A Dynamic Instrumentation Amplifier for Low-Power and Low-Noise Biopotential Acquisition
Abstract
:1. Introduction
2. Circuit Design
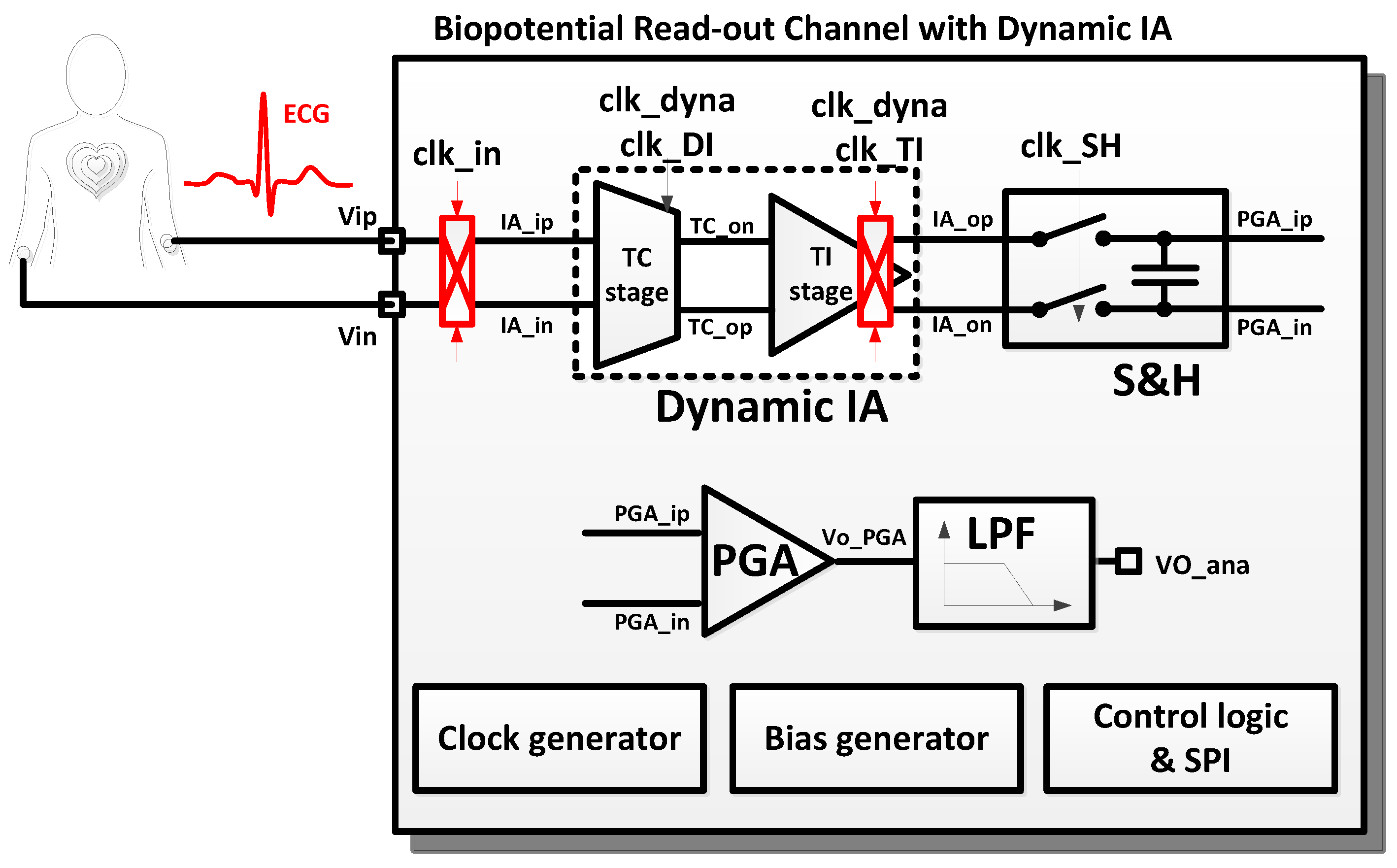
2.1. Top Level Architecture
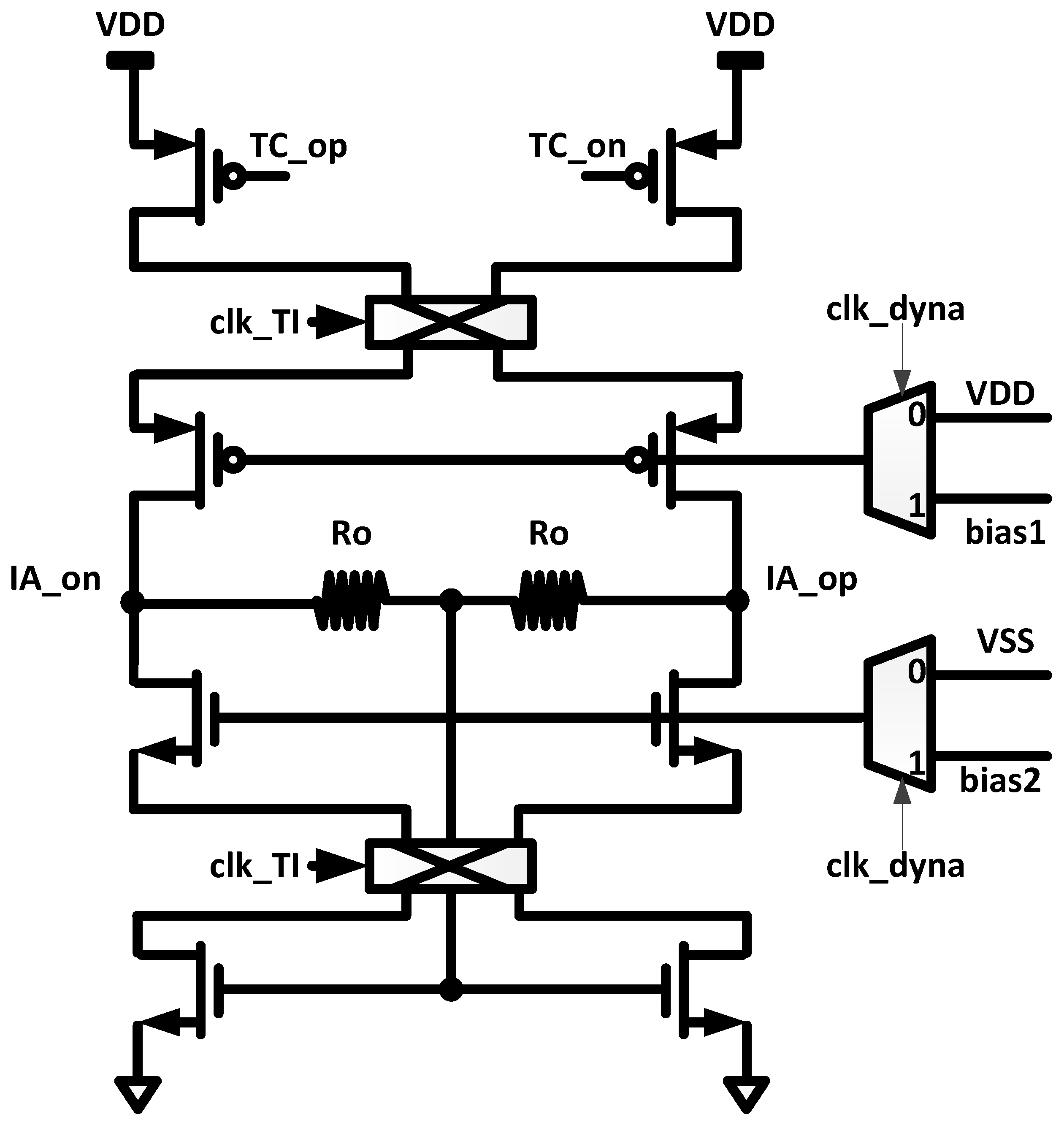
2.2. Dynamic IA
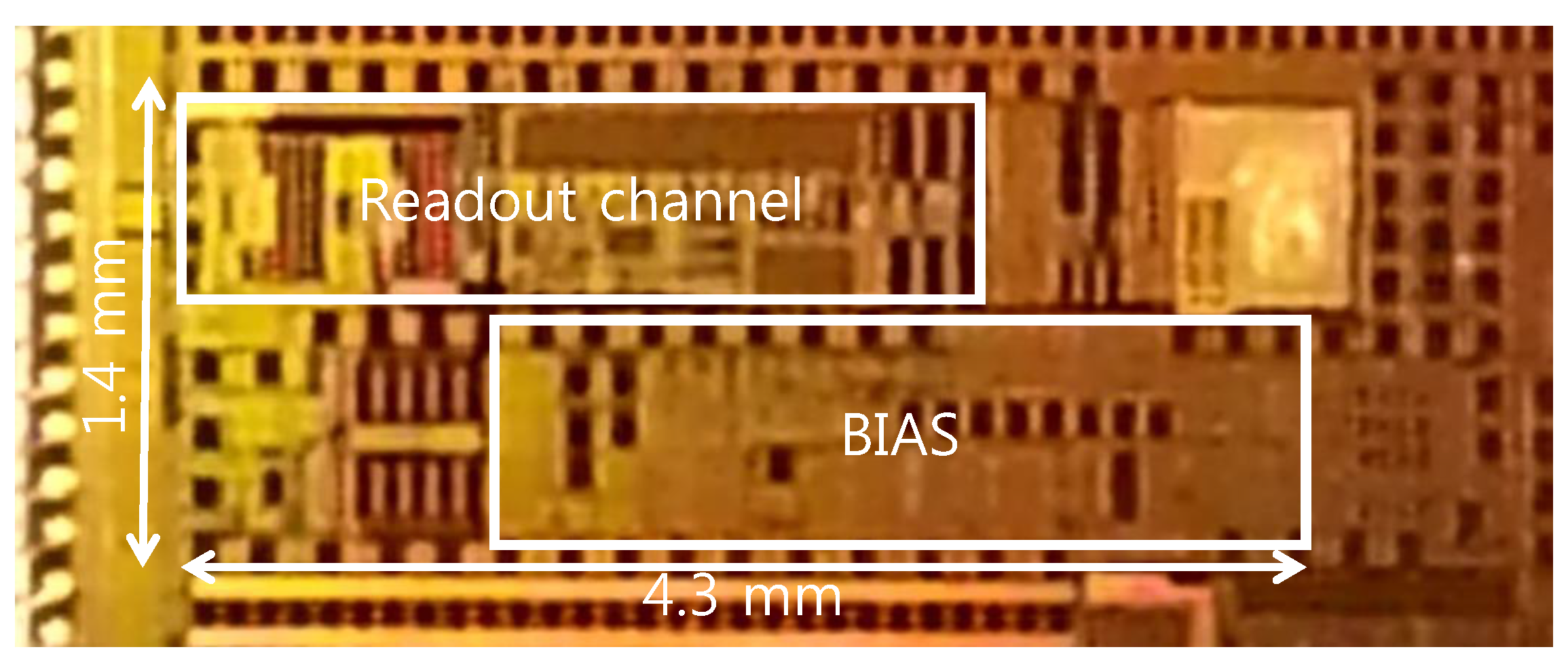
3. Experimental Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shin, K.; Park, G.; Kim, J.; Lee, T.; Ko, B.; Kim, Y. An ultra-low power (ULP) bandage-type ECG sensor for efficient cardiac disease management. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 1474–1477.
- Wong, A.; McDonagh, D.; Omeni, O.; Nunn, C.; Hernandez-Silveira, M.; Burdett, A. An ultra-low-power wireless body sensor network platform: Design & application challenges. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 6576–6579.
- Miao, F.; Cheng, Y.; He, Y.; He, Q.; Li, Y. A wearable context-aware ECG monitoring system integrated with built-in kinematic sensors of the smartphone. Sensors 2015, 15, 11465–11484. [Google Scholar] [CrossRef] [PubMed]
- Van Helleputte, N.; Konijnenburg, M.; Pettine, J.; Jee, D.-W.; Kim, H.; Morgado, A.; Van Wegberg, R.; Torfs, T.; Mohan, R.; Breeschoten, A. A 345 µw multi-sensor biomedical soc with bio-impedance, 3-channel ECG, motion artifact reduction, and integrated DSP. IEEE J. Solid-State Circuits 2015, 50, 230–244. [Google Scholar] [CrossRef]
- Baig, M.M.; Gholamhosseini, H.; Connolly, M.J. A comprehensive survey of wearable and wireless ECG monitoring systems for older adults. Med. Biol. Eng. Comput. 2013, 51, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Stoppa, M.; Chiolerio, A. Wearable electronics and smart textiles: A critical review. Sensors 2014, 14, 11957–11992. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Cadmus-Bertram, L.A.; Natarajan, L.; White, M.M.; Madanat, H.; Nichols, J.F.; Ayala, G.X.; Pierce, J.P. Wearable sensor/device (Fitbit one) and SMS text-messaging prompts to increase physical activity in overweight and obese adults: A randomized controlled trial. Telemed. e-Health 2015, 21, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.C. Wearable sensors for human activity monitoring: A review. IEEE Sens. 2015, 15, 1321–1330. [Google Scholar] [CrossRef]
- Hung, K.K.; Ko, P.K.; Hu, C.; Cheng, Y.C. A unified model for the flicker noise in metal-oxide-semiconductor field-effect transistors. IEEE Trans. Electron Devices 1990, 37, 654–665. [Google Scholar] [CrossRef]
- Enz, C.C.; Temes, G.C. Circuit techniques for reducing the effects of op-amp imperfections: Autozeroing, correlated double sampling, and chopper stabilization. Proc. IEEE 1996, 84, 1584–1614. [Google Scholar] [CrossRef]
- Tseng, Y.; Ho, Y.; Kao, S.; Su, C. A 0.09 W low power front-end biopotential amplifier for biosignal recording. IEEE Trans. Biomed. Circuits Syst. 2012, 6, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Holleman, J.; Otis, B.P. Design of ultra-low power biopotential amplifiers for biosignal acquisition applications. IEEE Trans. Biomed. Circuits Syst. 2012, 6, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fan, Q.; Huijsing, J.H.; Van Hoof, C.; Yazicioglu, R.F.; Makinwa, K. Measurement and analysis of current noise in chopper amplifiers. IEEE J. Solid-State Circuits 2013, 48, 1575–1584. [Google Scholar]
- Kusuda, Y. A 60 V auto-zero and chopper operational amplifier with 800 kHz interleaved clocks and input bias current trimming. IEEE J. Solid-State Circuits 2015, 50, 2804–2813. [Google Scholar] [CrossRef]
- Wirth, G.; Koh, J.; Silva, R.D.; Thewes, R.; Brederlow, R. Modeling of statistical low-frequency noise of deep-submicrometer MOSFETS. IEEE Trans. Electron Devices 2005, 52, 1576–1588. [Google Scholar] [CrossRef]
- Koh, J.; Schmitt-Landsiedel, D.; Thewes, R.; Brederlow, R. A complementary switched mosfet architecture for the 1/f noise reduction in linear analog CMOS ICS. IEEE J. Solid-State Circuits 2007, 42, 1352–1361. [Google Scholar]
- Han, M.; Kim, B.; Chen, Y.-A.; Lee, H.; Park, S.-H.; Cheong, E.; Hong, J.; Han, G.; Chae, Y. Bulk switching instrumentation amplifier for a high-impedance source in neural signal recording. IEEE Trans. Circuits Syst. II Exp. Briefs 2015, 62, 194–198. [Google Scholar] [CrossRef]
- Van Der Wel, A.P.; Klumperink, E.A.; Kolhatkar, J.S.; Hoekstra, E.; Snoeij, M.F.; Salm, C.; Wallinga, H.; Nauta, B. Low-frequency noise phenomena in switched mosfets. IEEE J. Solid-State Circuits 2007, 42, 540–550. [Google Scholar] [CrossRef]
- Luo, S.; Johnston, P. A review of electrocardiogram filtering. J. Electrocardiol. 2010, 43, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Buendía-Fuentes, F.; Arnau-Vives, M.; Arnau-Vives, A.; Jiménez-Jiménez, Y.; Rueda-Soriano, J.; Zorio-Grima, E.; Osa-Sáez, A.; Martínez-Dolz, L.; Almenar-Bonet, L.; Palencia-Pérez, M. High-bandpass filters in electrocardiography: Source of error in the interpretation of the st segment. ISRN Cardiol. 2012, 2012, 706217. [Google Scholar] [CrossRef] [PubMed]
- 3M™ Red Dot™ Monitoring Electrodes. Available online: http://multimedia.3m.com/mws/media/513875O/red-dot-electrodes.pdf (accessed on 18 February 2016).
- Van Helleputte, N.; Kim, S.; Kim, H.; Kim, J.P.; Van Hoof, C.; Yazicioglu, R.F. A 160 μa biopotential acquisition ASIC with fully integrated IA and motion-artifact suppression. In Proceedings of the IEEE International Solid-State Circuits Conference Digest of Technical Papers (ISSCC), San Francisco, CA, USA, 19–23 February 2012; pp. 118–120.
- Fan, Q.; Sebastiano, F.; Huijsing, J.H.; Makinwa, K.A. A 1.8 W 60 nV Hz capacitively-coupled chopper instrumentation amplifier in 65 nm CMOS for wireless sensor nodes. IEEE J. Solid-State Circuits 2011, 46, 1534–1543. [Google Scholar] [CrossRef]
- Verma, N.; Shoeb, A.; Bohorquez, J.; Dawson, J.; Guttag, J.; Chandrakasan, A.P. A micro-power EEG acquisition SOC with integrated feature extraction processor for a chronic seizure detection system. IEEE J. Solid-State Circuits 2010, 45, 804–816. [Google Scholar] [CrossRef]
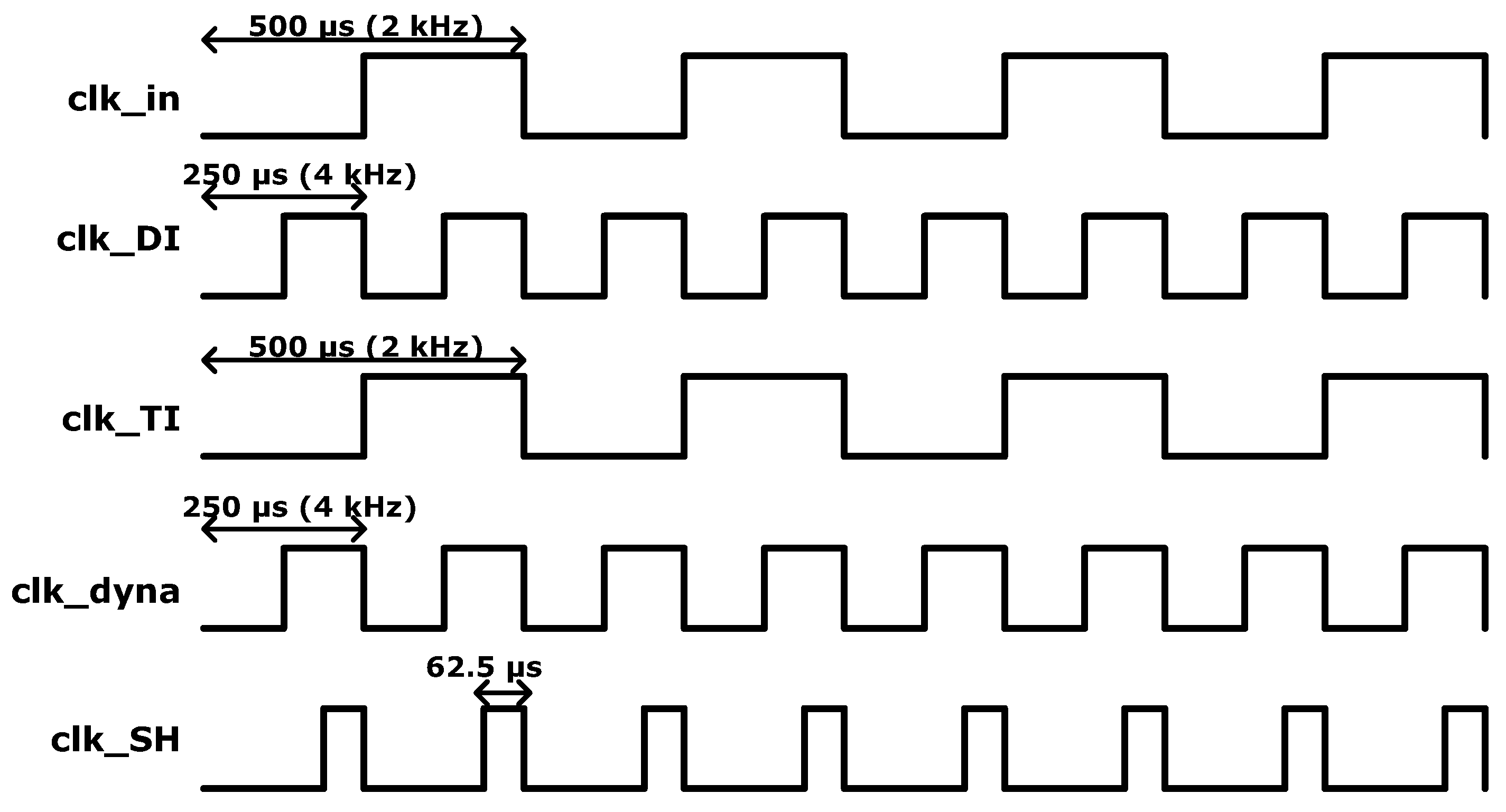

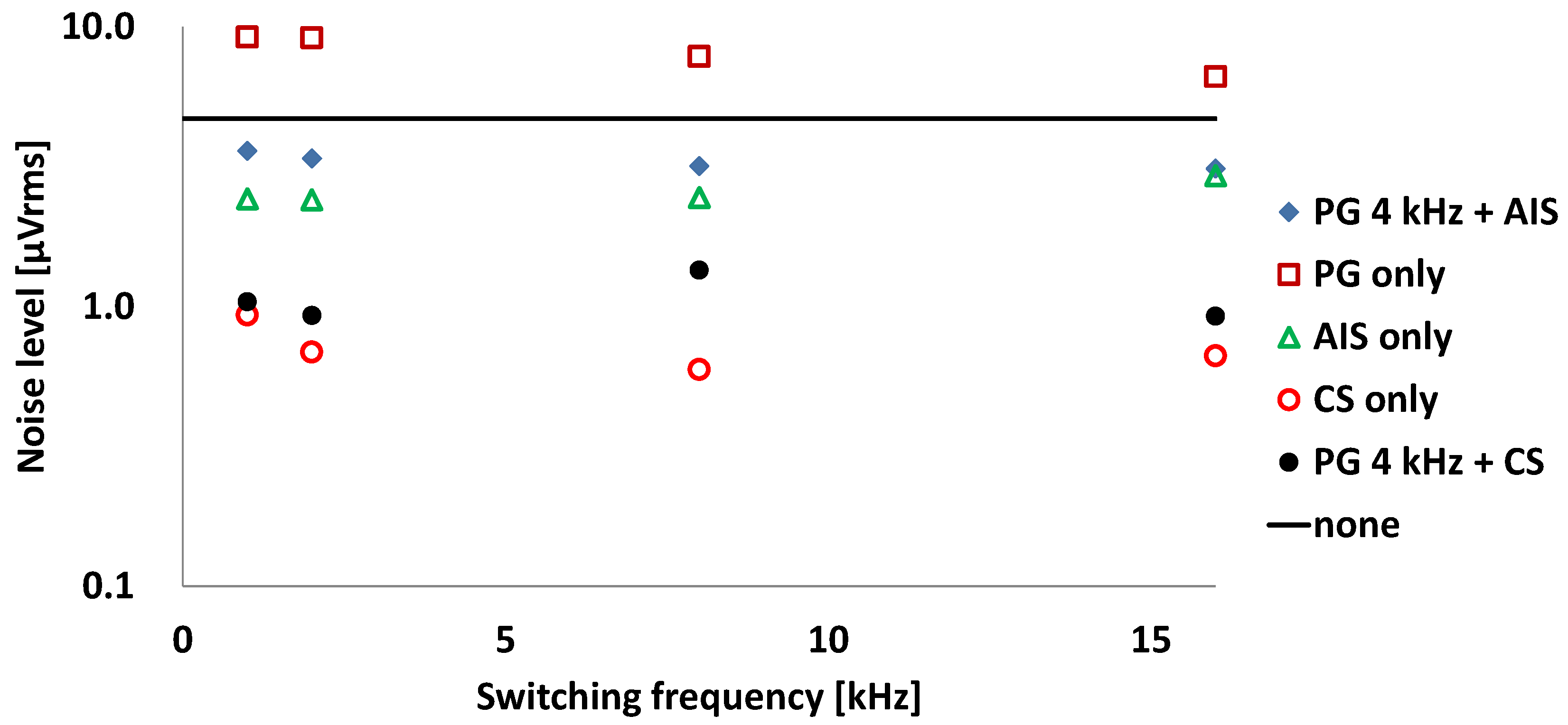


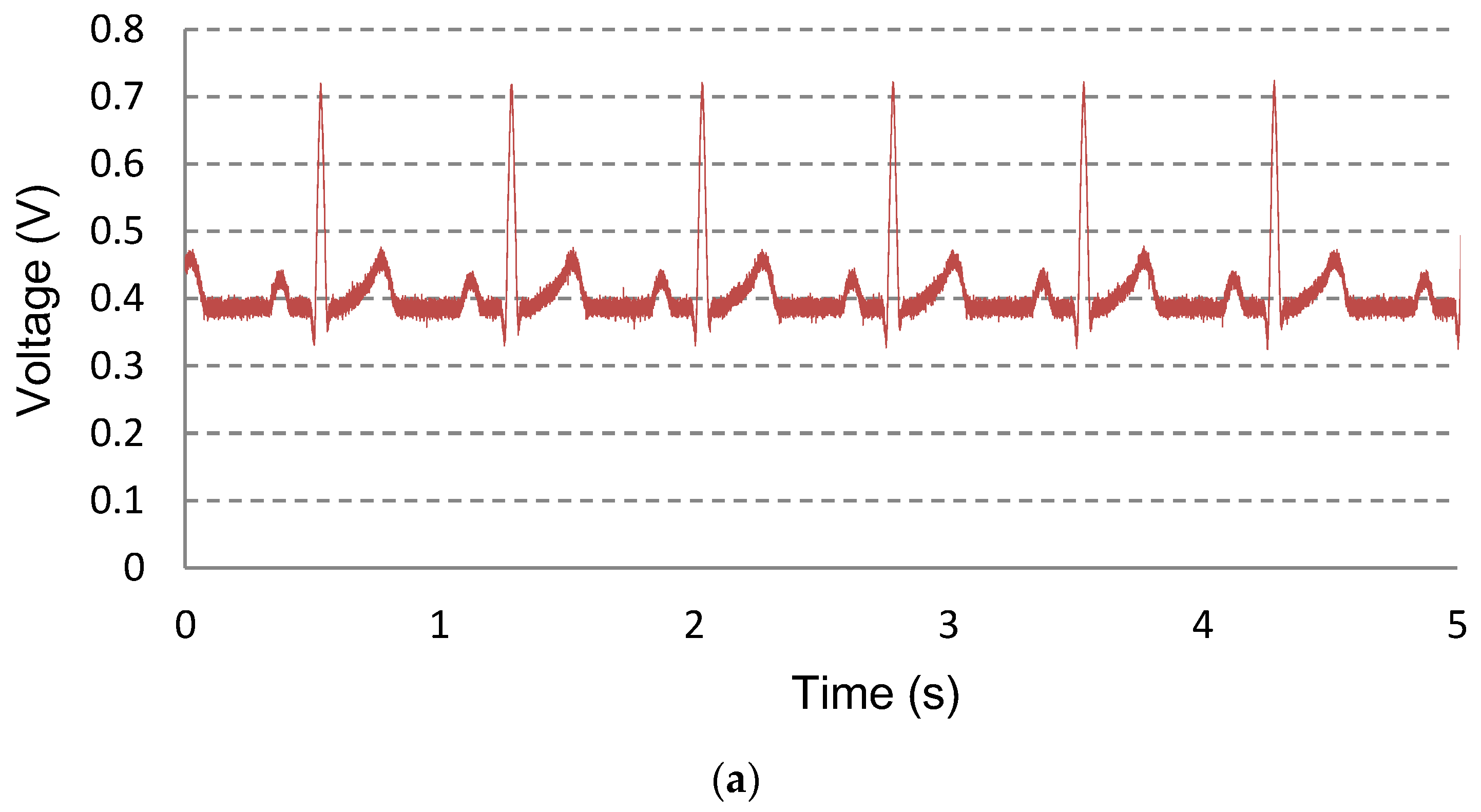
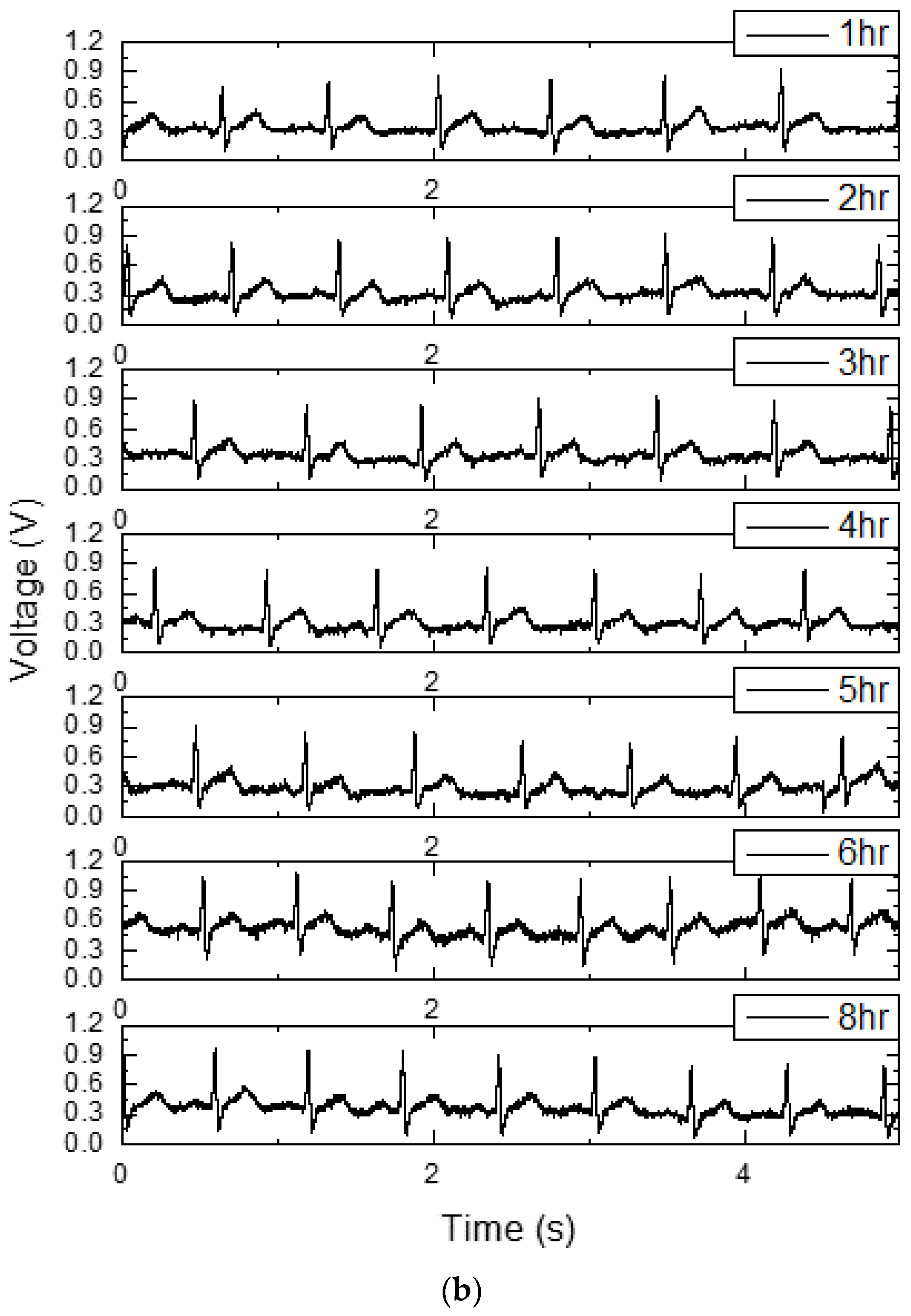

| PG 4 kHz + AIS | PG only | AIS only | CS only | PG 4 kHz + CS | |
|---|---|---|---|---|---|
| clk_in | Static “H” | Static “H” | Static “H” | Chopper freq. clock | Chopper freq. clock |
| clk_DI | AIS freq. clock | Static “H” | AIS freq. clock | Static “H” | Static “H” |
| clk_TI | Static “H” | Static “H” | Static “H” | Chopper freq. clock | Chopper freq. clock |
| clk_dyna | 4 kHz clock | PG freq. clock | Static “H” | Static “H” | Static “H” |
| clk_SH | 4 kHz clock with 1/4 duty ratio | PG freq. clock with 1/4 duty ratio | Static “H” | Static “H” | 4 kHz clock with 1/4 duty ratio |
| PG 4 kHz + AIS 2 kHz | PG only 2 kHz | AIS only 2 kHz | CS only 2 kHz | PG 4 kHz + CS 2 kHz | |
|---|---|---|---|---|---|
| Input noise (μVrms) | 3.4 | 9.1 | 2.4 | 0.69 | 0.92 |
| Supply current (μA) | 5 | 5 | 10 | 10 | 5 |
| Bandwidth (Hz) | 100 | 100 | 100 | 100 | 100 |
| NEF | 29.8 | 79.9 | 29.8 | 8.6 | 7.9 |
| This Work | [22] | [23] | [24] | |
|---|---|---|---|---|
| Technology | 130 nm | 180 nm | 65 nm | 180 nm |
| Supply | 1.2 V | 1.2 V | 1 V | 1 V |
| IA current | 10 µA (static mode) 5 µA (half duty-cycled power gating mode) | 5 µA | 1.8 µA | 3.5 µA |
| Input-referred noise (~100 Hz) | 0.6 µVrms (static mode) 0.9 µVrms (half duty-cycled power gating mode) | 1.3 µVrms | 6.7 µVrms | 1.3 µVrms |
| Input impedance | 415 MΩ (simulated) | 1 GΩ | N/A | 700 MΩ |
| CMRR | 91.3 dB | 120 dB | 134 dB | 60 dB |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Ko, H. A Dynamic Instrumentation Amplifier for Low-Power and Low-Noise Biopotential Acquisition. Sensors 2016, 16, 354. https://doi.org/10.3390/s16030354
Kim J, Ko H. A Dynamic Instrumentation Amplifier for Low-Power and Low-Noise Biopotential Acquisition. Sensors. 2016; 16(3):354. https://doi.org/10.3390/s16030354
Chicago/Turabian StyleKim, Jongpal, and Hyoungho Ko. 2016. "A Dynamic Instrumentation Amplifier for Low-Power and Low-Noise Biopotential Acquisition" Sensors 16, no. 3: 354. https://doi.org/10.3390/s16030354






