Sinabro: A Smartphone-Integrated Opportunistic Electrocardiogram Monitoring System
Abstract
:1. Introduction
2. Potential Opportunities for Daily ECG Monitoring
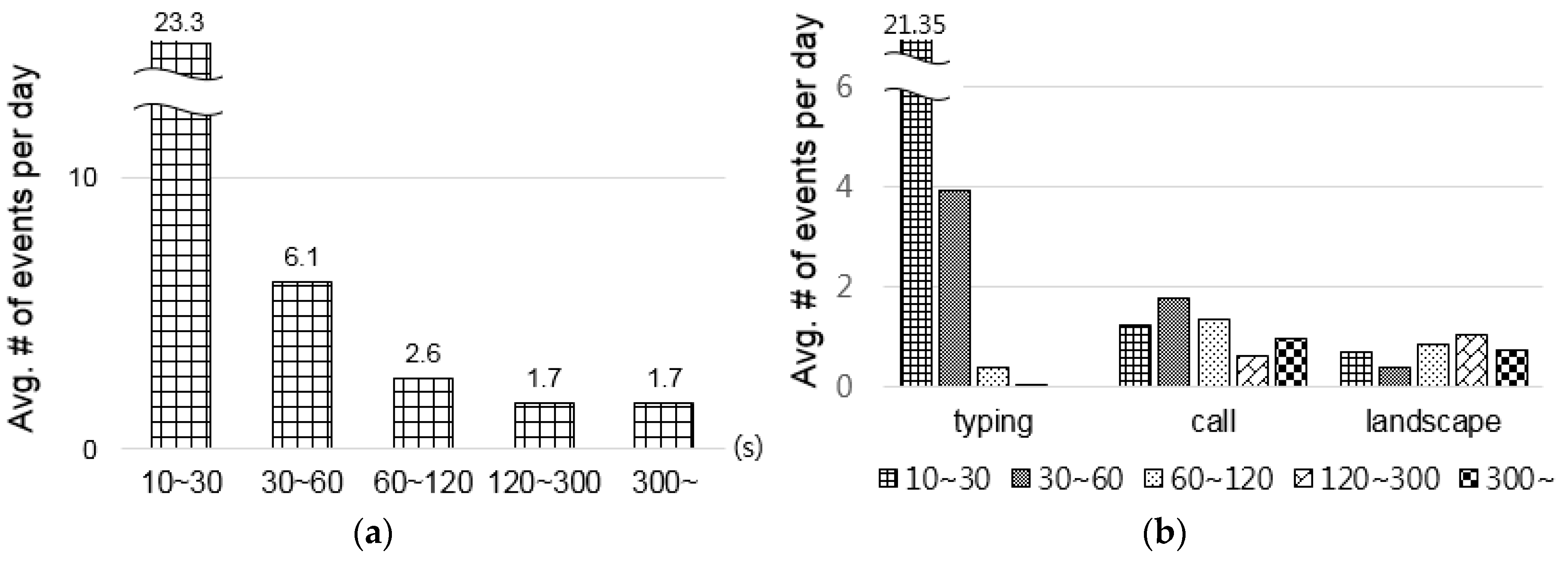
- How many potential opportunities exist per day?
- How long does each opportunity last?
- How reliably can ECG signals be captured during the opportunities?
3. Sinabro Design and Prototype
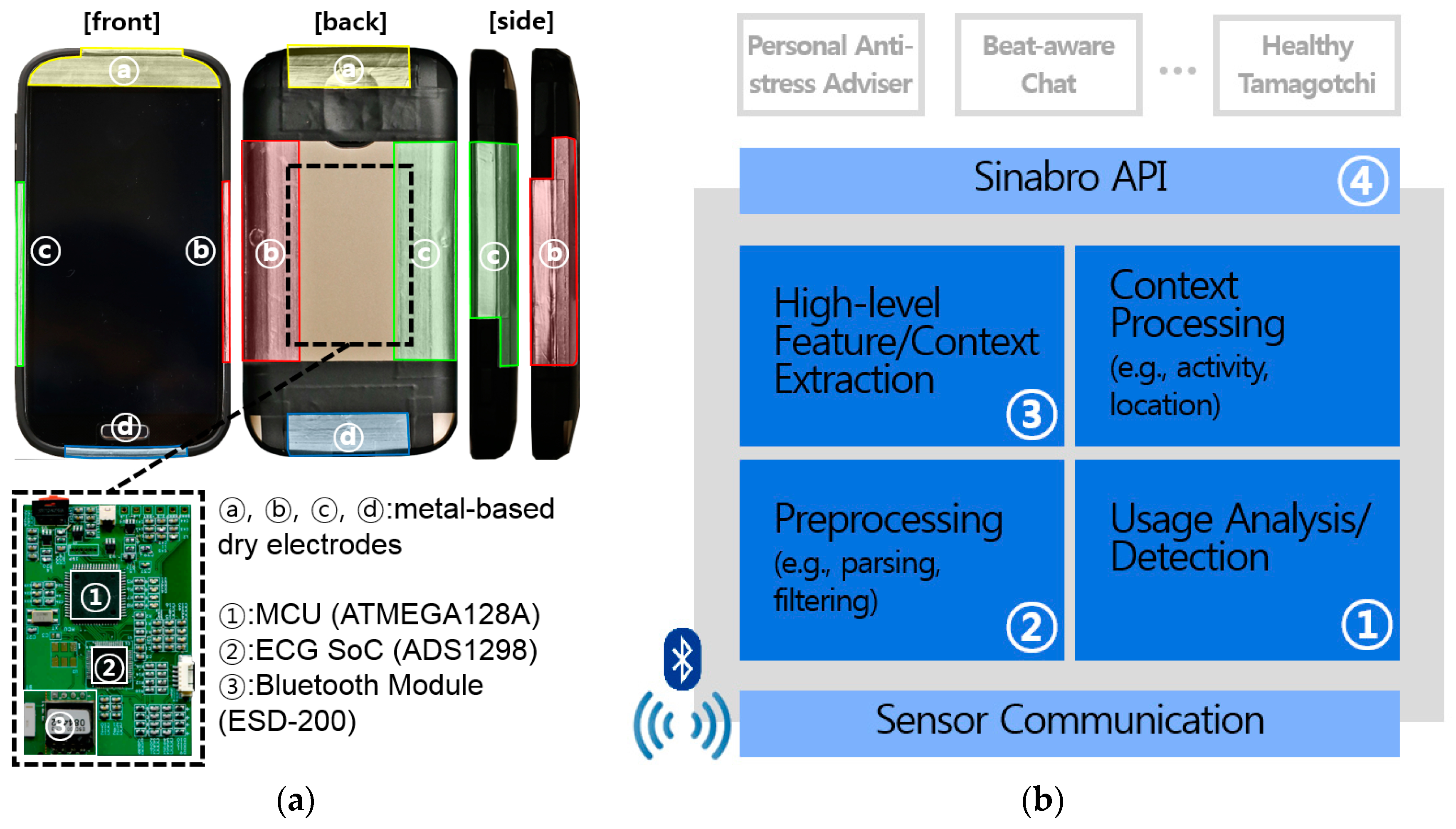
3.1. Phone-Case-Type ECG Sensor
3.2. Sinabro Middleware for Extracting ECG-Derived Features
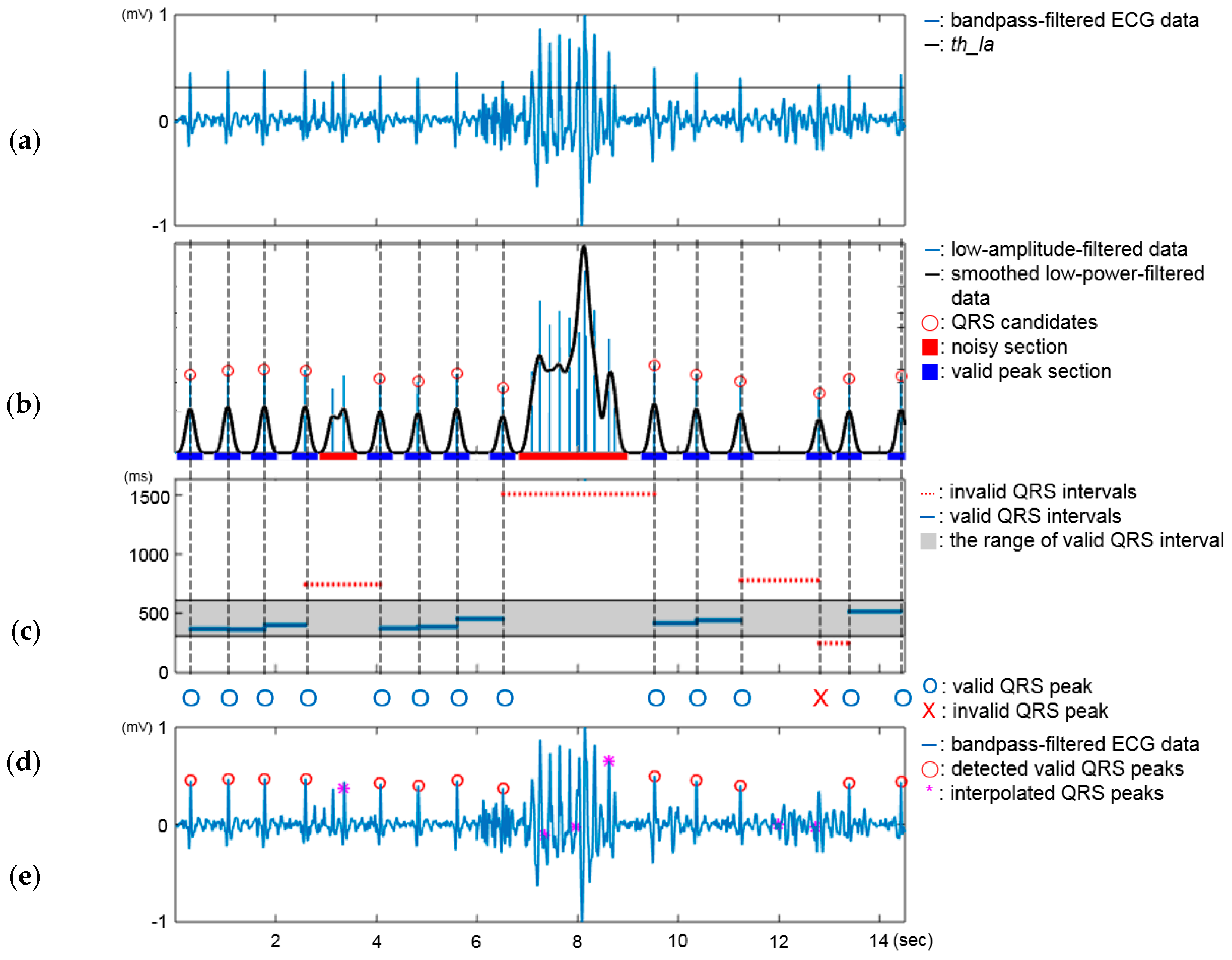
3.2.1. QRS Peak Detector
3.2.2. Sinabro APIs
4. Evaluation
4.1. Experimental Setup
4.2. Parameter Setup in the QRS Peak Detector
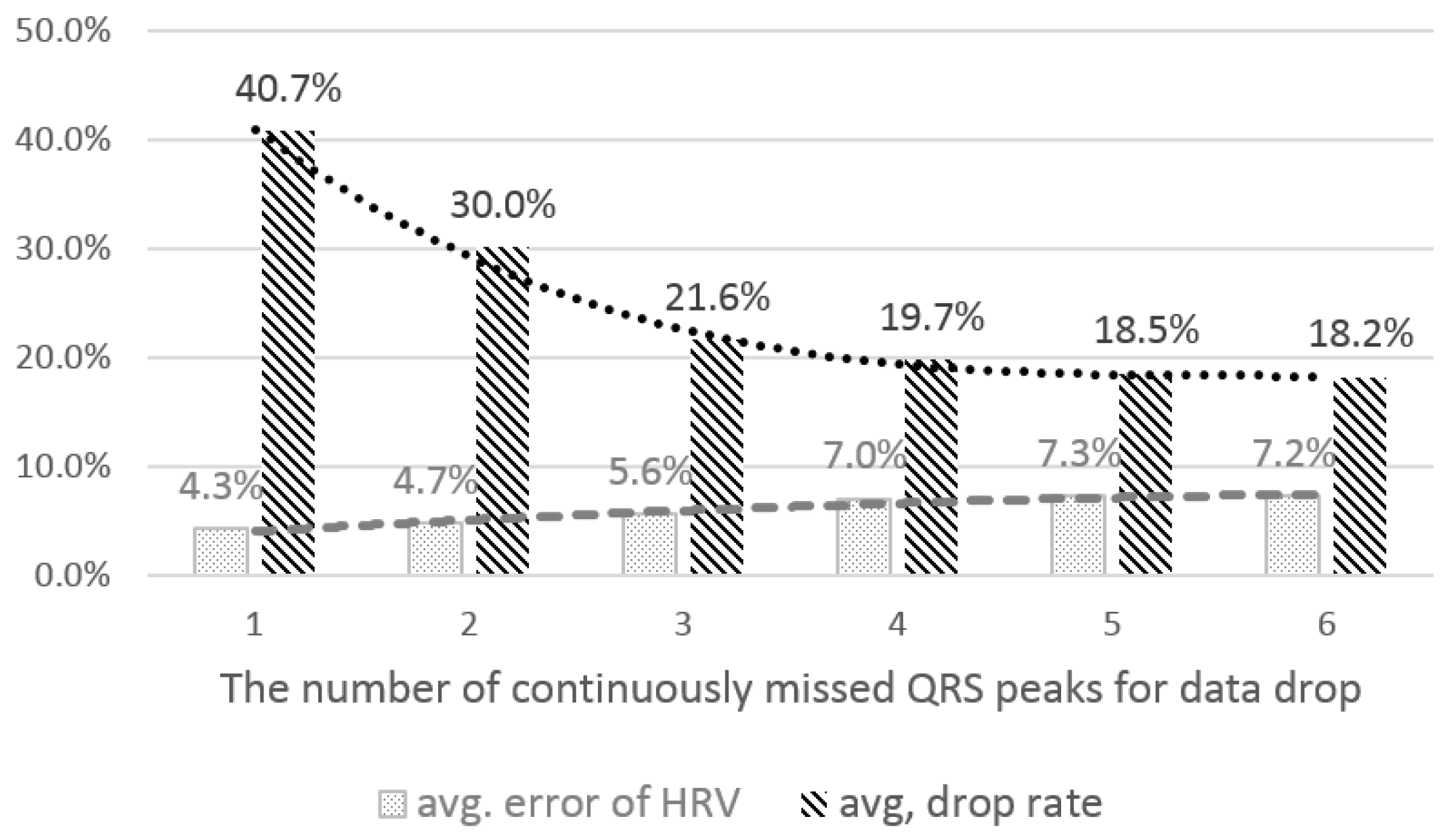
4.2.1. The Number of Consecutively-Missed QRS Peaks for Data Drop
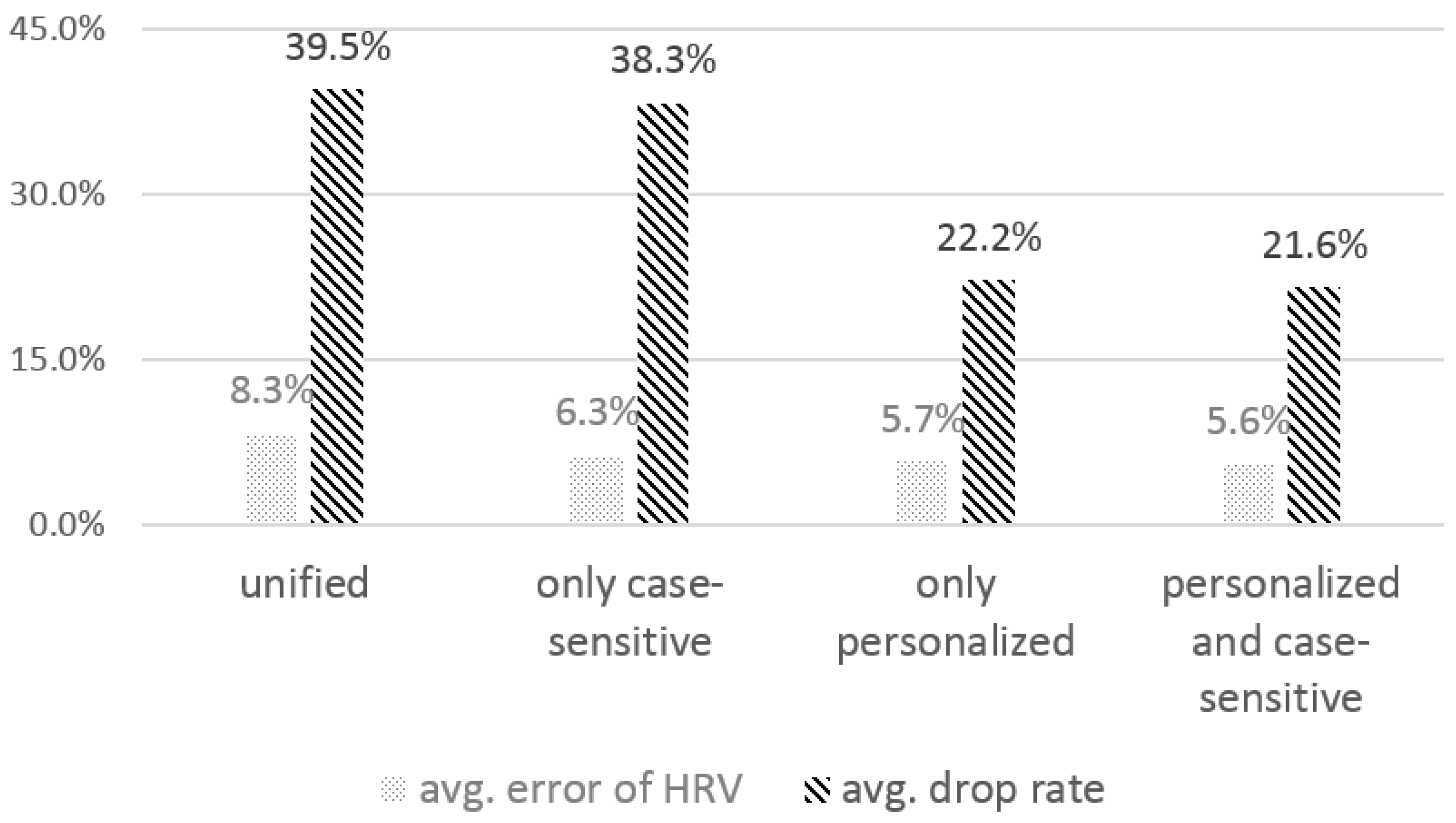
4.2.2. The Effect of the Personalized, Case-Sensitive Threshold
4.2.3. The Coefficient Value to Derive the Threshold for Low-Amplitude Filtering
4.3. Sensing and Feature Extraction in Actual Opportunities
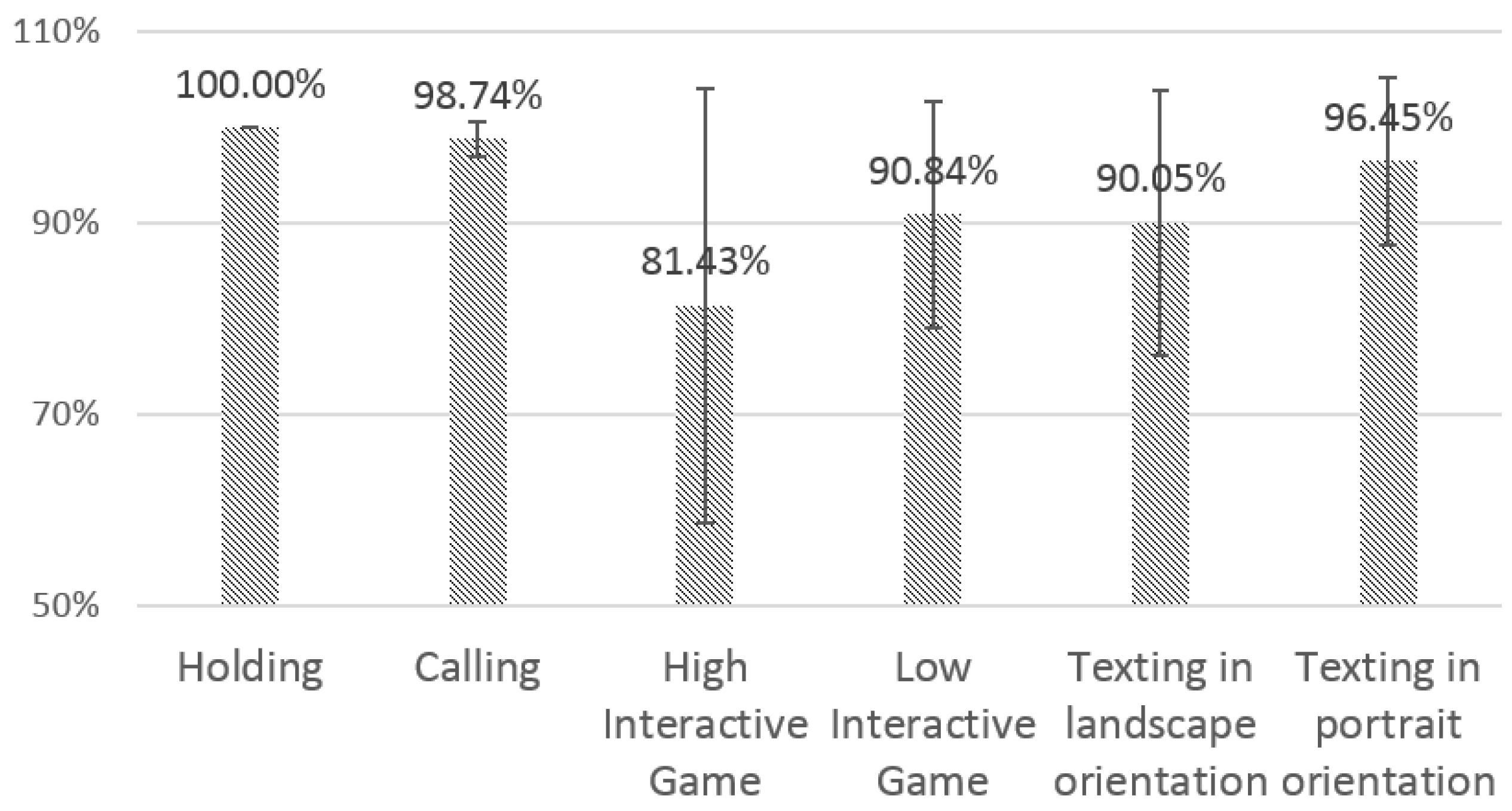
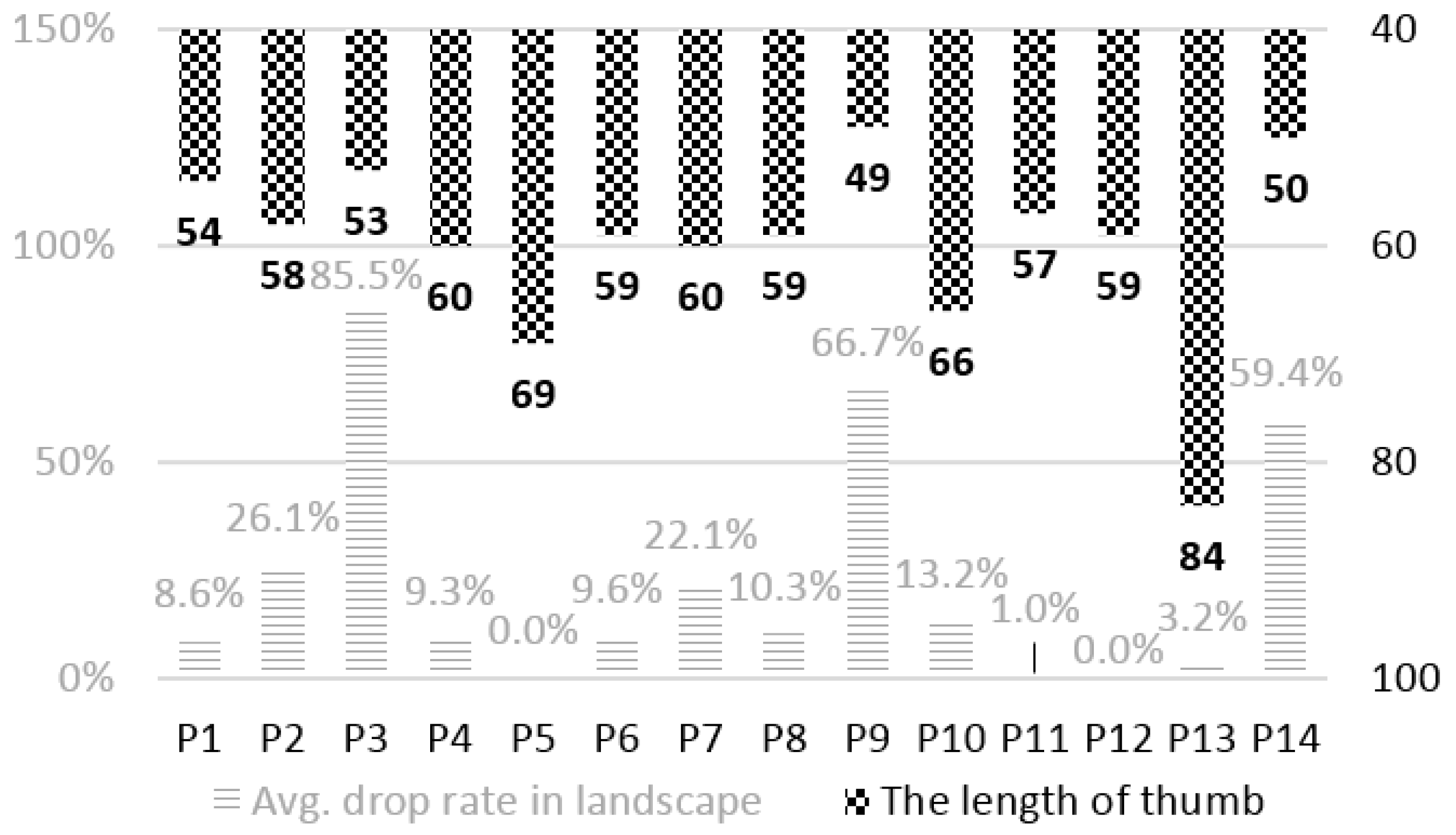
4.3.1. Sensing Reliability
4.3.2. Performance of the QRS Peak Detector
4.3.3. Performance of the Feature Extraction
5. Discussion and Future Work
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Task Force of the European Society of Cardiology. Heart rate variability standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar]
- Oresko, J.J.; Jin, Z.; Cheng, J.; Huang, S.; Sun, Y.; Duschl, H.; Cheng, A.C. A wearable smartphone-based platform for real-time cardiovascular disease detection via electrocardiogram processing. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Ertin, E.; Stohs, N.; Kumar, S.; Raij, A.; Al’Absi, M.; Shas, S. AutoSense: Unobtrusively wearable sensor suite for inferring the onset, causality, and consequences of stress in the field. In Proceedings of the 9th ACM Conference on Embedded Networked Sensor Systems, Seattle, WA, USA, 1–4 November 2011.
- Riener, A.; Ferscha, A.; Aly, M. Heart on the road: HRV analysis for monitoring a driver’s affective state. In Proceedings of the 1st International Conference on Automotive User Interfaces and Interactive Vehicular Applications, Essen, Germany, 21–22 September 2009.
- Salahuddin, L.; Cho, J.; Jeong, M.G.; Kim, D. Ultra short term analysis of heart rate variability for monitoring mental stress in mobile settings. In Proceedings of the Engineering in Medicine and Biology Society, 29th Annual International Conference of the IEEE, Lyon, France, 22–26 August 2007.
- Polar Heart Rate Sensor. Available online: http://www.polar.com/us-en/products/accessories/H1_heart_rate_sensor (accesssed on 8 March 2016).
- Kwon, S.; Kim, J.; Kang, S.; Lee, Y.; Baek, H.; Park, K. CardioGuard: A Brassiere-Based Reliable ECG Monitoring Sensor System for Supporting Daily Smartphone Healthcare Applications. Telemedicine e-Health 2014, 20, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- AliveCor. Available online: http://www.alivecor.com/ (accesssed on 8 March 2016).
- Kang, S.; Kwon, S.; Yoo, C.; Seo, S.; Park, K.; Song, J.; Lee, Y. Sinabro: Opportunistic and unobtrusive mobile electrocardiogram monitoring system. In Proceedings of the 15th Workshop on Mobile Computing Systems and Applications, Santa Barbara, CA, USA, 26–27 February 2014.
- Kwon, S.; Kang, S.; Lee, Y.; Yoo, C. Unobtrusive monitoring of ECG-derived features during daily smartphone use. In Proceedings of the Engineering in Medicine and Biology Society (EMBC), 36th Annual International Conference of the IEEE, Chicago, IL, USA, 26–30 August 2014.
- Salahuddin, L.; Jeong, M.G.; Kim, D. Ultra short term analysis of heart rate variability using normal sinus rhythm and atrial fibrillation ECG data. In Proceedings of the 9th IEEE International Conference on e-Health Networking, Application and Services, Taipei, Taiwan, 19–22 June 2007.
- Poh, M.-Z.; McDuff, D.J.; Picard, R.W. Non-contact, automated cardiac pulse measurements using video imaging and blind source separation. Opt. Express 2010, 18, 10762–10774. [Google Scholar] [CrossRef] [PubMed]
- Seo, S. Performance Enhancement in Heart Rate Variability Analysis with Constrained Missing RR Interval Estimation. Thesis, Seoul National University, Seoul, Korea, 2015. [Google Scholar]
- Kim, K.K.; Kim, J.S.; Lim, Y.G.; Park, K.S. The effect of missing RR-interval data on heart rate variability analysis in the frequency domain. Physiol. Meas. 2009, 30, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- MP150, BIOPAC. Available online: http://www.biopac.com/ (accesssed on 8 March 2016).
- Kim, K.-E.; Chang, L.; Cho, S.-J.; Shim, J.; Lee, H.; Park, J.; Lee, Y.; Kim, S. Hand grip pattern recognition for mobile user interfaces. In Proceedings of the National Conference on Artificial Intelligence; Boston, MA, USA, 16–20 July 2006, AAAI Press: Menlo Park, CA, USA; MIT Press: Cambridge, MA, USA; London, UK, 1999. [Google Scholar]
- Goel, M.; Wobbrock, J.; Patel, S. GripSense: Using built-in sensors to detect hand posture and pressure on commodity mobile phones. In Proceedings of the 25th Annual ACM Symposium on User Interface Software and Technology, Cambridge, MA, USA, 7–10 October 2012.



| Current Interval | Head Peak | Tail Peak |
|---|---|---|
| valid | valid | valid |
| invalid | valid | |
| not fixed → valid | valid | |
| invalid | valid | not fixed |
| invalid | not fixed | |
| not fixed → invalid | not fixed |
| Monitoring HR and HRV | registerHRListener(callback(HR), condition) registerHRVListener(callback(HRV), condition) * condition = TARGET_APP|TARGET_MODE class HR {long timestamp; int HR;} class HRV {long timestamp; float LF; float HF; float LF/HF; float RMSSD; float SDNN; …}; |
| Monitoring HR-/HRV-derived contexts | registerContextListener(callback(Context), condition, type) * type = STRESS|AFFECTIVE_STATE|… |
| SDNN | RMSSD | Mean HR | LF | HF | TF | nLF | nHF | LF/HF | Average | |
|---|---|---|---|---|---|---|---|---|---|---|
| avg. correlation | 0.99 | 0.95 | 1.00 | 0.98 | 0.95 | 0.98 | 0.98 | 0.98 | 0.96 | 0.97 |
| SD | 0.05 | 0.09 | 0.01 | 0.06 | 0.06 | 0.05 | 0.02 | 0.02 | 0.03 | 0.04 |
| ratio, p < 0.01 | 96.9% | 96.9% | 98.4% | 96.9% | 95.3% | 98.4% | 96.9% | 96.9% | 96.9% | 97.0% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, S.; Lee, D.; Kim, J.; Lee, Y.; Kang, S.; Seo, S.; Park, K. Sinabro: A Smartphone-Integrated Opportunistic Electrocardiogram Monitoring System. Sensors 2016, 16, 361. https://doi.org/10.3390/s16030361
Kwon S, Lee D, Kim J, Lee Y, Kang S, Seo S, Park K. Sinabro: A Smartphone-Integrated Opportunistic Electrocardiogram Monitoring System. Sensors. 2016; 16(3):361. https://doi.org/10.3390/s16030361
Chicago/Turabian StyleKwon, Sungjun, Dongseok Lee, Jeehoon Kim, Youngki Lee, Seungwoo Kang, Sangwon Seo, and Kwangsuk Park. 2016. "Sinabro: A Smartphone-Integrated Opportunistic Electrocardiogram Monitoring System" Sensors 16, no. 3: 361. https://doi.org/10.3390/s16030361





