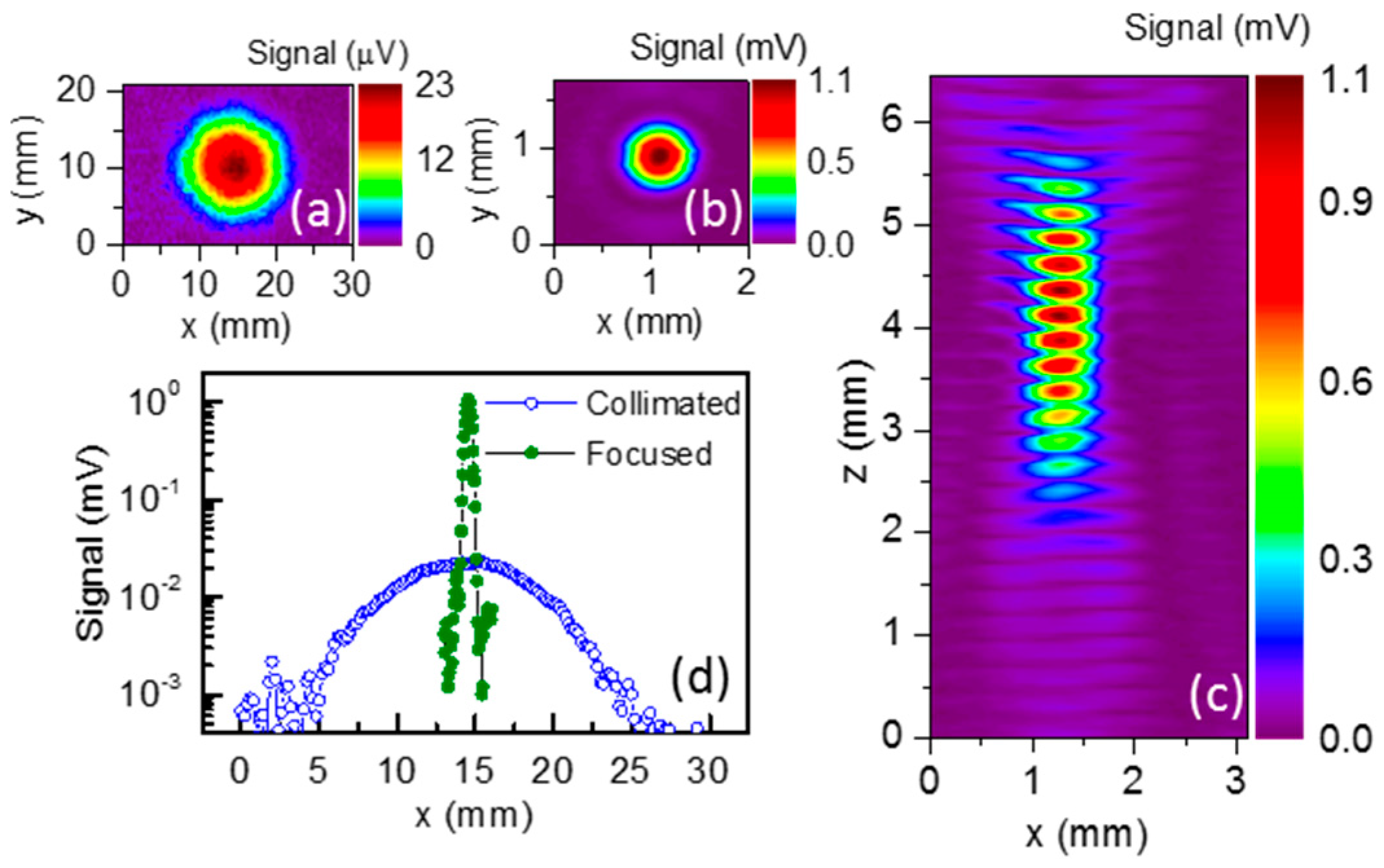
4. Diffractive THz Components for High Spatial Resolution Imaging
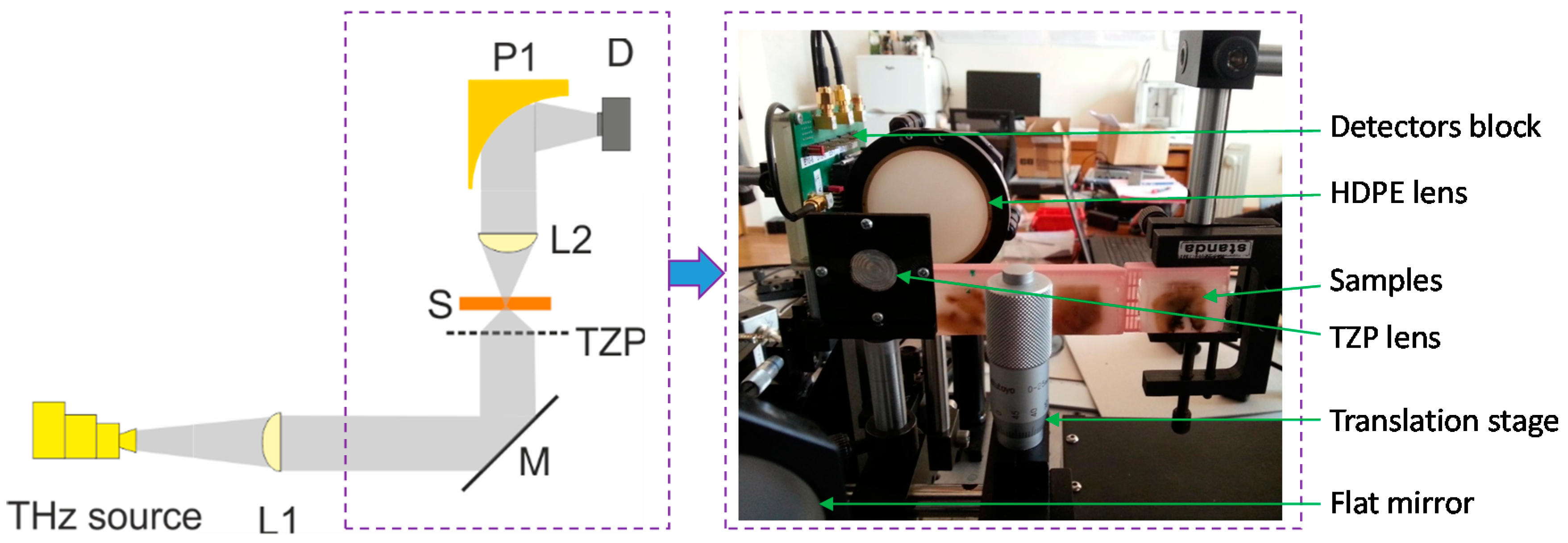
5. A Compact THz Imaging System for Medical Applications
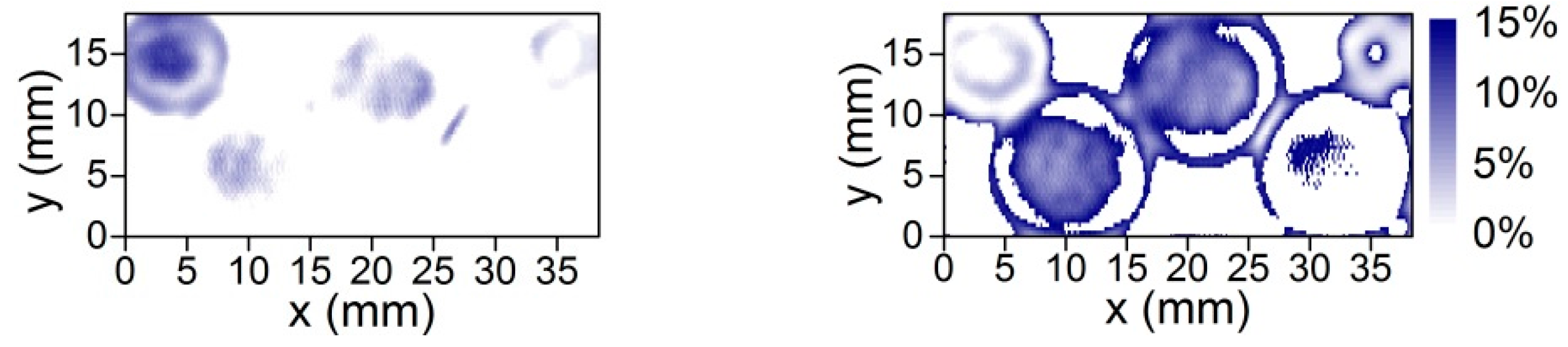
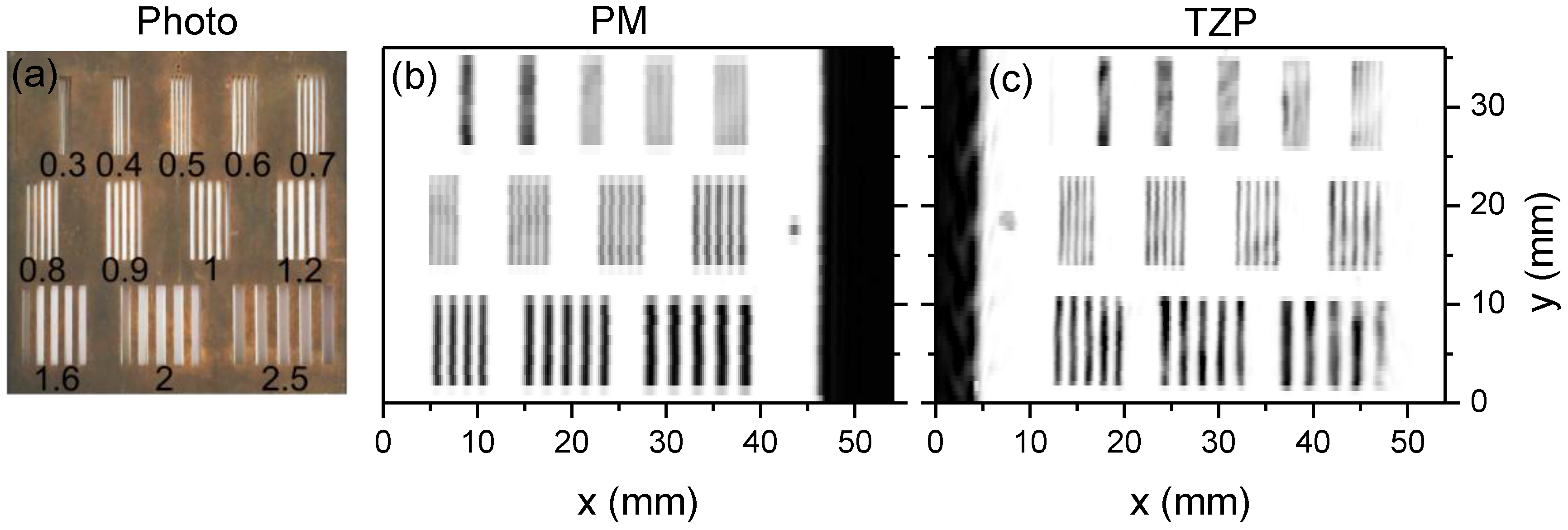
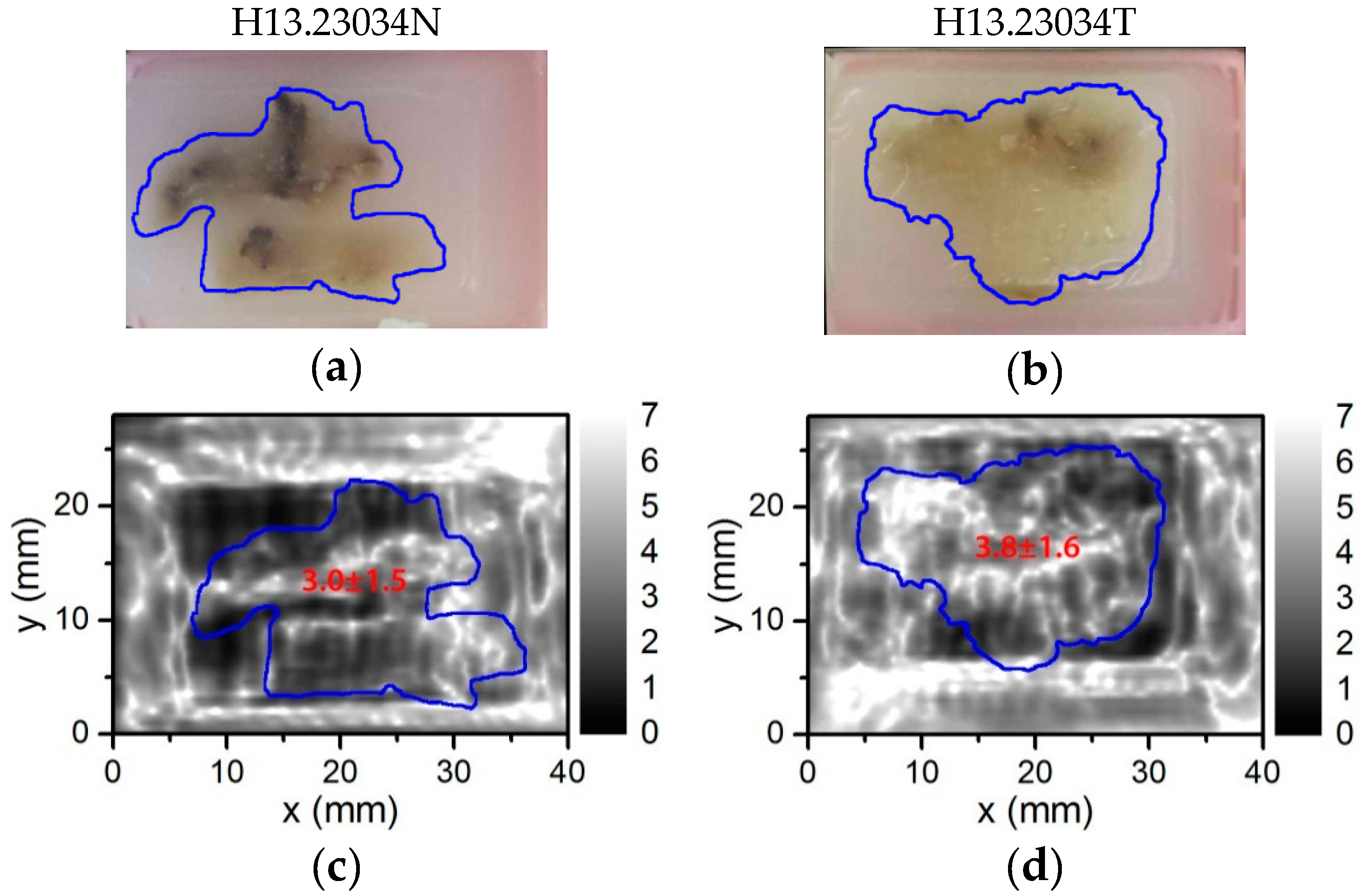
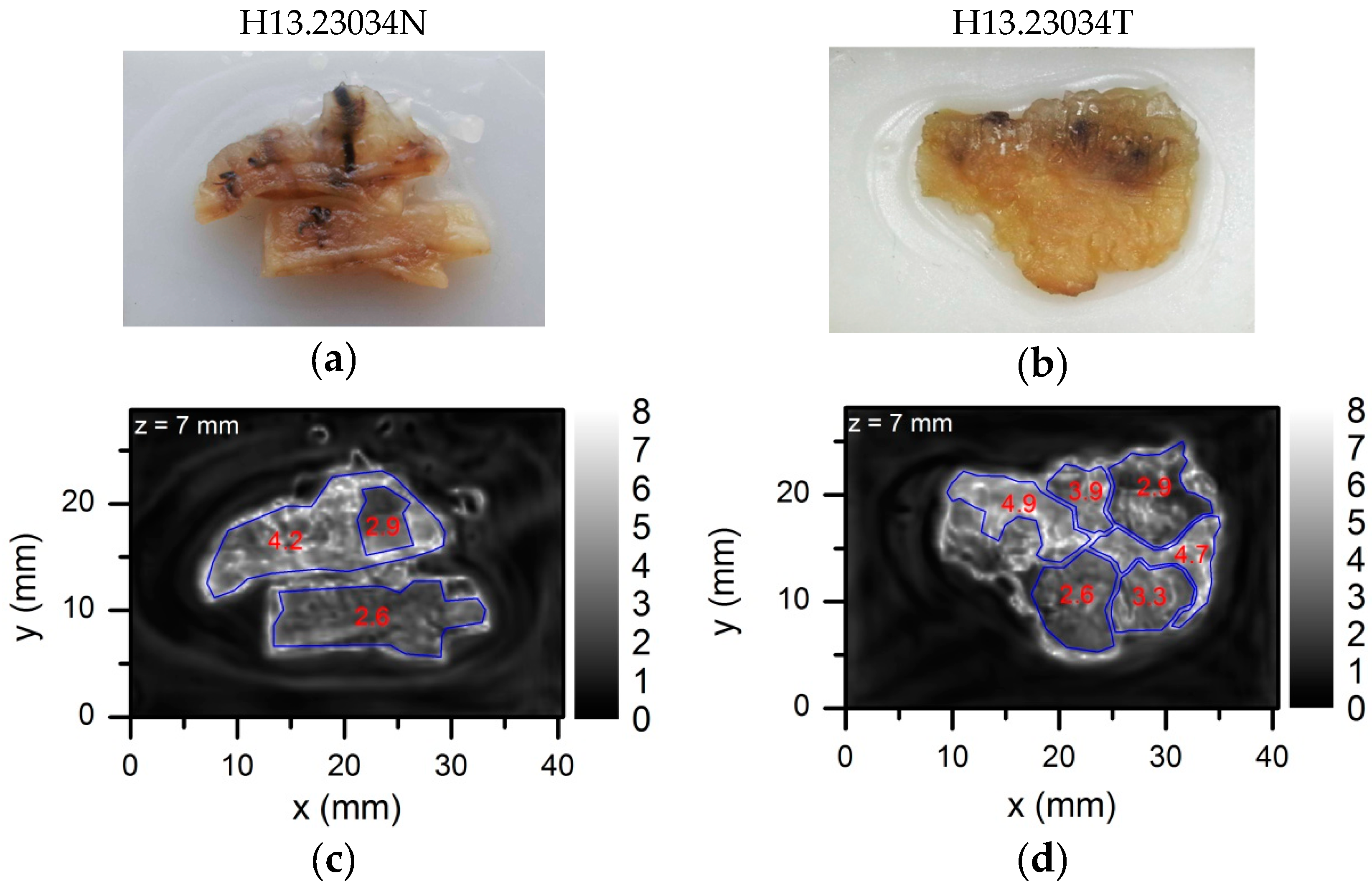
5.1. Demonstration of Compact THz Imaging System Performance
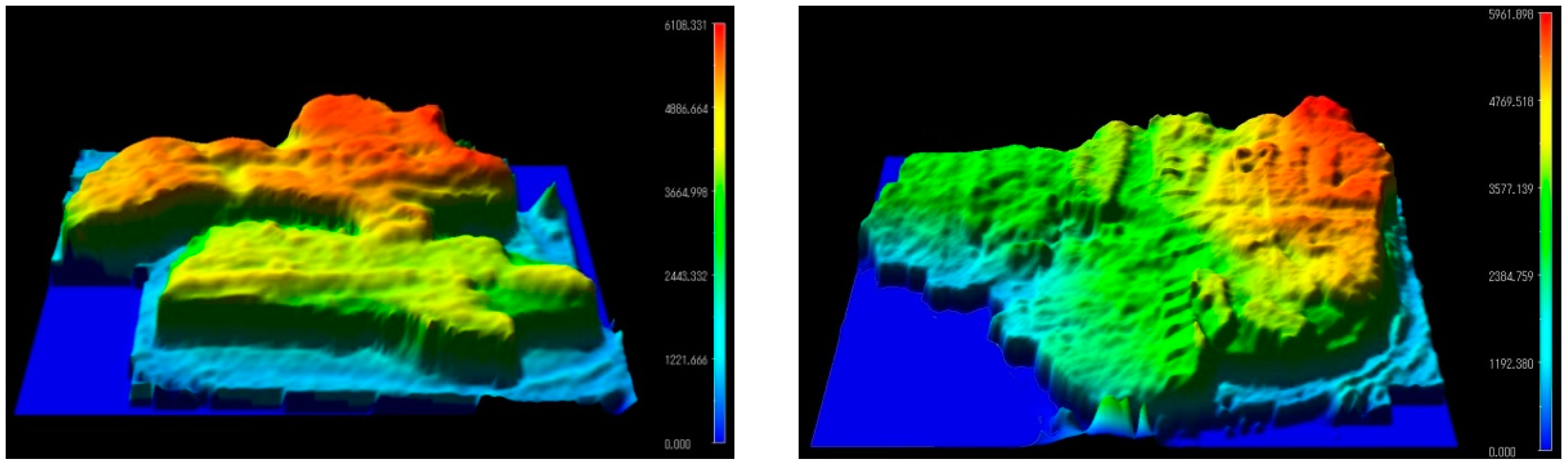
5.2. Towards Confocal THz Microscopy
6. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| THz | Terahertz |
| RT | Room temperature |
| NEP | Noise-equivalent-power |
| FET | Fielf effect transistor |
| TeraFET | Terahertz antenna-coupled fielf effect transistor |
| QCL | Quantum cascade laser |
| PTFE | Polytetrafluoroethylene |
| PE | Polyethylene |
| FTIR | Fourier transform infrared |
| NBMS | Narrow band microbolometer terahertz sensor |
| THz TDS | Terahertz time domain spectrometer |
| TZP | Terahertz zone plate with integrated resonant filter apertures |
| CMOS | Complementary metal-oxide semiconductor |
| DA | Double dipole antenna |
| CDA | Cross dipole antenna |
| PM | Parabolic mirror |
| D∗ | Relative detectivity |
| SNR | Signal to noise ratio |
| ROI | Range of interest |
References
- Chan, W.L.; Deibel, J.; Mittleman, D.M. Imaging with Terahertz Radiation. Reports Prog. Phys. 2007, 70, 1325–1379. [Google Scholar] [CrossRef]
- Siegel, P.H. Terahertz Technology in Biology and Medicine. IEEE Trans. Microw. Theory Techn. 2004, 52, 2438–2447. [Google Scholar] [CrossRef]
- Yu, C.; Fan, S.; Sun, Y.; Pickwell-Macpherson, E. The Potential of Terahertz Imaging for Cancer Diagnosis: A Review of Investigations to Date. Quant. Imaging Med. Surg. 2012, 2, 33–45. [Google Scholar] [PubMed]
- Mickan, S.P.; Menikh, A.; Liu, H.; Mannella, C.A.; MacColl, R.; Abbott, D.; Munch, J.; Zhang, X.C. Label-Free Bioaffinity Detection Using Terahertz Technology. Phys. Med. Biol. 2002, 47, 3789–3795. [Google Scholar] [CrossRef] [PubMed]
- Dean, P.; Valavanis, A.; Keeley, J.; Bertling, K.; Lim, Y.L.; Alhathlool, R.; Burnett, A.D.; Li, L.H.; Khanna, S.P.; Indjin, D.; et al. Terahertz Imaging Using Quantum Cascade Lasers—a Review of Systems and Applications. J. Phys. D. Appl. Phys. 2014, 47, 374008. [Google Scholar] [CrossRef]
- Kasalynas, I.; Venckevicius, R.; Valusis, G. Continuous Wave Spectroscopic Terahertz Imaging With InGaAs Bow-Tie Diodes at Room Temperature. IEEE Sens. J. 2013, 13, 50–54. [Google Scholar] [CrossRef]
- Bauer, M.; Venckevičius, R.; Kašalynas, I.; Boppel, S.; Mundt, M.; Minkevičius, L.; Lisauskas, A.; Valušis, G.; Krozer, V.; Roskos, H.G. Antenna-Coupled Field-Effect Transistors for Multi-Spectral Terahertz Imaging up to 4.25 THz. Opt. Express 2014, 22, 19235–19241. [Google Scholar] [CrossRef] [PubMed]
- Simoens, F.; Meilhan, J.; Nicolas, J.-A. Terahertz Real-Time Imaging Uncooled Arrays Based on Antenna-Coupled Bolometers or FET Developed at CEA-Leti. J. Infrared, Millimeter, Terahertz Waves 2015, 36, 961–985. [Google Scholar] [CrossRef]
- Oda, N.; Kurashina, S.; Miyoshi, M.; Doi, K.; Ishi, T.; Sudou, T.; Morimoto, T.; Goto, H.; Sasaki, T. Microbolometer Terahertz Focal Plane Array and Camera with Improved Sensitivity in the Sub-Terahertz Region. J. Infrared, Millimeter, Terahertz Waves 2015, 36, 947–960. [Google Scholar] [CrossRef]
- Al Hadi, R.; Sherry, H.; Grzyb, J.; Zhao, Y.; Förster, W.; Keller, H.M.; Cathelin, A.; Kaiser, A.; Pfeiffer, U.R. A 1 K-Pixel Video Camera for 0.7-1.1 Terahertz Imaging Applications in 65-Nm CMOS. IEEE J. Solid-State Circuits 2012, 47, 2999–3012. [Google Scholar] [CrossRef]
- Dufour, D.; Marchese, L.; Terroux, M.; Oulachgar, H.; Généreux, F.; Doucet, M.; Mercier, L.; Tremblay, B.; Alain, C.; Beaupré, P.; et al. Review of Terahertz Technology Development at INO. J. Infrared, Millimeter, Terahertz Waves 2015, 36, 922–946. [Google Scholar] [CrossRef]
- Kasalynas, I.; Adam, A.J.L.; Klaassen, T.O.; Hovenier, J.N.; Pandraud, G.; Iordanov, V.P.; Sarro, P.M. Design and Performance of a Room-Temperature Terahertz Detection Array for Real-Time Imaging. IEEE J. Sel. Top. Quantum Electron. 2008, 14, 363–369. [Google Scholar] [CrossRef]
- Grant, J.; Escorcia-Carranza, I.; Li, C.; Mccrindle, I.J.H.; Gough, J.; Cumming, D.R.S. A Monolithic Resonant Terahertz Sensor Element Comprising a Metamaterial Absorber and Micro-Bolometer. Laser Photonics Rev. 2013, 7, 1043–1048. [Google Scholar] [CrossRef]
- Hack, E.; Valzania, L.; Gäumann, G.; Shalaby, M.; Hauri, C.; Zolliker, P. Comparison of Thermal Detector Arrays for Off-Axis THz Holography and Real-Time THz Imaging. Sensors 2016, 16, 221. [Google Scholar] [CrossRef] [PubMed]
- Dyakonov, M.I.; Shur, M.S. Plasma Wave Electronics: Novel Terahertz Devices Using Two Dimensional Electron Fluid. IEEE Trans. Electron Devices 1996, 43, 1640–1645. [Google Scholar] [CrossRef]
- Lisauskas, A.; Pfeiffer, U.; Öjefors, E.; Bolívar, P.H.; Glaab, D.; Roskos, H.G. Rational Design of High-Responsivity Detectors of Terahertz Radiation Based on Distributed Self-Mixing in Silicon Field-Effect Transistors. J. Appl. Phys. 2009, 105, 114511. [Google Scholar] [CrossRef]
- Schuster, F.; Coquillat, D.; Videlier, H.; Sakowicz, M.; Teppe, F.; Dussopt, L.; Giffard, B.; Skotnicki, T.; Knap, W. Broadband Terahertz Imaging with Highly Sensitive Silicon CMOS Detectors. Opt. Express 2011, 19, 7827. [Google Scholar] [CrossRef] [PubMed]
- Boppel, S.; Lisauskas, A.; Mundt, M.; Seliuta, D.; Minkevičius, L.; Kašalynas, I.; Valušis, G.; Mittendorff, M.; Winnerl, S.; Krozer, V.; et al. CMOS Integrated Antenna-Coupled Field-Effect Transistors for the Detection of Radiation from 0.2 to 4.3 THz. IEEE Trans. Microw. Theory Tech. 2012, 60, 3834–3843. [Google Scholar] [CrossRef]
- Sakhno, M.; Gumenjuk-Sichevska, J.; Sizov, F. Modeling of the Substrate Influence on Multielement THz Detector Operation. J. Infrared, Millimeter, Terahertz Waves 2014, 35, 703–719. [Google Scholar] [CrossRef]
- Zdanevičius, J.; Bauer, M.; Boppel, S.; Palenskis, V.; Lisauskas, A.; Krozer, V.; Roskos, H.G. Camera for High-Speed THz Imaging. J. Infrared, Millimeter, Terahertz Waves 2015, 36, 986–997. [Google Scholar] [CrossRef]
- Seliuta, D.; Kašalynas, I.; Tamošiūnas, V.; Balakauskas, S.; Martūnas, Z.; Ašmontas, S.; Valušis, G.; Lisauskas, A.; Roskos, H.G.; Köhler, K. Silicon Lens-Coupled Bow-Tie InGaAs-Based Broadband Terahertz Sensor Operating at Room Temperature. Electron. Lett. 2006, 42, 825. [Google Scholar] [CrossRef]
- Kašalynas, I.; Venckevičius, R.; Seliuta, D.; Grigelionis, I.; Valušis, G. InGaAs-Based Bow-Tie Diode for Spectroscopic Terahertz Imaging. J. Appl. Phys. 2011, 110, 114505. [Google Scholar] [CrossRef]
- Minkevičius, L.; Madeikis, K.; Kašalynas, I.; Venckevičius, R.; Seliuta, D.; Tamošiūnas, V.; Valušis, G. Discrete spectrum terahertz imaging using bow-tie diodes: Optimized antenna designs and arrays. Proc. SPIE 2013. [Google Scholar] [CrossRef]
- Amanti, M.I.; Scalari, G.; Beck, M.; Faist, J. Stand-Alone System for High-Resolution, Real-Time Terahertz Imaging. Opt. Express 2012, 20, 2772. [Google Scholar] [CrossRef] [PubMed]
- De Cumis, U.S.; Xu, J.-H.; Masini, L.; Degl’Innocenti, R.; Pingue, P.; Beltram, F.; Tredicucci, A.; Vitiello, M.S.; Benedetti, P.A.; Beere, H.E.; et al. Terahertz Confocal Microscopy with a Quantum Cascade Laser Source. Opt. Express 2012, 20, 21924. [Google Scholar] [CrossRef] [PubMed]
- Trontelj, J.; Sešek, A. Micro-machined millimeter wave sensor array for FM radar application. Proc. SPIE 2012. [Google Scholar] [CrossRef]
- Simoens, F.; Meilhan, J. Terahertz Real-Time Imaging Uncooled Array Based on Antenna- and Cavity-Coupled Bolometers. Philos. Trans. A. Math. Phys. Eng. Sci. 2014, 372, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Trontelj, J.; Valušis, G.; Venckevičius, R.; Kašalynas, I.; Sešek, A.; Švigelj, A. A high performance room temperature THz sensor. Proc. SPIE 2014. [Google Scholar] [CrossRef]
- Kasalynas, I.; Svigelj, A.; Adamonis, J.; Kornijcuk, V. Performance of the antenna coupled microbolometers characterized by the quasi-optical measurements at frequencies 0.1–1.0 THz. In Proceedings of the 2013 38th International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), Mainz, Germany, 1–6 September 2013.
- Minkevičius, L.; Voisiat, B.; Mekys, A.; Venckevičius, R.; Kašalynas, I.; Seliuta, D.; Valušis, G.; Račiukaitis, G.; Tamošiūnas, V. Terahertz Zone Plates with Integrated Laser-Ablated Bandpass Filters. Electron. Lett. 2013, 49, 49–50. [Google Scholar] [CrossRef]
- Minkevičius, L.; Madeikis, K.; Voisiat, B.; Kašalynas, I.; Venckevičius, R.; Račiukaitis, G.; Tamošiūnas, V.; Valušis, G. Focusing Performance of Terahertz Zone Plates with Integrated Cross-Shape Apertures. J. Infrared, Millimeter, Terahertz Waves 2014, 35, 699–702. [Google Scholar] [CrossRef]
- Minkevičius, L.; Tamošiūnas, V.; Madeikis, K.; Voisiat, B.; Kašalynas, I.; Valušis, G. On-Chip Integration of Laser-Ablated Zone Plates for Detection Enhancement of InGaAs Bow-Tie Terahertz Detectors. Electron. Lett. 2014, 50, 1367–1369. [Google Scholar] [CrossRef]
- Löffler, T.; Bauer, T.; Siebert, K.; Roskos, H.; Fitzgerald, A.; Czasch, S. Terahertz Dark-Field Imaging of Biomedical Tissue. Opt. Express 2001, 9, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Woodward, R.M.; Wallace, V.P.; Arnone, D.D.; Linfield, E.H.; Pepper, M. Terahertz Pulsed Imaging of Skin Cancer in the Time and Frequency Domain. J. Biol. Phys. 2003, 29, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Wahaia, F.; Valusis, G.; Bernardo, L.M.; Almeida, A.; Moreira, J.A.; Lopes, P.C.; Macutkevic, J.; Kasalynas, I.; Seliuta, D.; Adomavicius, R.; et al. Detection of Colon Cancer by Terahertz Techniques. J. Mol. Struct. 2011, 1006, 77–82. [Google Scholar] [CrossRef]
- Png, G.M.; Choi, J.W.; Ng, B.W.-H.; Mickan, S.P.; Abbott, D.; Zhang, X.-C. The Impact of Hydration Changes in Fresh Bio-Tissue on THz Spectroscopic Measurements. Phys. Med. Biol. 2008, 53, 3501–3517. [Google Scholar] [CrossRef] [PubMed]
- Wallace, V.P.; Fitzgerald, A.J.; Pickwell, E.; Pye, R.J.; Taday, P.F.; Flanagan, N.; Ha, T. Terahertz Pulsed Spectroscopy of Human Basal Cell Carcinoma. Appl. Spectrosc. 2006, 60, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Doradla, P.; Alavi, K.; Joseph, C.; Giles, R. Detection of Colon Cancer by Continuous-Wave Terahertz Polarization Imaging Technique. J. Biomed. Opt. 2013, 18, 090504. [Google Scholar] [CrossRef]
- Minkevicius, L.; Madeikis, K.; Voisiat, B.; Tamosnmas, V. Compact diffractive optical components for terahertz beam manipulation. In Proceedings of the 2015 40th International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), Hong Kong, China, 23–28 August 2015.
- Kasalynas, I.; Venckevičius, R.; Adomavičius, R.; Valušis, G. Antenna-coupled microbolometer-based THz detectors for room temperature beam profile imaging of the photoconductive THz pulse emitters. In Proceedings of the 2014 39th International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), Tucson, AZ, USA, 14–19 September 2014.
- Voisiat, B.; Račiukaitis, G.; Kašalynas, I. THz components and plasmonic structures fabricated by direct laser patterning of metals. In Proceedings of the 2014 39th International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), Tucson, AZ, USA, 14–19 September 2014.
- Salhi, M.A.; Pupeza, I.; Koch, M. Confocal THz laser microscope. J. Infrared Millim. Terahertz Waves 2010, 31, 358–366. [Google Scholar] [CrossRef]
- Wahaia, F.; Kasalynas, I.; Venckevicius, R.; Seliuta, D.; Valusis, G.; Urbanowicz, A.; Molis, G.; Carneiro, F.; Carvalho Silva, C.D.; Granja, P.L. Terahertz absorption and reflection imaging of carcinoma-affected colon tissues embedded in paraffin. J. Mol. Struct. 2016, 1107, 214–219. [Google Scholar] [CrossRef]
- Wahaia, F.; Kasalynas, I.; Seliuta, D.; Molis, G.; Urbanowicz, A.; Carvalho Silva, C.D.; Carneiro, F.; Valusis, G.; Granja, P.L. Study of paraffin-embedded colon cancer tissue using terahertz spectroscopy. J. Mol. Struct. 2015, 1079, 448–453. [Google Scholar] [CrossRef]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kašalynas, I.; Venckevičius, R.; Minkevičius, L.; Sešek, A.; Wahaia, F.; Tamošiūnas, V.; Voisiat, B.; Seliuta, D.; Valušis, G.; Švigelj, A.; et al. Spectroscopic Terahertz Imaging at Room Temperature Employing Microbolometer Terahertz Sensors and Its Application to the Study of Carcinoma Tissues. Sensors 2016, 16, 432. https://doi.org/10.3390/s16040432
Kašalynas I, Venckevičius R, Minkevičius L, Sešek A, Wahaia F, Tamošiūnas V, Voisiat B, Seliuta D, Valušis G, Švigelj A, et al. Spectroscopic Terahertz Imaging at Room Temperature Employing Microbolometer Terahertz Sensors and Its Application to the Study of Carcinoma Tissues. Sensors. 2016; 16(4):432. https://doi.org/10.3390/s16040432
Chicago/Turabian StyleKašalynas, Irmantas, Rimvydas Venckevičius, Linas Minkevičius, Aleksander Sešek, Faustino Wahaia, Vincas Tamošiūnas, Bogdan Voisiat, Dalius Seliuta, Gintaras Valušis, Andrej Švigelj, and et al. 2016. "Spectroscopic Terahertz Imaging at Room Temperature Employing Microbolometer Terahertz Sensors and Its Application to the Study of Carcinoma Tissues" Sensors 16, no. 4: 432. https://doi.org/10.3390/s16040432





