A Highly Selective Sensor for Cyanide in Organic Media and on Solid Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Preparation of IR-786 Stock Solutions
2.3. Qualitative Response of IR-786 to Anions in Solution Phase and on Solid Surfaces
2.4. Quantitative Response of IR-786 to Anions in Solution Phase and on Solid Surfaces
2.5. Characterization of IR-786-(CN) Complex Using 1H-NMR
2.6. Characterization of IR-786-(OH) Complex Using 1H-NMR
2.7. Characterization of IR-786 in the Presence of Cyanide and Hydroxyl Anions IR-786-(CN)-(OH) Using 1H-NMR
3. Results
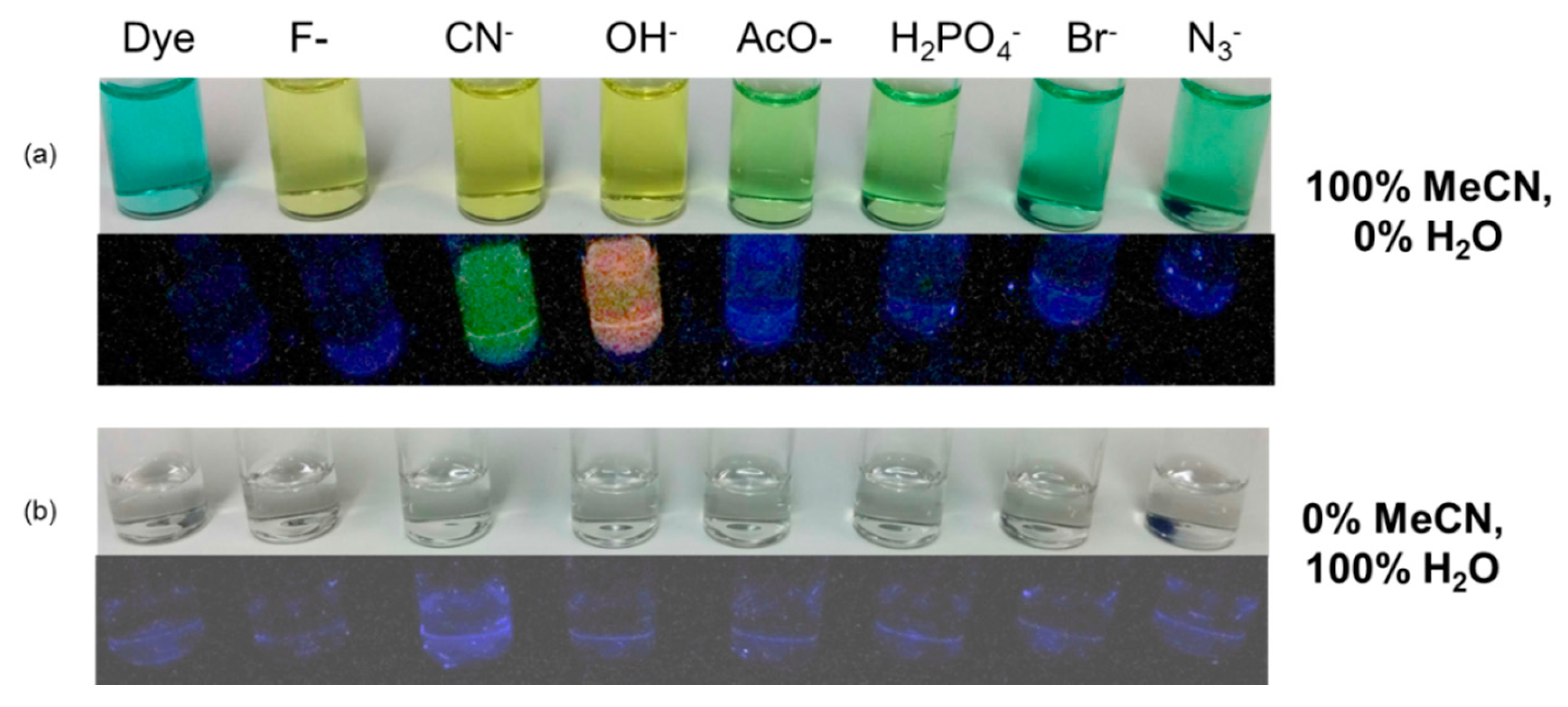
3.1. Qualitative Assessment of Selectivity of IR-786 for Anions in Organic (MeCN) Media
3.2. Quantitative Assessment of Selectivity of IR-786 for Anions in MeCN
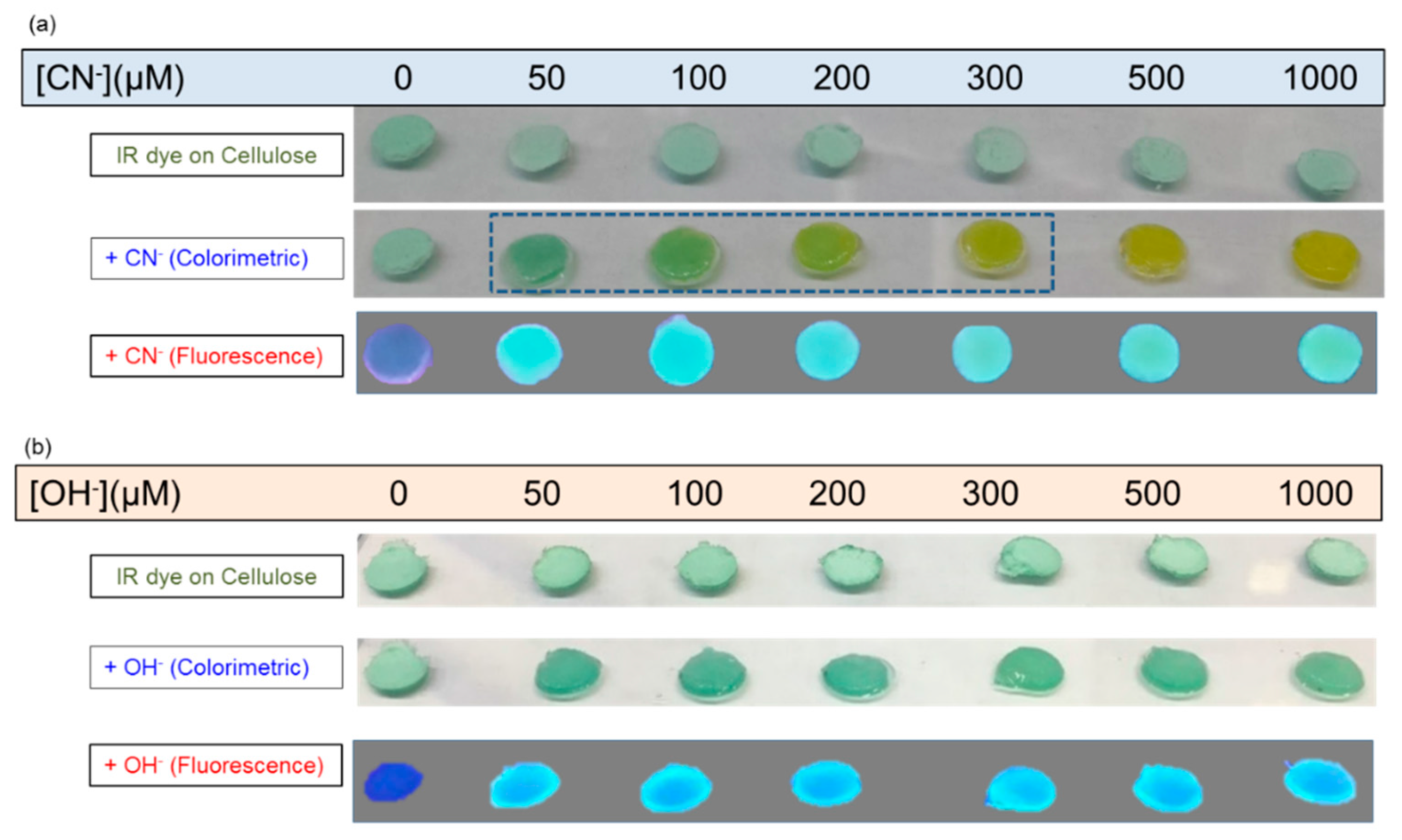
3.3. Quantitative Response of IR-786 to Anions on Solid Surfaces
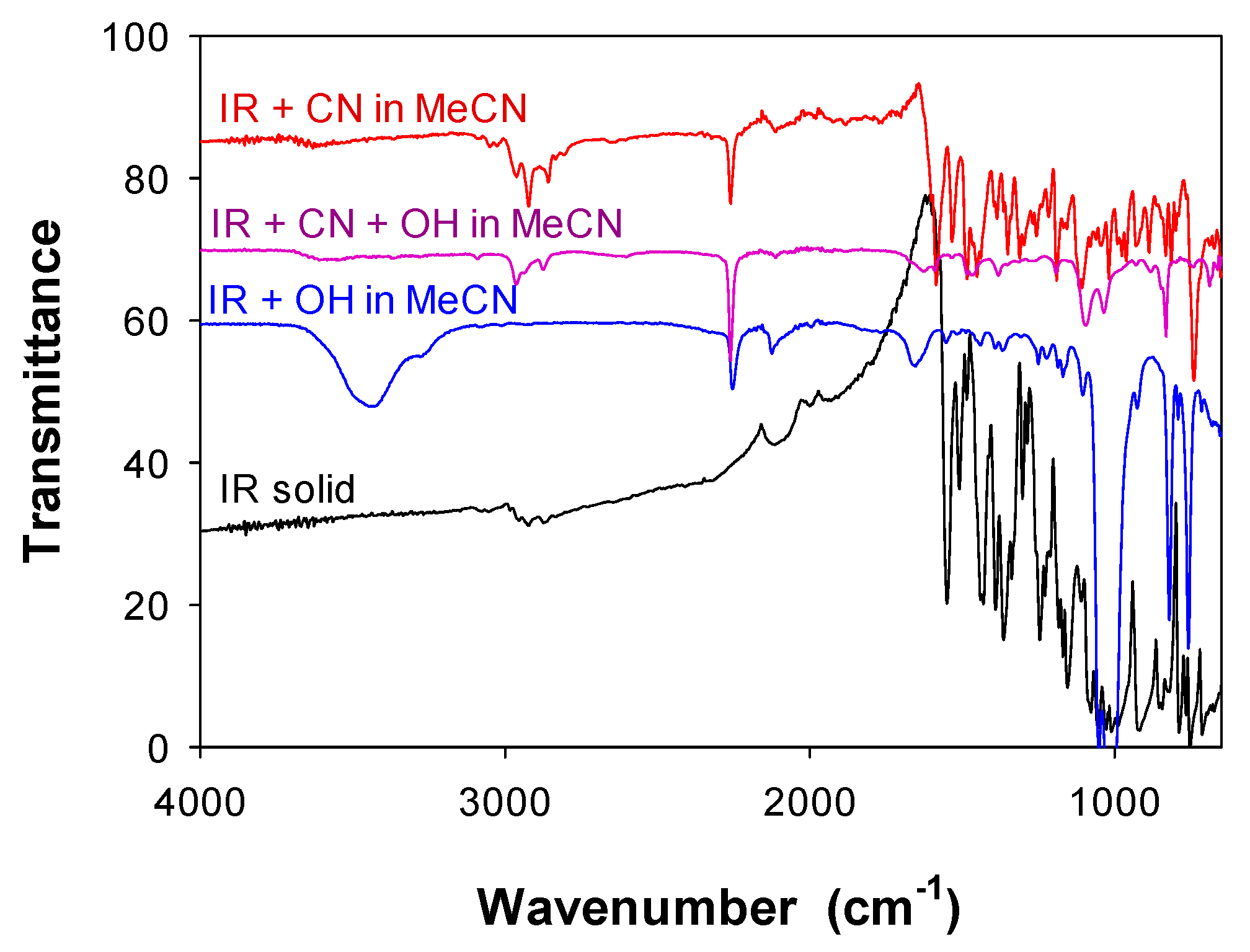
3.4. Elucidation of Binding Mechanism for Anions in MeCN
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IR 786 | IR 786 perchlorate or 2-(2-[2-Chloro-3-([1,3-dihydro-1,3,3-trimethyl-2H-indol-2-ylidene]ethylidene)-1-cyclohexen-1-yl]ethenyl)-1,3,3-trimethylindolium perchlorate |
| MeCN | Acetonitrile |
References
- Gale, P.A.; Busschaert, N.; Haynes, C.J.; Karagiannidis, L.E.; Kirby, I.L. Anion receptor chemistry: Highlights from 2011 and 2012. Chem. Soc. Rev. 2014, 43, 205–241. [Google Scholar] [CrossRef] [PubMed]
- Anzenbacher, P.; Tyson, D.S.; Jursíková, K.; Castellano, F.N. Luminescence lifetime-based sensor for cyanide and related anions. J. Am. Chem. Soc. 2002, 124, 6232–6233. [Google Scholar] [CrossRef] [PubMed]
- Isaad, J.; Perwuelz, A. New color chemosensors for cyanide based on water soluble azo dyes. Tetrahedron Lett. 2010, 51, 5810–5814. [Google Scholar] [CrossRef]
- Rogers, K. Recent advances in biosensor techniques for environmental monitoring. Anal. Chim. Acta 2006, 568, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Gunnlaugsson, T.; Glynn, M.; Kruger, P.E.; Pfeffer, F.M. Anion recognition and sensing in organic and aqueous media using luminescent and colorimetric sensors. Coord. Chem. Rev. 2006, 250, 3094–3117. [Google Scholar] [CrossRef]
- Kulig, K.W.; Ballantyne, B. Cyanide Toxicity; US Department of Health & Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 1991; Volume 15. [Google Scholar]
- Xu, Z.; Chen, X.; Kim, H.N.; Yoon, J. Sensors for the optical detection of cyanide ion. Chem. Soc. Rev. 2010, 39, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Sareen, D.; Kaur, S.; Singh, K. An efficacious “naked-eye” selective sensing of cyanide from aqueous solutions using a triarylmethane leuconitrile. Inorg. Chem. Commun. 2009, 12, 272–275. [Google Scholar] [CrossRef]
- Niu, H.-T.; Jiang, X.; He, J.; Cheng, J.-P. Cyanine dye-based chromofluorescent probe for highly sensitive and selective detection of cyanide in water. Tetrahedron Lett. 2009, 50, 6668–6671. [Google Scholar] [CrossRef]
- Qiao, Y.-H.; Lin, H.; Lin, H.-K. Design, synthesis and recognition properties of a new acetate ion receptor based on shiff-base derivative. Chem. Res. Chin. Univ. 2011, 27, 574–577. [Google Scholar]
- Chapman, G.; Henary, M.; Patonay, G. The effect of varying short-chain alkyl substitution on the molar absorptivity and quantum yield of cyanine dyes. Anal. Chem. Insights 2011, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Isaad, J.; El Achari, A.; Malek, F. Bio-polymer starch thin film sensors for low concentration detection of cyanide anions in water. Dyes Pigment. 2013, 97, 134–140. [Google Scholar] [CrossRef]
- Isaad, J.; El Achari, A. Colorimetric sensing of cyanide anions in aqueous media based on functional surface modification of natural cellulose materials. Tetrahedron 2011, 67, 4939–4947. [Google Scholar] [CrossRef]
- Isaad, J.; Malek, F.; El Achari, A. Water soluble and fluorescent copolymers as highly sensitive and selective fluorescent chemosensors for the detection of cyanide anions in biological media. RSC Adv. 2013, 3, 22168–22175. [Google Scholar] [CrossRef]
- Panigrahi, M.; Dash, S.; Patel, S.; Mishra, B.K. Tetrahedron report number 960: Syntheses of cyanines: A review. Tetrahedron 2012, 68, 781–805. [Google Scholar] [CrossRef]
- Li, Q.; Tan, J.; Peng, B.-X. Synthesis and characterization of heptamethine cyanine dyes. Molecules 1997, 2, 91–98. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A review of nir dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-H.; Wang, L.-Y.; Zhai, G.-H.; Wen, Z.-Y.; Zhang, Z.-X. Microwave-assisted solvent-free synthesis of some dimethine cyanine dyes, spectral properties and td-dft/pcm calculations. Bull. Korean Chem. Soc. 2007, 28, 2382–2388. [Google Scholar] [CrossRef]
- Narayanan, N.; Patonay, G. A new method for the synthesis of heptamethine cyanine dyes: Synthesis of new near-infrared fluorescent labels. J. Org. Chem. 1995, 60, 2391–2395. [Google Scholar] [CrossRef]
- Winstead, A.J.; Williams, R.; Zhang, Y.; McLean, C.; Oyaghire, S. Microwave synthesis of cyanine dyes. J. Microw. Power Electromagn. Energy 2010, 44, 207–212. [Google Scholar] [PubMed]
- Leung, K. Ir-786 Perchlorate. Available online: http://www.ncbi.nlm.nih.gov/books/NBK23223/ (accessed on 12 December 2015).
- Winstead, A.; Williams, R. Application of Microwave Assisted Organic Synthesis to the Development of Near-Ir Cyanine Dye Probes; INTECH Open Access Publisher: Rijeka, Croatia, 2011. [Google Scholar]
- Hijji, Y.M.; Barare, B.; Zhang, Y. Lawsone (2-hydroxy-1, 4-naphthoquinone) as a sensitive cyanide and acetate sensor. Sens. Actuators B Chem. 2012, 169, 106–112. [Google Scholar] [CrossRef]
- Peng, X.; Song, F.; Lu, E.; Wang, Y.; Zhou, W.; Fan, J.; Gao, Y. Heptamethine cyanine dyes with a large stokes shift and strong fluorescence: A paradigm for excited-state intramolecular charge transfer. J. Am. Chem. Soc. 2005, 127, 4170–4171. [Google Scholar] [CrossRef] [PubMed]
- Haav, K.; Kadam, S.A.; Toom, L.; Gale, P.A.; Busschaert, N.; Wenzel, M.; Hiscock, J.R.; Kirby, I.L.; Haljasorg, T.; Lõkov, M.; et al. Accurate method to quantify binding in supramolecular chemistry. J. Org. Chem. 2013, 78, 7796–7808. [Google Scholar] [CrossRef] [PubMed]
- Sayın, E.; Kürkçüoğlu, G.S.; Yeşilel, O.Z.; Hökelek, T. 1d cyanide complexes with 2-pyridinemethanol: Synthesis, crystal structures and spectroscopic properties. J. Mol. Struct. 2015, 1101, 73–81. [Google Scholar] [CrossRef]
- Grabowska, B.; Sitarz, M.; Olejnik, E.; Kaczmarska, K.; Tyliszczak, B. Ft-ir and ft-raman studies of cross-linking processes with Ca2+ ions, glutaraldehyde and microwave radiation for polymer composition of poly(acrylic acid)/sodium salt of carboxymethyl starch—In moulding sands, part ii. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 151, 27–33. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barare, B.; Babahan, I.; Hijji, Y.M.; Bonyi, E.; Tadesse, S.; Aslan, K. A Highly Selective Sensor for Cyanide in Organic Media and on Solid Surfaces. Sensors 2016, 16, 271. https://doi.org/10.3390/s16030271
Barare B, Babahan I, Hijji YM, Bonyi E, Tadesse S, Aslan K. A Highly Selective Sensor for Cyanide in Organic Media and on Solid Surfaces. Sensors. 2016; 16(3):271. https://doi.org/10.3390/s16030271
Chicago/Turabian StyleBarare, Belygona, Ilknur Babahan, Yousef M. Hijji, Enock Bonyi, Solomon Tadesse, and Kadir Aslan. 2016. "A Highly Selective Sensor for Cyanide in Organic Media and on Solid Surfaces" Sensors 16, no. 3: 271. https://doi.org/10.3390/s16030271






