A Novel Gas Sensor Based on MgSb2O6 Nanorods to Indicate Variations in Carbon Monoxide and Propane Concentrations
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of MgSb2O6 Nanorods
2.2. Physical Characterization of MgSb2O6 Powders
2.3. Pellets Preparation for Gas Sensitivity Analysis
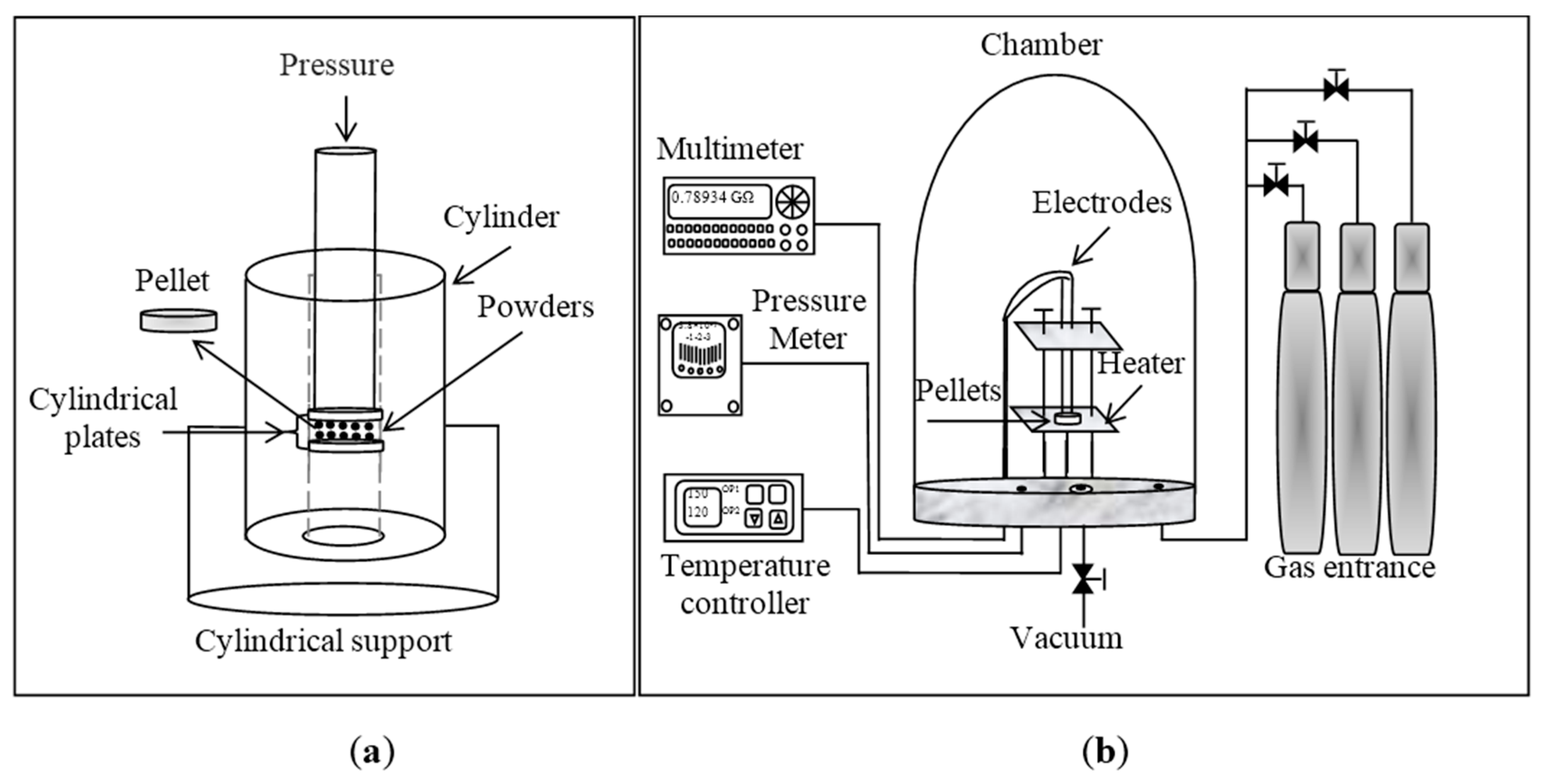
3. Results and Discussion
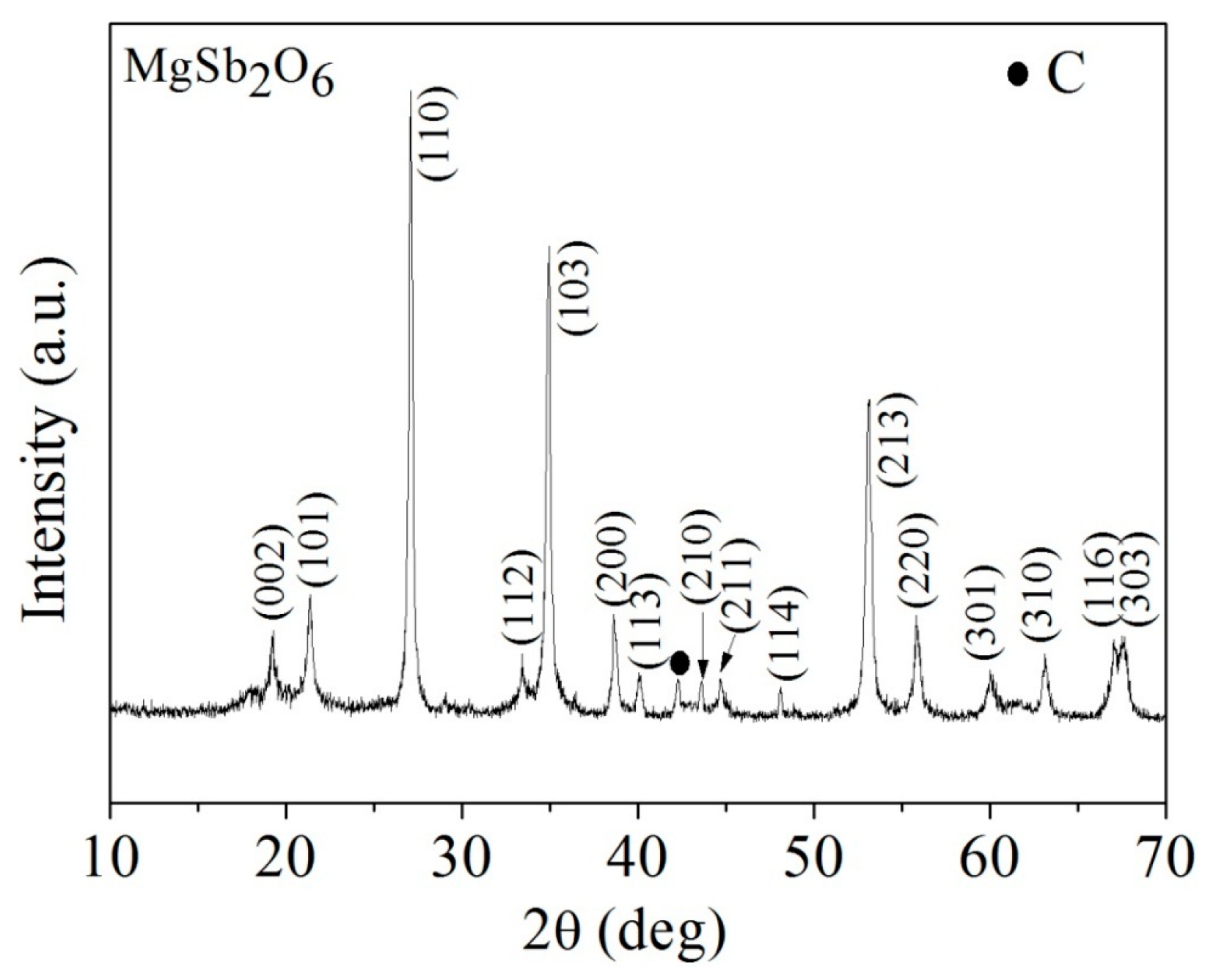
3.1. XRD Analysis
3.2. Scanning Electron Microscopy Analysis

3.3. Transmission Electron Microscopy Analysis
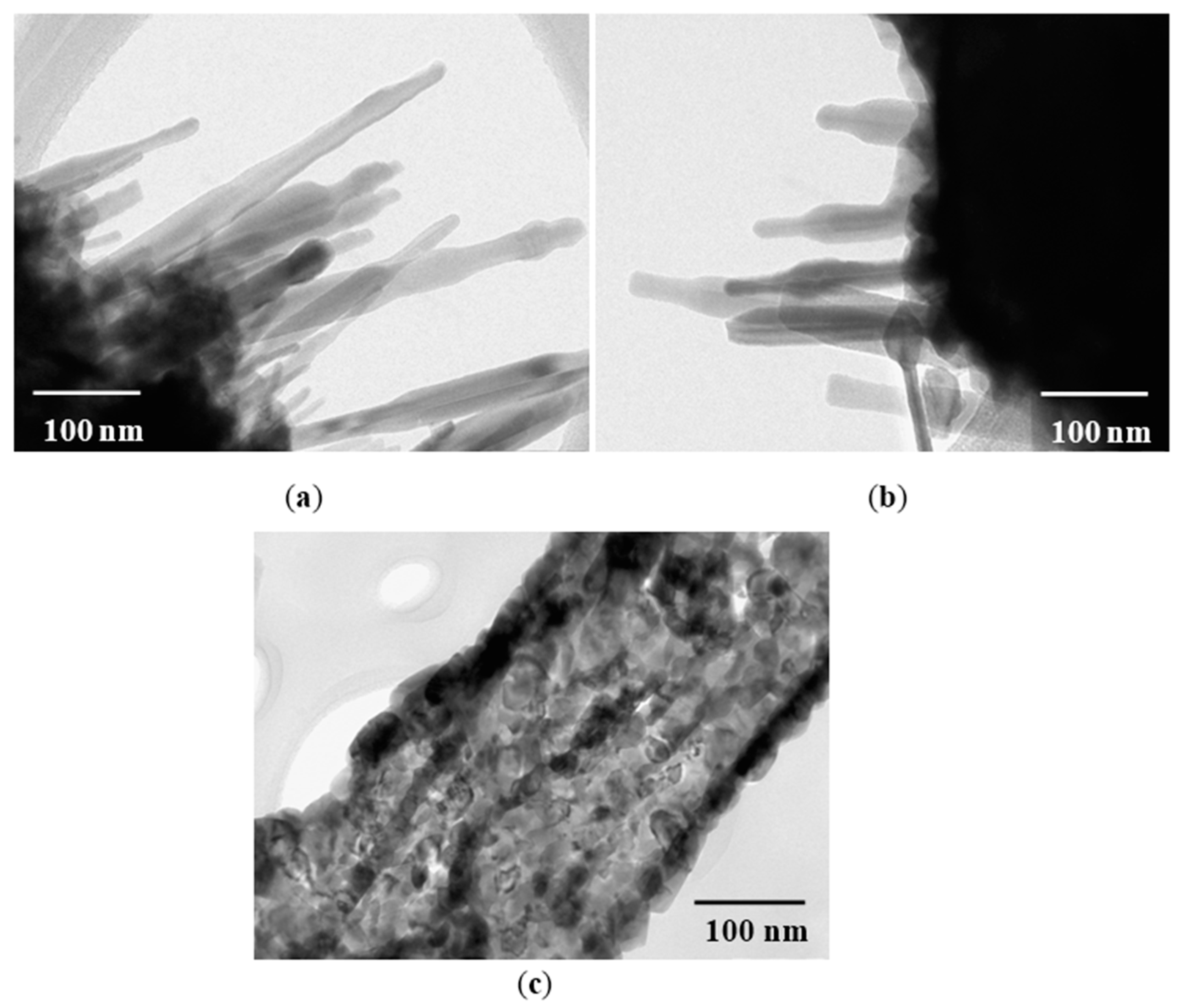

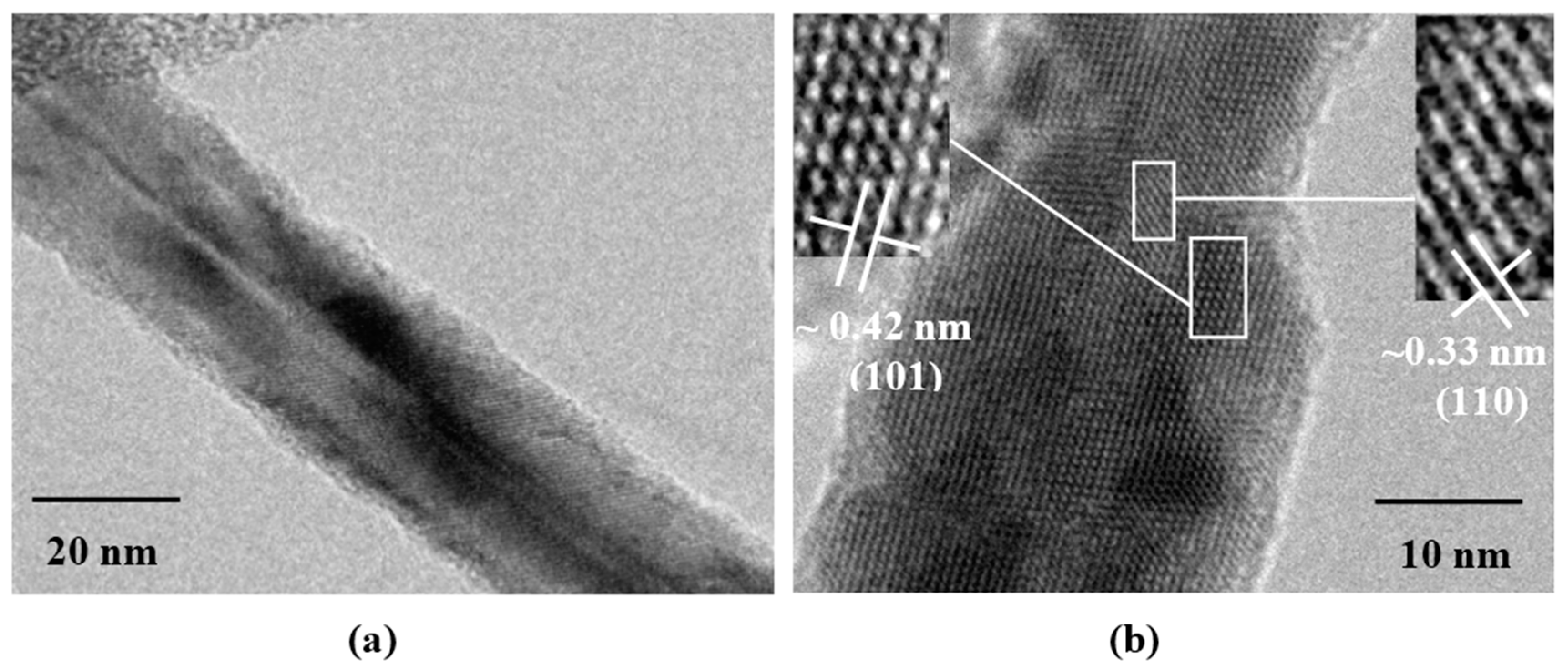
3.4. Sensing Properties
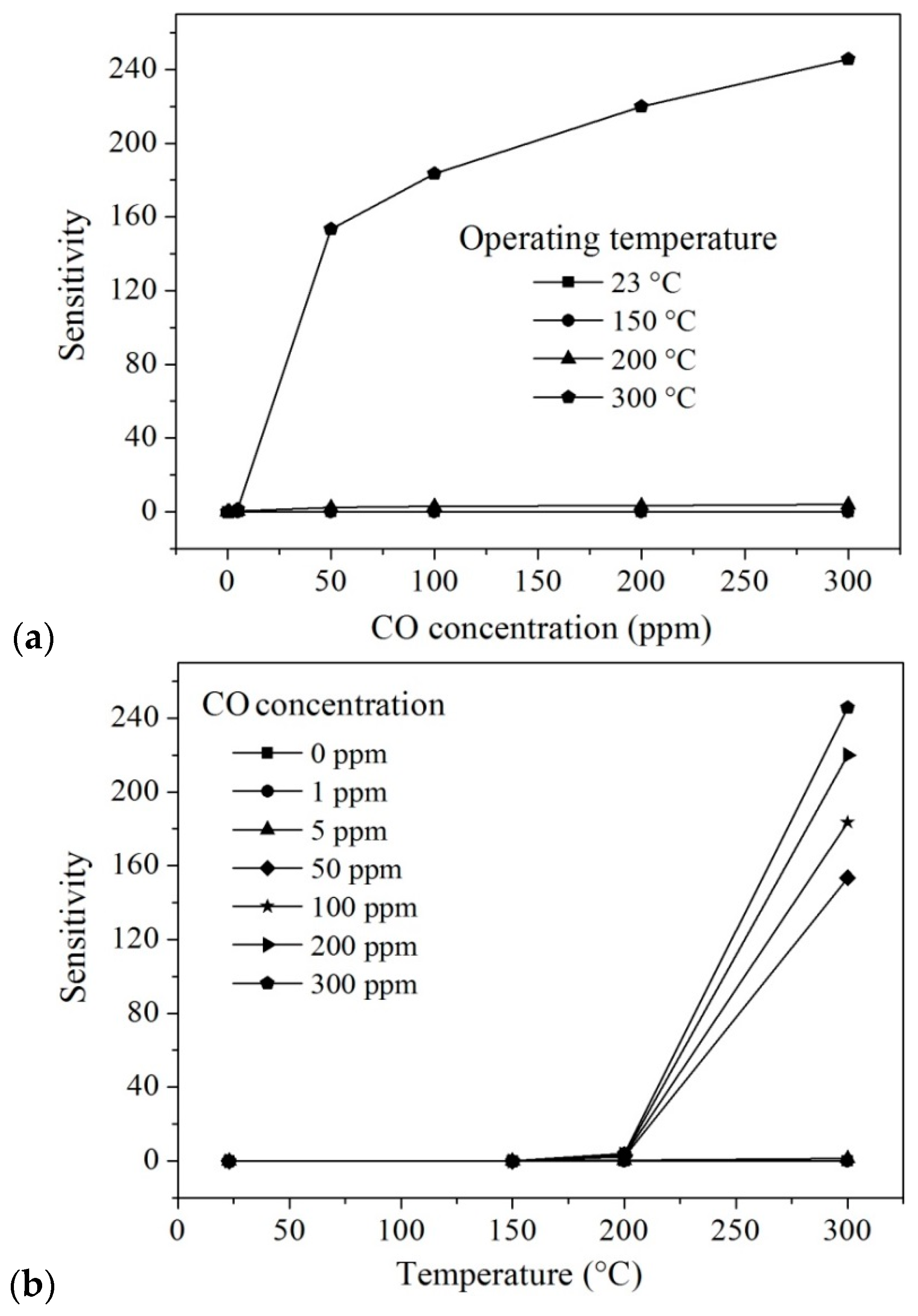
| Temperature (°C) | Concentration CO (ppm) | Sensitivity (S) | Temperature (°C) | Concentration CO (ppm) | Sensitivity (S) |
|---|---|---|---|---|---|
| 200 | 0 | 0 | 300 | 0 | 0 |
| 5 | 0.36 | 5 | 1.37 | ||
| 50 | 2.40 | 50 | 153.47 | ||
| 100 | 2.84 | 100 | 183.46 | ||
| 200 | 3.39 | 200 | 219.93 | ||
| 300 | 3.87 | 300 | 245.75 |
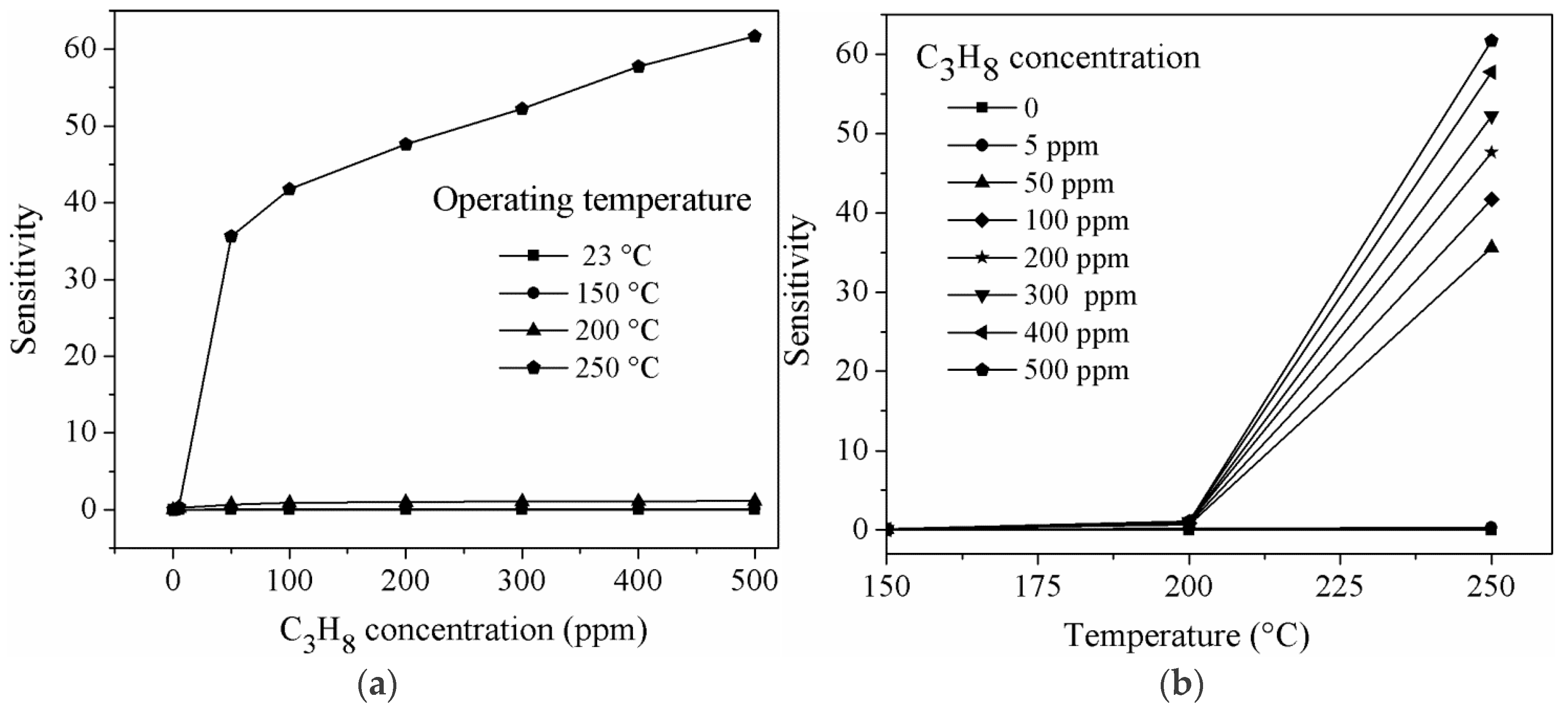
| Temperature (°C) | Concentration C3H8 (ppm) | Sensitivity (S) |
|---|---|---|
| 250 | 5 | 0.311 |
| 50 | 35.62 | |
| 100 | 41.72 | |
| 200 | 47.62 | |
| 300 | 52.20 | |
| 400 | 57.75 | |
| 500 | 61.66 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fleischer, M.; Meixner, H. Fast gas sensors based on metal oxides which are stable at high temperatures. Sens. Actuators B Chem. 1997, 43, 1–10. [Google Scholar] [CrossRef]
- Bartolomeo, E.D.; Grilli, M.L.; Traversa, E. Sensing mechanism of potentiometric gas sensors based on stabilized zirconia with oxide electrodes is it always mixed potential? J. Electrochem. Soc. 2004, 151, H133–H139. [Google Scholar] [CrossRef]
- Grilli, M.L.; Bartolomeo, E.D.; Traversa, E. Electrochemical NOx sensors based on interfacing nanosized LaFeO3 perovskite-type oxide and ionic conductors. J. Electrochem. Soc. 2001, 148, H98–H102. [Google Scholar] [CrossRef]
- Carotta, M.C.; Butturi, M.A.; Martinelli, G.; Sadaoka, Y.; Nunziante, P.; Traversa, E. Microstructural evolution of nanosized LaFeO3 powders from the thermal decomposition of a cyano-complex for thick film gas sensors. Sens. Actuators B Chem. 1999, 44, 590–594. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A Survey on gas sensing technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Zhou, H.J.; Jin, Z.L.; Savinell, R.F.; Liu, C.C. Application of nano-crystalline porous tin oxide thin film for CO sensing. Sens. Actuators B Chem. 1998, 52, 188–194. [Google Scholar] [CrossRef]
- Guillén-Bonilla, A.; Rodríguez-Betancourtt, V.-M.; Flores-Martínez, M.; Blanco-Alonso, O.; Reyes-Gómez, J.; Gildo-Ortiz, L.; Guillén-Bonilla, H. Dynamic Response of CoSb2O6 Trirutile-Type Oxides in a CO2 Atmosphere at Low-Temperatures. Sensors 2014, 14, 15802–15814. [Google Scholar] [CrossRef] [PubMed]
- Moseley, P.T.; Williams, D.E.; Norris, J.O.W.; Tofield, B.C. Electrical conductivity and gas sensitivity of some transition metal tantalates. Sens. Actuators B Chem. 1988, 14, 79–91. [Google Scholar] [CrossRef]
- Tamaki, J.; Yamada, Y.; Yamamoto, Y.; Matsuoka, M.; Ota, I. Sensing properties to dilute hydrogen sulfide of ZnSb2O6 thick-film prepared by dip-coating method. Sens. Actuators B Chem. 2000, 66, 70–73. [Google Scholar] [CrossRef]
- Gurav, A.S.; Duan, Z.; Wang, L.; Hampden-Smith, M.J.; Kodas, T.T. Synthesis of fullerene-rhodium nanocomposites via aerosol decomposition. Chem. Mater. 1993, 5, 214–216. [Google Scholar] [CrossRef]
- Cao, M.; Djerdj, I.; Antonietti, M.; Niederberger, M. Nonaqueouss synthesis of colloidal ZnGa2O4 nanocrystals and their photoluminescence properties. Chem. Mater. 2007, 19, 5830–5832. [Google Scholar] [CrossRef]
- Gulgun, M.A.; Nguyen, M.H.; Kriven, W.M. Polymerized organic-inorganic synthesis of mixed oxides. J. Am. Ceram. Soc. 1999, 82, 556–560. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q.; Tanaka, H.; Iwasa, M. Improvement of the dispersion of Al2O3 slurries using EDTA-4Na. J. Am. Ceram. Soc. 2006, 89, 1440–1442. [Google Scholar] [CrossRef]
- Strizhak, P.E.; Didenko, O.Z.; Kosmambetova, G.R. Synthesis and characterization of ZnO/MgO solids prepared by deposition of preformed colloidal ZnO nanoparticles. Mater. Lett. 2008, 62, 4094–4096. [Google Scholar] [CrossRef]
- Gildo-Ortiz, L.; Guillén-Bonilla, H.; Santoyo-Salazar, J.; Olvera, M.L.; Karthik, T.V.K.; Campos-González, E.; Reyes-Gómez, J. Low-temperature synthesis and gas sensitivity of perovskite-type LaCoO3 nanoparticles. J. Nanomater. 2014, 2014. [Google Scholar] [CrossRef]
- Matijevic, E. Preparation and properties of uniform size colloids. Chem. Mater. 1993, 5, 412–426. [Google Scholar] [CrossRef]
- Libert, S.; Goia, D.V.; Matijevic, E. Internally Composite Uniform Colloidal Cadmium Sulfide Spheres. Langmuir 2003, 19, 10673–10678. [Google Scholar] [CrossRef]
- Guillén-Bonilla, H.; Gildo-Ortiz, L.; Olvera, M.L.; Santoyo-Salazar, J.; Rodríguez-Betancourtt, V.-M.; Guillen-Bonilla, A.; Reyes-Gómez, J. Sensitivity of mesoporous CoSb2O6 nanoparticles to gaseous CO and C3H8 at low temperatures. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef]
- Haeuseler, H. Infrared and Raman spectra and normal coordinate calculations on trirutile-type compounds. Spectrochim. Acta Part A Mol. Spectrosc. 1981, 37, 487–495. [Google Scholar] [CrossRef]
- Delgado, E.; Michel, C.R. CO2 and O2 sensing behavior of nanostructured barium-doped SmCoO3. Mater. Lett. 2006, 60, 1613–1616. [Google Scholar] [CrossRef]
- Michel, C.R.; Lopez-Contreras, N.L.; Lopez-Alvarez, M.A.; Martinez-Preciado, A.H. Gas selectivity of nanostructured ZnSb2O6 synthesized by a colloidal method. Sens. Actuators B Chem. 2012, 171–172, 686–690. [Google Scholar] [CrossRef]
- Husson, E.; Repelin, Y.; Brusset, H.; Cerez, A. Spectres de vibration et calcul du champ de force des antimoniates et des tantalates de structure trirutile. Spectrochim. Acta Part A Mol. Spectrosc. 1979, 35, 1171–1187. (In French) [Google Scholar] [CrossRef]
- Mizoguchi, H.; Woodward, P.M. Electronic structure studies of main group oxides possessing edge-sharing octahedra: Implications for the design of transparent conducting oxides. Chem. Mater. 2004, 16, 5233–5248. [Google Scholar] [CrossRef]
- Deng, Z.-X.; Wang, C.; Sun, X.-M.; Li, Y.D. Structure-directing coordination template effect of ethylenediamine in formations of ZnS and ZnSe nanocrystallites via solvothermal route. Inorg. Chem. 2002, 41, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y. Solution-based synthetic strategies for 1-D nanostructures. Inorg. Chem. 2006, 45, 7522–7534. [Google Scholar] [CrossRef] [PubMed]
- LaMer, V.K.; Dinegar, R.H. Theory, production and mechanism of formation of monodispersed hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Chittofrati, A.; Matijevic, E. Uniform particles of zinc oxide of different morphologies. Colloids Surf. 1990, 48, 65–78. [Google Scholar] [CrossRef]
- Sampanthar, J.T.; Zeng, H.C. Arresting butterfly-like intermediate nanocrystals of β-Co(OH)2 via ethylenediamine-mediated synthesis. J. Am. Chem. Soc. 2002, 124, 6668–6675. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pozos, H.; González-Vidal, J.L.; Torres, G.A.; Olvera, M.L.; Castañeda, L. Physical characterization and effect of effective surface area on the sensing properties of tin dioxide thin solid films in a propane atmosphere. Sensors 2014, 14, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pozos, H.; González-Vidal, J.L.; Torres, G.A.; Rodríguez-Baez, J.; Maldonado, A.; Olvera, M.L.; Acosta, D.R.; Avendaño-Alejo, M.; Castañeda, L. Chromium and ruthenium-doped zinc oxide thin films for propane sensing applications. Sensors 2013, 13, 3432–3444. [Google Scholar] [CrossRef] [PubMed]
- Fields, L.L.; Zheng, J.P.; Cheng, Y.; Xiong, P. Room-temperature low-power hydrogen sensor based on a single tin dioxide nanobelt. Appl. Phys. Lett. 2006, 88, 263102. [Google Scholar] [CrossRef]
- Hsiao, C.-C.; Luo, L.-S. A rapid process for fabricating gas sensors. Sensors 2014, 14, 12219–12232. [Google Scholar] [CrossRef] [PubMed]
- Baraton, M.-I.; Merhari, L. Electrical behavior of semiconducting nanopowders versus environment. Adv. Mater. Sci. 2003, 4, 15–24. [Google Scholar]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Yamaura, H.; Jinkawa, T.; Tamaki, J.; Moriya, K.; Miura, N.; Yamazoe, N. Indium oxide-based gas sensor for selective detection of CO. Sens. Actuators B Chem. 1996, 35–36, 325–332. [Google Scholar] [CrossRef]
- Wetchakun, K.; Samerjai, T.; Tamaekong, N.; Liewhiran, C.; Siriwong, C.; Kruefua, V.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Semiconducting metal oxides as sensors for environmentally hazardous gases. Sens. Actuators B Chem. 2011, 160, 580–591. [Google Scholar] [CrossRef]
- Siemons, M.; Simon, U. High throughput screening of the sensing properties of doped SmFeO3. Solid State Phenomena 2007, 128, 225–236. [Google Scholar] [CrossRef]
- Chang, S.C. Oxygen chemisorption on tin oxide: Correlation between electrical conductivity and EPR measurements. J. Vac. Sci. Technol. 1979, 17, 366–369. [Google Scholar] [CrossRef]
- Toan, N.N.; Saukko, S.; Lantto, V. Gas sensing with semiconducting perovskite oxide LaFeO3. Phys. B 2003, 327, 279–282. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-F.; Liu, S.-B.; Meng, F.-L.; Liu, J.-Y.; Jin, Z.; Kong, L.-T.; Liu, J.-H. Metal oxide nanostructures and their gas sensing properties: A Review. Sensors 2012, 12, 2610–2631. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wan, Q. Gas sensors based on semiconducting metal oxide one-dimensional nanostructures. Sensors 2009, 9, 9903–9924. [Google Scholar] [CrossRef] [PubMed]
- Kerlau, M.; Reichel, P.; Bârsan, N.; Weimar, U.; Delsarte-Gueguen, S.; Merdrignac-Conanec, O. Detection of propane by “GaON” thick-film gas sensors. Sens. Actuators B Chem. 2007, 122, 14–19. [Google Scholar] [CrossRef]
- Wei, M.-D.; Teraoka, Y.; Kagawa, S. Catalytic property of AII2BIIBVIO6 double perovskites (BVI = Mo, W) for the reduction of nitric oxide with propane in the presence of oxygen. Mater. Res. Bull. 2000, 35, 521–530. [Google Scholar] [CrossRef]
- Bahrami, B.; Khodadadi, A.; Kazemeini, M.; Mortazavi, Y. Enhanced CO sensitivity and selectivity of gold nanoparticles-doped SnO2 sensor in presence of propane and methane. Sens. Actuators B Chem. 2008, 133, 352–356. [Google Scholar] [CrossRef]
- Saberi, M.H.; Mortazavi, Y.; Khodadadi, A.A. Dual selective Pt/SnO2 sensor to CO and propane in exhaust gases of gasoline engines using Pt/LaFeO3 filter. Sens. Actuators B Chem. 2015, 206, 617–623. [Google Scholar] [CrossRef]
- Koziej, D.; Bârsan, N.; Volker Hoffmann, V.; Szuber, J.; Weimar, U. Complementary phenomenological and spectroscopic studies of propane sensing with tin dioxide based. Sens. Actuators B Chem. 2005, 108, 75–83. [Google Scholar] [CrossRef]
- Yamazoe, N. New approaches for improving semiconductor gas sensors. Sens. Actuators B Chem. 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Engineering B Chem. 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Bochenkov, V.E.; Sergeev, G.B. Preparation and chemiresistive properties of nanostructured materials. Adv. Colloid Interface Sci. 2005, 116, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, N. Toward innovations of gas sensor technology. Sens. Actuators B Chem. 2005, 108, 2–14. [Google Scholar] [CrossRef]
- Neri, G. First fifty years of chemoresistive gas sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillén-Bonilla, H.; Flores-Martínez, M.; Rodríguez-Betancourtt, V.-M.; Guillen-Bonilla, A.; Reyes-Gómez, J.; Gildo-Ortiz, L.; De la Luz Olvera Amador, M.; Santoyo-Salazar, J. A Novel Gas Sensor Based on MgSb2O6 Nanorods to Indicate Variations in Carbon Monoxide and Propane Concentrations. Sensors 2016, 16, 177. https://doi.org/10.3390/s16020177
Guillén-Bonilla H, Flores-Martínez M, Rodríguez-Betancourtt V-M, Guillen-Bonilla A, Reyes-Gómez J, Gildo-Ortiz L, De la Luz Olvera Amador M, Santoyo-Salazar J. A Novel Gas Sensor Based on MgSb2O6 Nanorods to Indicate Variations in Carbon Monoxide and Propane Concentrations. Sensors. 2016; 16(2):177. https://doi.org/10.3390/s16020177
Chicago/Turabian StyleGuillén-Bonilla, Héctor, Martín Flores-Martínez, Verónica-María Rodríguez-Betancourtt, Alex Guillen-Bonilla, Juan Reyes-Gómez, Lorenzo Gildo-Ortiz, María De la Luz Olvera Amador, and Jaime Santoyo-Salazar. 2016. "A Novel Gas Sensor Based on MgSb2O6 Nanorods to Indicate Variations in Carbon Monoxide and Propane Concentrations" Sensors 16, no. 2: 177. https://doi.org/10.3390/s16020177





