Evaluation of Laser-Assisted Trans-Nail Drug Delivery with Optical Coherence Tomography
Abstract
:1. Introduction
2. Experiment Method and Setup
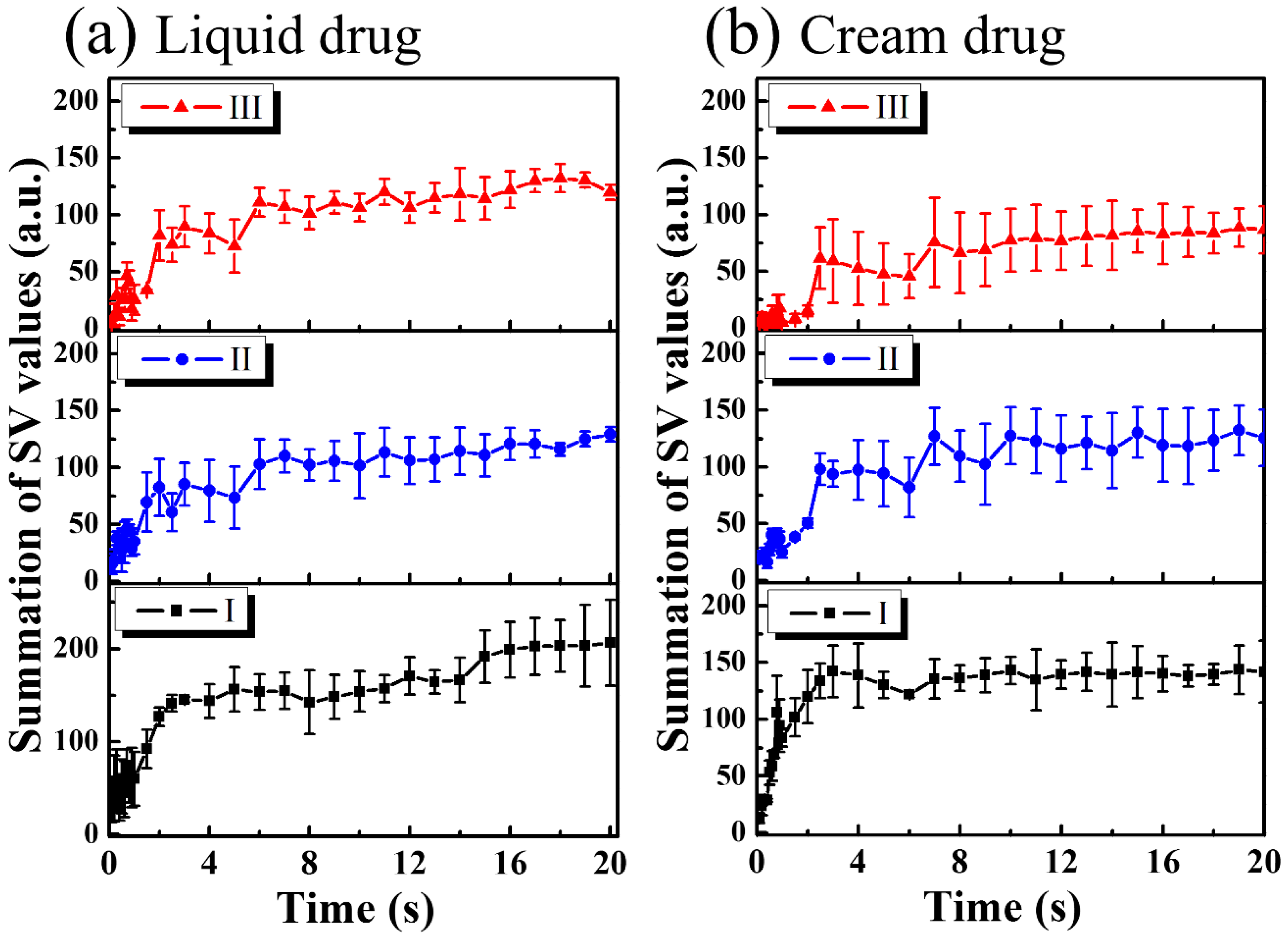
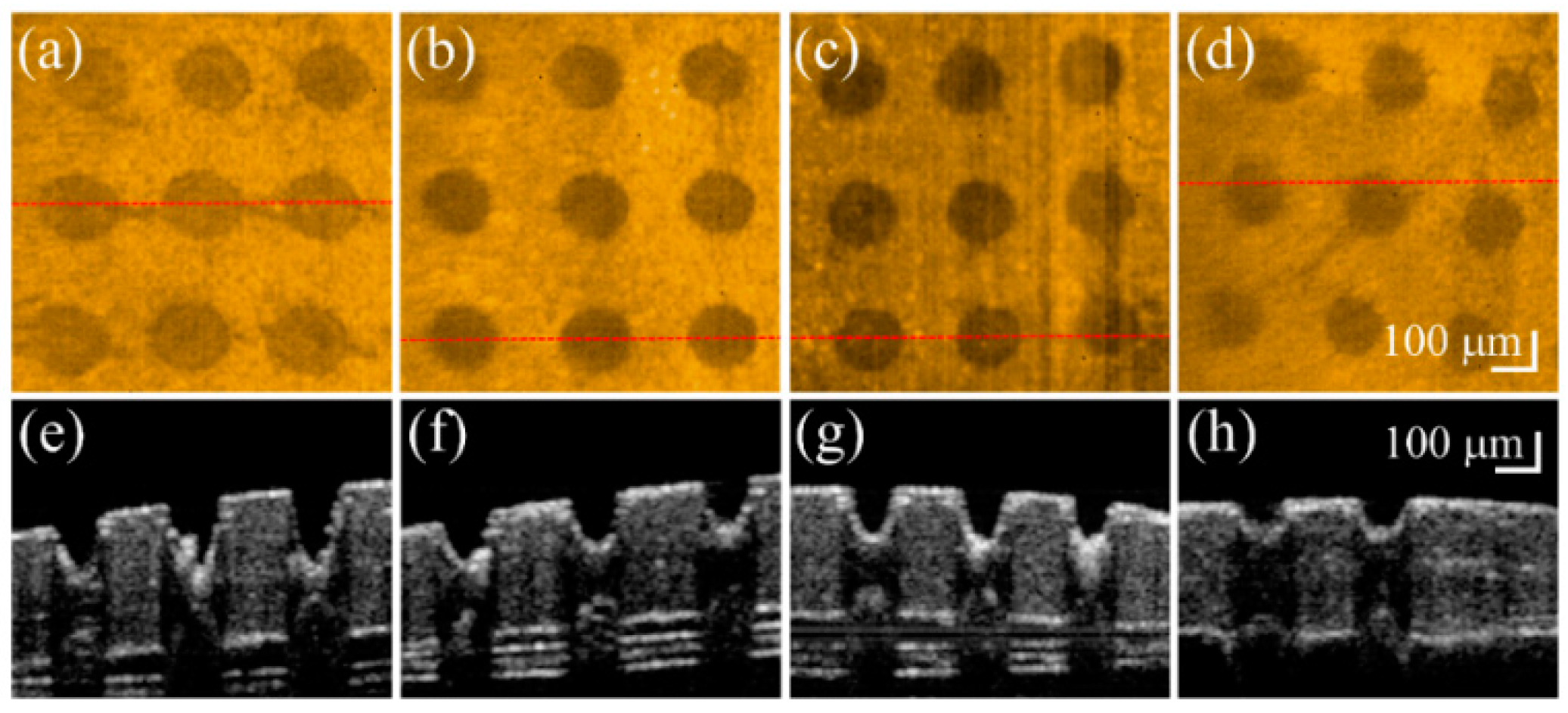
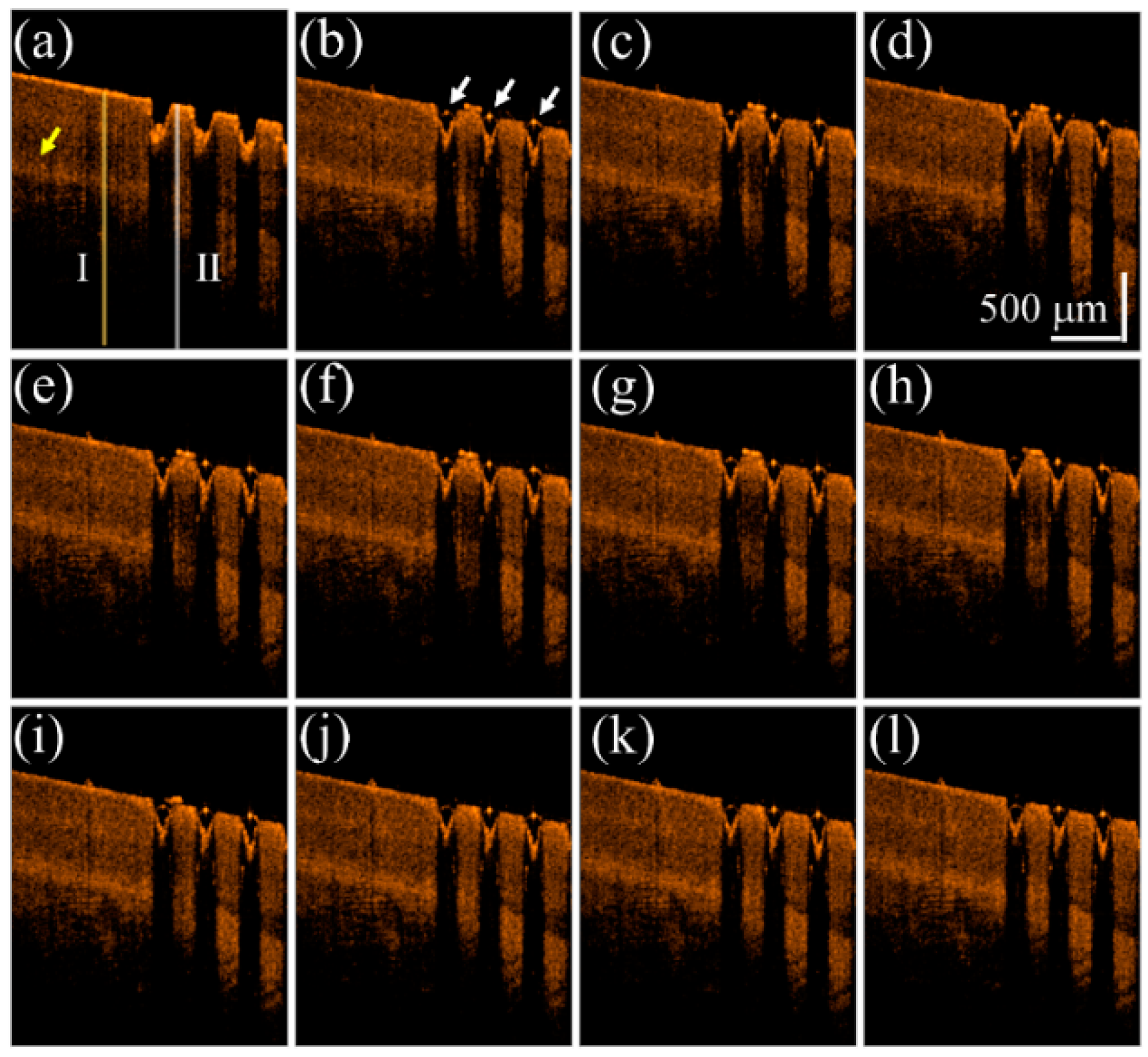
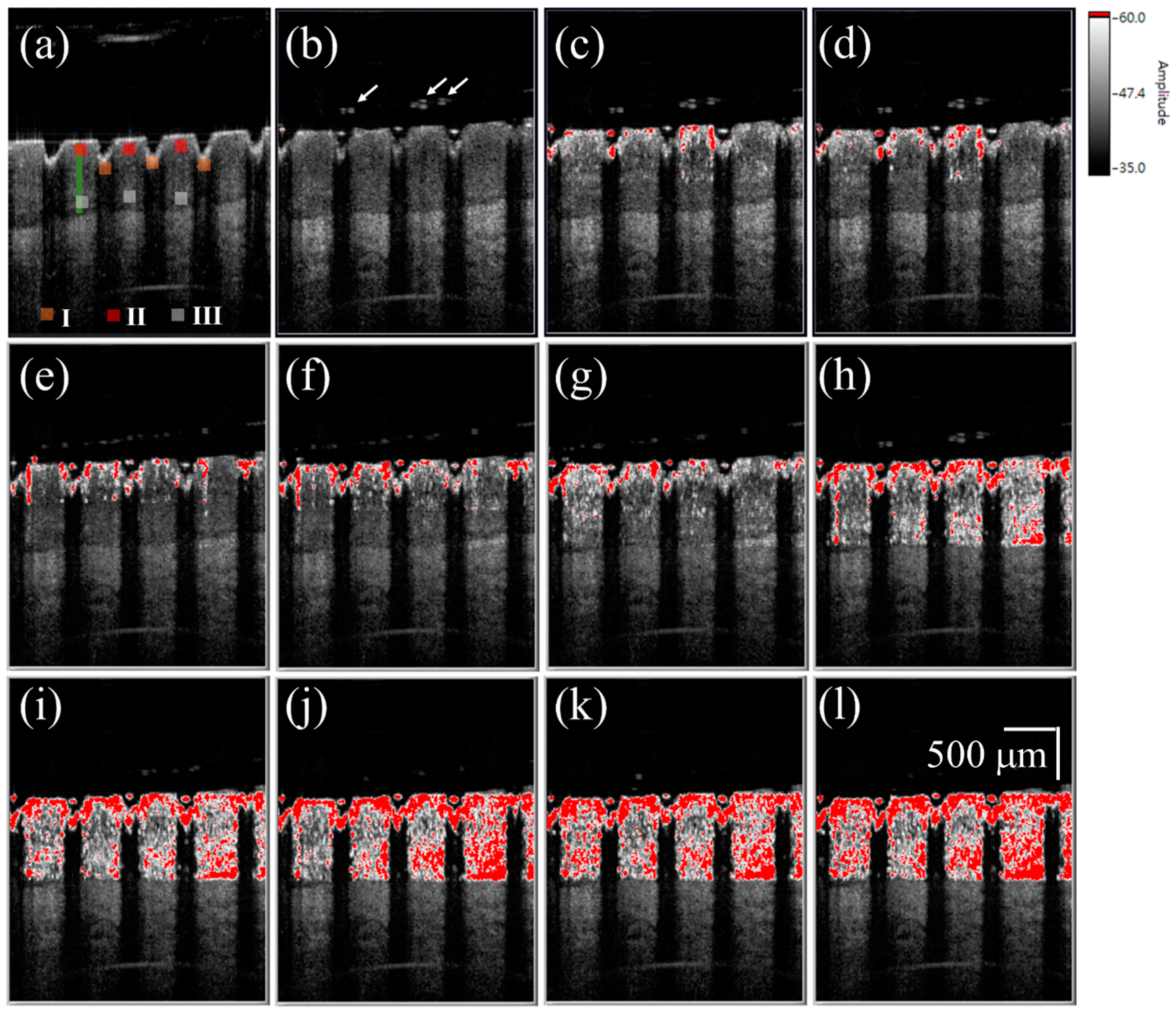
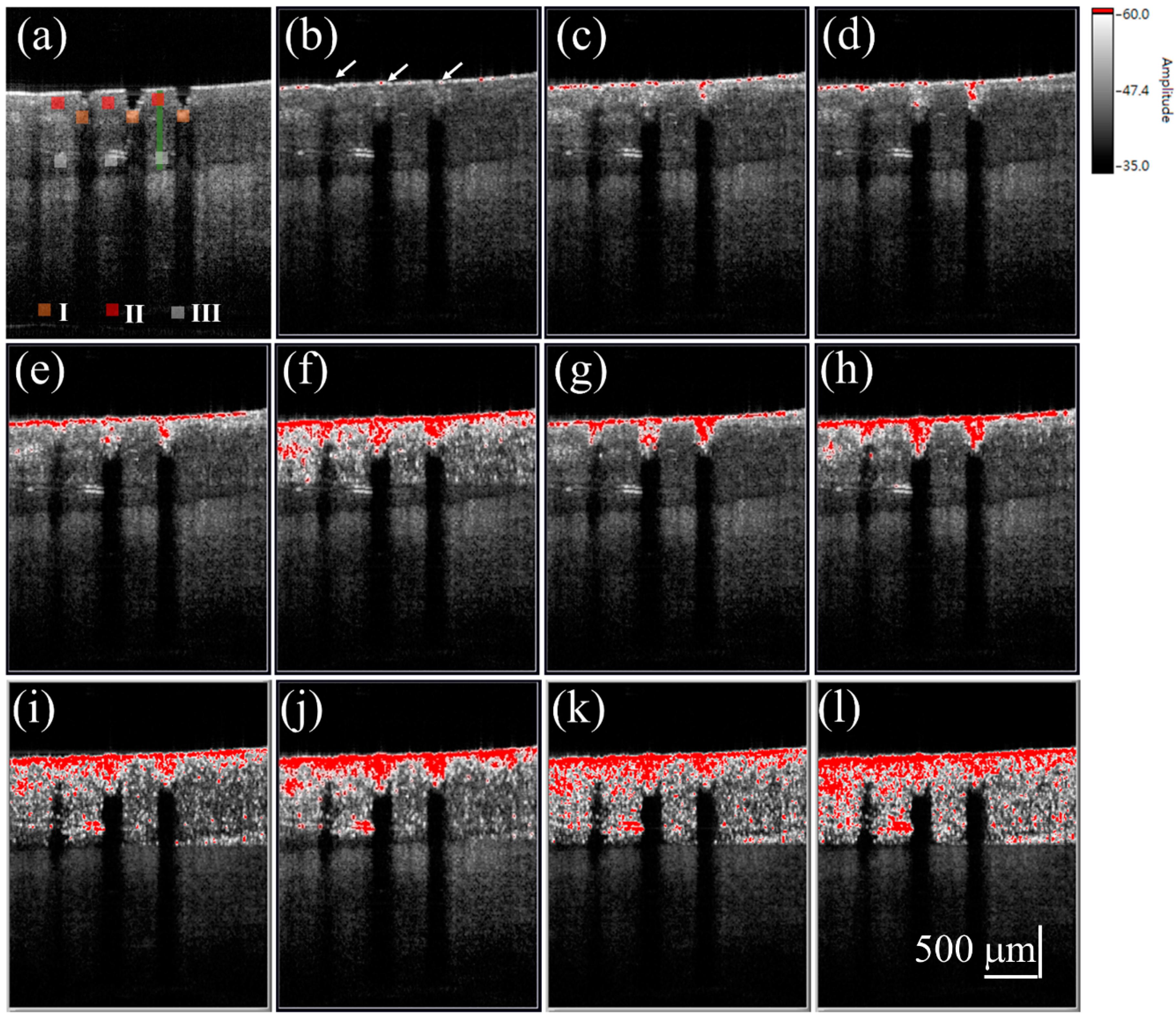
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gupchup, G.V.; Zatz, J.L. Structural characteristics and permeability properties of the human nail: A review. J. Cosmet. Sci. 1999, 50, 363–385. [Google Scholar]
- Repka, M.A.; O’Haver, J.; See, C.H.; Gutta, K.; Munjal, M. Nail morphology studies as assessments for onychomycosis treatment modalities. Int. J. Pharm. 2002, 245, 25–36. [Google Scholar] [CrossRef]
- Shivakumar, H.; Juluri, A.; Desai, B.; Murthy, S.N. Ungual and transungual drug delivery. Drug Dev. Ind. Pharm. 2012, 38, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Paquet, M. Improved efficacy in onychomycosis therapy. Clin. Dermatol. 2013, 31, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Elkeeb, R.; AliKhan, A.; Elkeeb, L.; Hui, X.; Maibach, H.I. Transungual drug delivery: Current status. Int. J. Pharm. 2010, 384, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.S.; Belsey, N.A.; Garrett, N.L.; Moger, J.; Price, G.J.; Delgado-Charro, M.B.; Guy, R.H. Drug delivery into microneedle-porated nails from nanoparticle reservoirs. J. Control. Release 2015, 220, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.W. Electrical, magnetic, photomechanical and cavitational waves to overcome skin barrier for transdermal drug delivery. J. Control. Release 2014, 193, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Man, G.; Elias, P.M.; Man, M.-Q. Therapeutic benefits of enhancing permeability barrier for atopic eczema. Dermatol. Sin. 2015, 33, 84–89. [Google Scholar] [CrossRef]
- Hu, L.; Man, H.; Elias, P.M.; Man, M.-Q. Herbal medicines that benefit epidermal permeability barrier function. Dermatol. Sin. 2015, 33, 90–95. [Google Scholar] [CrossRef]
- Merino, V.; Escobar-Chávez, J.J. Current Technologies to Increase the Transdermal Delivery of Drugs; Bentham Science Publishers: Sharjah, United Arab Emirates, 2010. [Google Scholar]
- Goswami, S.; Bajpai, J.; Bajpai, A. Designing gelatin nanocarriers as a swellable system for controlled release of insulin: An in vitro kinetic study. J. Macromol. Sci. A 2009, 47, 119–130. [Google Scholar] [CrossRef]
- Van der Maaden, K.; Jiskoot, W.; Bouwstra, J. Microneedle technologies for (trans) dermal drug and vaccine delivery. J. Control. Release 2012, 161, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Tsioris, K.; Raja, W.K.; Pritchard, E.M.; Panilaitis, B.; Kaplan, D.L.; Omenetto, F.G. Fabrication of silk microneedles for controlled-release drug delivery. Adv. Funct. Mater. 2012, 22, 330–335. [Google Scholar] [CrossRef]
- Smith, N.B. Perspectives on transdermal ultrasound mediated drug delivery. Int. J. Nanomed. 2007, 2, 585–594. [Google Scholar]
- Azagury, A.; Khoury, L.; Enden, G.; Kost, J. Ultrasound mediated transdermal drug delivery. Adv. Drug Del. Rev. 2014, 72, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Bounoure, F.; Skiba, M.L.; Besnard, M.; Arnaud, P.; Mallet, E.; Skiba, M. Effect of iontophoresis and penetration enhancers on transdermal absorption of metopimazine. J. Dermatol. Sci. 2008, 52, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Chavez, J.J.; Merino, V.; López-Cervantes, M.; Rodriguez-Cruz, I.M.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. The use of iontophoresis in the administration of nicotine and new non-nicotine drugs through the skin for smoking cessation. Curr. Drug Disc. Technol. 2009, 6, 171–185. [Google Scholar] [CrossRef]
- Jelínková, H. Lasers for Medical Applications: Diagnostics, Therapy and Surgery; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Stafford, R.J.; Fuentes, D.; Elliott, A.A.; Weinberg, J.S.; Ahrar, K. Laser-induced thermal therapy for tumor ablation. Crit. Rev. Biomed. Eng. 2010, 38, 79–100. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.; Galimberti, M.; De Pace, B.; Pellacani, G.; Bencini, P.L. Laser skin rejuvenation: Epidermal changes and collagen remodeling evaluated by in vivo confocal microscopy. Laser Med. Sci. 2013, 28, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Mazur, E. Surgical Applications of femtosecond lasers. J. Biophotonics 2009, 2, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Garvie-Cook, H.; Stone, J.M.; Yu, F.; Guy, R.H.; Gordeev, S.N. Femtosecond pulsed laser ablation to enhance drug delivery across the skin. J. Biophotonics 2016, 9, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-R.; Shen, S.-C.; Al-Suwayeh, S.A.; Yang, H.-H.; Yuan, C.-Y.; Fang, J.-Y. Laser-assisted topical drug delivery by using a low-fluence fractional laser: Imiquimod and macromolecules. J. Control. Release 2011, 153, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-H.; Kim, H.-R.; Park, Y.-O.; Lee, Y.; Seo, Y.-J.; Kim, C.-D.; Lee, J.-H.; Im, M. Toenail onychomycosis treated with a fractional carbon-dioxide laser and topical antifungal cream. J. Am. Acad. Dermatol. 2014, 70, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Stumpp, O.F.; Bedi, V.P.; Wyatt, D.; Lac, D.; Rahman, Z.; Chan, K.F. In vivo confocal imaging of epidermal cell migration and dermal changes post nonablative fractional resurfacing: Study of the wound healing process with corroborated histopathologic evidence. J. Biomed. Opt. 2009, 14, 024018. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.M.; Behne, M.J.; Barry, N.P.; Mauro, T.M.; Gratton, E.; Clegg, R.M. Two-photon fluorescence lifetime imaging of the skin stratum corneum pH Gradient. Biophys. J. 2002, 83, 1682–1690. [Google Scholar] [CrossRef]
- Xiao, C.; Moore, D.J.; Rerek, M.E.; Flach, C.R.; Mendelsohn, R. Feasibility of tracking phospholipid permeation into skin using infrared and Raman microscopic imaging. J. Investig. Dermatol. 2005, 124, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Shemonski, N.D.; Adie, S.G.; Kim, H.-S.; Hwu, W.-M.W.; Carney, P.S.; Boppart, S.A. Real-time in vivo computed optical interferometric tomography. Nat. Photonics 2013, 7, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.T.; Tsai, M.T.; Lee, C.K. Two-level optical coherence tomography scheme for suppressing spectral saturation artifacts. Sensors 2014, 14, 13548–13555. [Google Scholar] [CrossRef] [PubMed]
- Deka, G.; Wu, W.-W.; Kao, F.-J. In vivo wound healing diagnosis with second harmonic and fluorescence lifetime imaging. J. Biomed. Opt. 2013, 18, 061222. [Google Scholar] [CrossRef] [PubMed]
- Yeh, A.T.; Kao, B.; Jung, W.G.; Chen, Z.P.; Nelson, J.S.; Tromberg, B.J. Imaging wound healing using optical coherence tomography and multiphoton microscopy in an in vitro skin-equivalent tissue model. J. Biomed. Opt. 2006, 9, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Cobb, M.J.; Chen, Y.; Underwood, R.A.; Usui, M.L.; Olerud, J.; Li, X.D. Noninvasive assessment of cutaneous wound healing using ultrahigh-resolution optical coherence tomography. J. Biomed. Opt. 2006, 11, 064002. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Baran, U.; Wang, R.K. In vivo blood flow imaging of inflammatory human skin induced by tape stripping using optical microangiography. J. Biophotonics 2015, 8, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Jia, W.; Sun, V.; Choi, B.; Chen, Z. High-resolution imaging of microvasculature in human skin in vivo with optical coherence tomography. Opt. Express 2012, 20, 7694–7705. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Yamanari, M.; Miyazawa, A.; Matsumoto, M.; Nakagawa, N.; Sugawara, T.; Kawabata, K.; Yatagai, T.; Yasuno, Y. In vivo three-dimensional birefringence analysis shows collagen differences between young and old photo-aged human skin. J. Investig. Dermatol. 2008, 128, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Yamanari, M.; Lim, Y.; Nakagawa, N.; Yasuno, Y. In vivo evaluation of human skin anisotropy by polarization-sensitive optical coherence tomography. Biomed. Opt. Express 2011, 2, 2623–2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.-M.; Song, S.; Arnal, B.; Huang, Z.; O’Donnell, M.; Wang, R.K. Visualizing ultrasonically induced shear wave propagation using phase-sensitive optical coherence tomography for dynamic elastography. Opt. Lett. 2014, 39, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Larin, K.V. Optical Coherence Elastography for Tissue Characterization: A Review. J. Biophotonics 2015, 8, 279–302. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-T.; Yang, C.-H.; Shen, S.-C.; Lee, Y.-J.; Chang, F.-Y.; Feng, C.-S. Monitoring of wound healing process of human skin after fractional laser treatments with optical coherence tomography. Biomed. Opt. Express 2013, 4, 2362–2375. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-H.; Tsai, M.-T.; Shen, S.-C.; Ng, C.Y.; Jung, S.-M. Feasibility of ablative fractional laser-assisted drug delivery with optical coherence tomography. Biomed. Opt. Express 2014, 5, 3949–3959. [Google Scholar] [CrossRef] [PubMed]
- Mariampillai, A.; Standish, B.A.; Moriyama, E.H.; Khurana, M.; Munce, N.R.; Leung, M.K.; Jiang, J.; Cable, A.; Wilson, B.C.; Vitkin, I.A. Speckle variance detection of microvasculature using swept-source optical coherence tomography. Opt. Lett. 2008, 33, 1530–1532. [Google Scholar] [CrossRef] [PubMed]
- Cadotte, D.W.; Mariampillai, A.; Cadotte, A.; Lee, K.K.; Kiehl, T.-R.; Wilson, B.C.; Fehlings, M.G.; Yang, V.X. Speckle variance optical coherence tomography of the rodent spinal cord: In vivo feasibility. Biomed. Opt. Express 2012, 3, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-K.; Tseng, H.-Y.; Lee, C.-Y.; Wu, S.-Y.; Chi, T.-T.; Yang, K.-M.; Chou, H.-Y.E.; Tsai, M.-T.; Wang, J.-Y.; Kiang, Y.-W. Characterizing the localized surface plasmon resonance behaviors of Au nanorings and tracking their diffusion in bio-tissue with optical coherence tomography. Biomed. Opt. Express 2010, 1, 1060–1074. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, M.S.; Cadotte, D.W.; Vuong, B.; Sun, C.; Luk, T.W.; Mariampillai, A.; Yang, V.X. Review of speckle and phase variance optical coherence tomography to visualize microvascular networks. J. Biomed. Opt. 2013, 18, 050901. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.N.; Vaka, S.R.K.; Sammeta, S.M.; Nair, A.B. Transcreen-N™: Method for rapid screening of trans-ungual drug delivery enhancers. J. Pharm. Sci. 2009, 98, 4264–4271. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-T.; Yang, C.-H.; Shen, S.-C.; Chang, F.-Y.; Yi, J.-Y.; Fan, C.-H. Noninvasive characterization of fractional photothermolysis induced by ablative and non-ablative lasers with optical coherence tomography. Laser Phys. 2013, 23, 075604. [Google Scholar] [CrossRef]
- Wijesinghe, R.E.; Lee, S.-Y.; Kim, P.; Jung, H.-Y.; Jeon, M.; Kim, J. Optical Inspection and Morphological Analysis of Diospyros kaki Plant Leaves for the Detection of Circular Leaf Spot Disease. Sensors 2016, 16, 1282. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, M.-T.; Tsai, T.-Y.; Shen, S.-C.; Ng, C.Y.; Lee, Y.-J.; Lee, J.-D.; Yang, C.-H. Evaluation of Laser-Assisted Trans-Nail Drug Delivery with Optical Coherence Tomography. Sensors 2016, 16, 2111. https://doi.org/10.3390/s16122111
Tsai M-T, Tsai T-Y, Shen S-C, Ng CY, Lee Y-J, Lee J-D, Yang C-H. Evaluation of Laser-Assisted Trans-Nail Drug Delivery with Optical Coherence Tomography. Sensors. 2016; 16(12):2111. https://doi.org/10.3390/s16122111
Chicago/Turabian StyleTsai, Meng-Tsan, Ting-Yen Tsai, Su-Chin Shen, Chau Yee Ng, Ya-Ju Lee, Jiann-Der Lee, and Chih-Hsun Yang. 2016. "Evaluation of Laser-Assisted Trans-Nail Drug Delivery with Optical Coherence Tomography" Sensors 16, no. 12: 2111. https://doi.org/10.3390/s16122111