Electrical Impedance Monitoring of C2C12 Myoblast Differentiation on an Indium Tin Oxide Electrode
Abstract
:1. Introduction
2. Materials and Methods
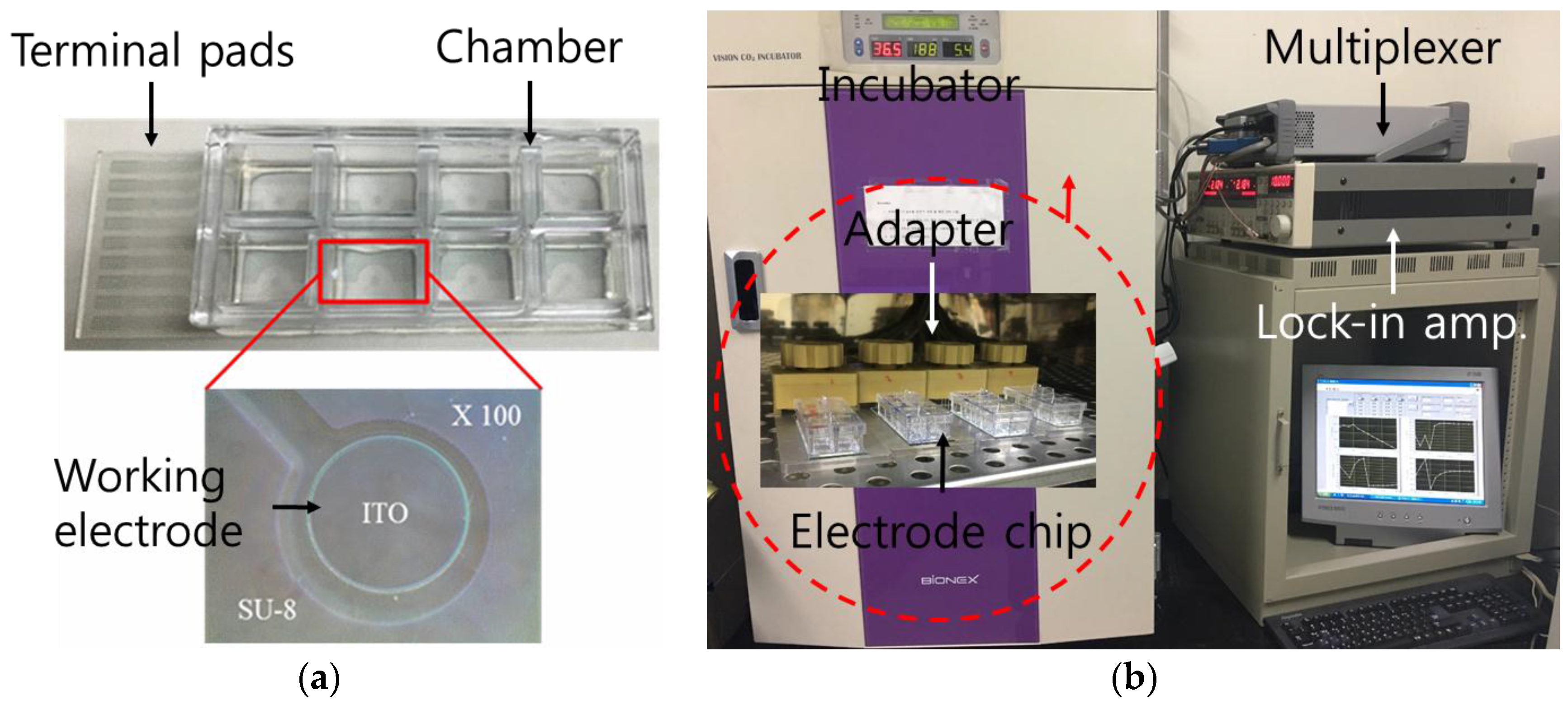
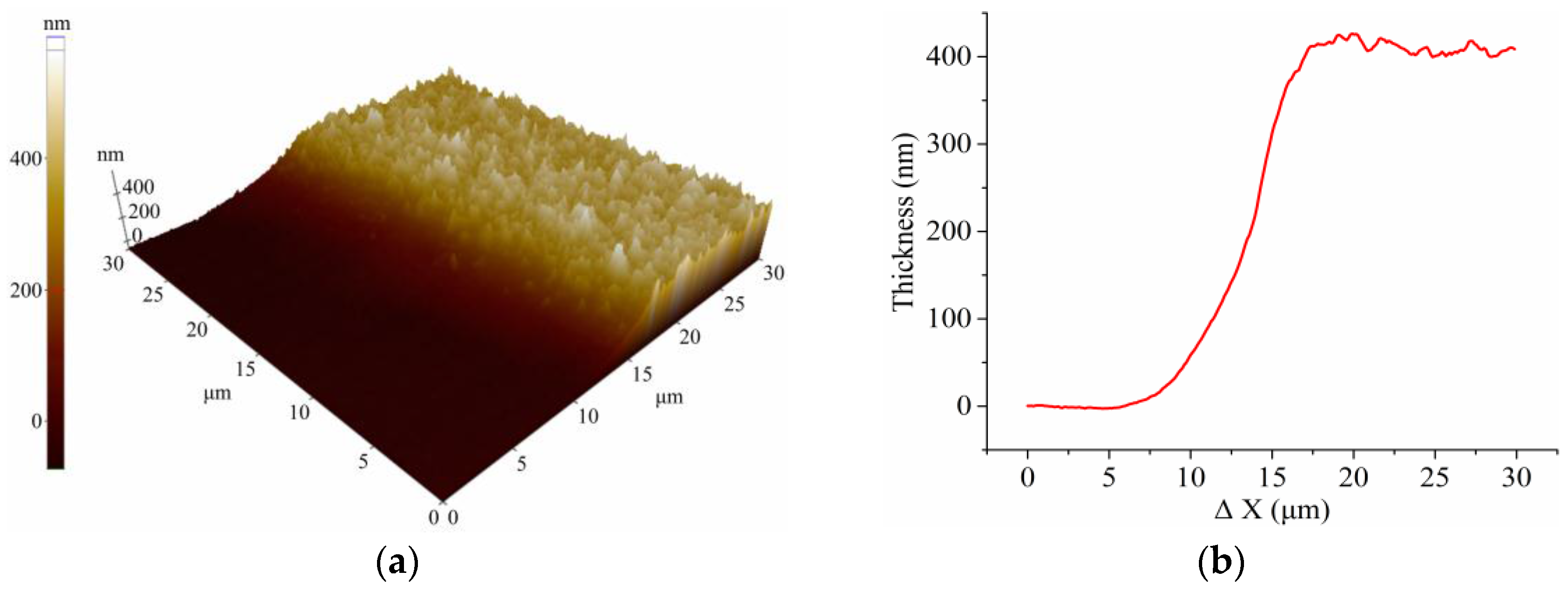
2.1. Fabrication of ITO Electrode-Based Chip
2.2. C2C12 Myoblast Cultivation and Differentiation
2.3. Electrical Impedance Measurement of Cells
2.4. RNA Isolation and Quantitative Real-Time PCR
2.5. Immunofluorescence Analysis
2.6. Statistical Analysis
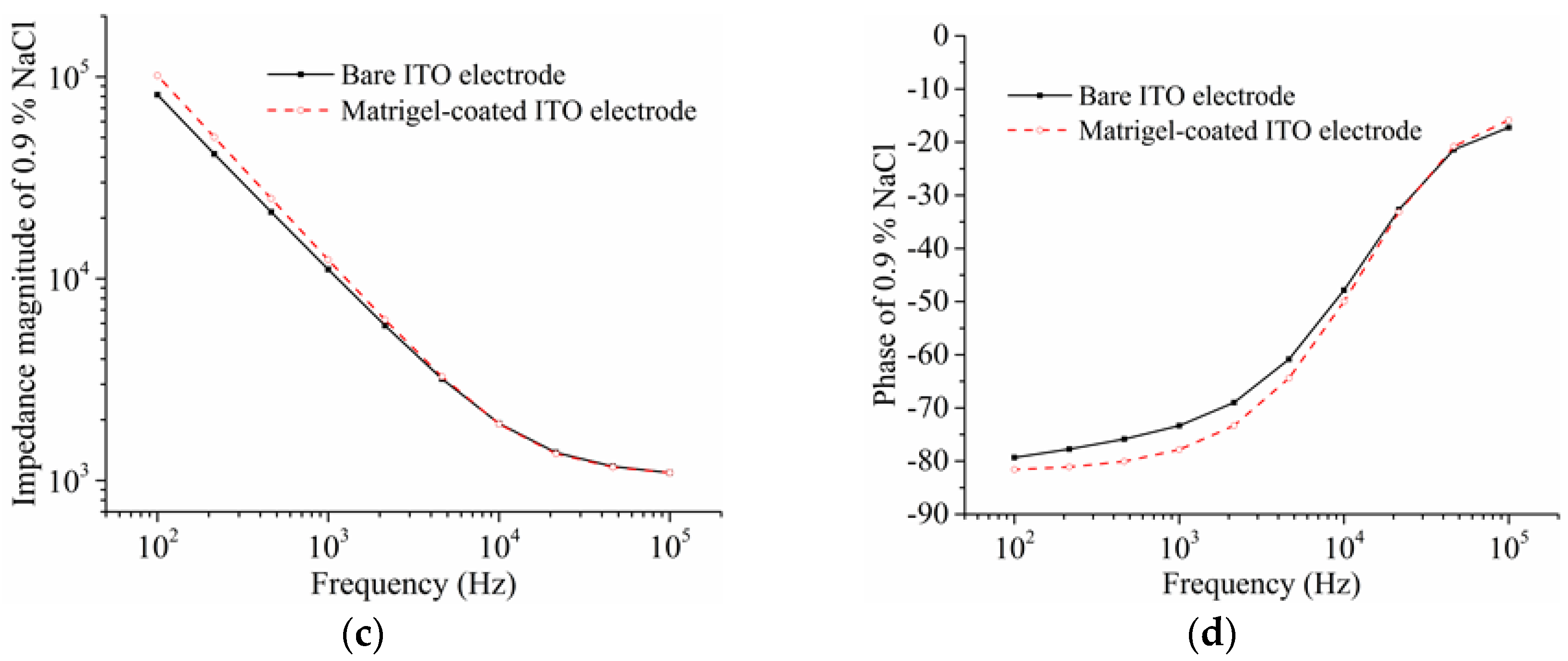
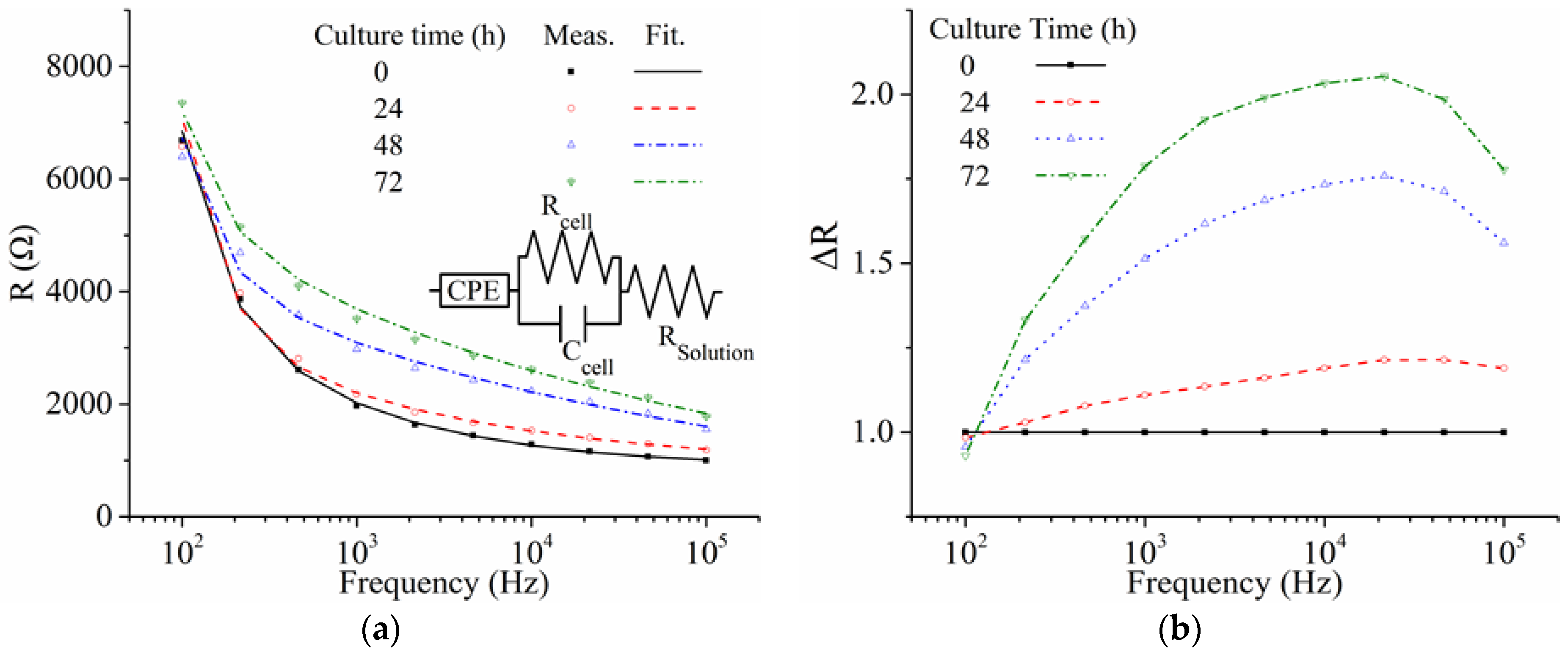
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lindstedt, S.L. Skeletal muscle tissue in movement and health: Positives and negatives. J. Exp. Biol. 2016, 219, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Mayeuf-Louchart, A.; Staels, B.; Duez, H. Skeletal muscle functions around the clock. Diabetes Obes. Metab. 2015, 17, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.P.; Foletta, V.C.; Snow, R.J.; Wadley, G.D. Skeletal muscle mitochondria: A major player in exercise, health and disease. BBA Gen. Subj. 2014, 1840, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Ryall, J.G. The role of sirtuins in the regulation of metabolic homeostasis in skeletal muscle. Curr. Opin. Clin. Nutr. 2012, 15, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Kinugawa, S.; Takada, S.; Matsushima, S.; Okita, K.; Tsutsui, H. Skeletal Muscle Abnormalities in Heart Failure. Int. Heart J. 2015, 56, 475–484. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, P.; Scaini, D.; Severino, L.U.; Borelli, V.; Passamonti, S.; Lorenzon, P.; Bandiera, A. In vitro myogenesis induced by human recombinant elastin-like proteins. Biomaterials 2015, 67, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Litwiniuk, A.; Pijet, B.; Pijet-Kucicka, M.; Gajewska, M.; Pajak, B.; Orzechowski, A. FOXO1 and GSK-3 beta Are Main Targets of Insulin-Mediated Myogenesis in C2C12 Muscle Cells. PLoS ONE 2016, 11, e0146726. [Google Scholar] [CrossRef] [PubMed]
- Rebbapragada, A.; Benchabane, H.; Wrana, J.L.; Celeste, A.J.; Attisano, L. Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol. Cell. Biol. 2003, 23, 7230–7242. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, D.M.; Al-Sajee, D.; Hawke, T.J. Diabetic rnyopathy: Impact of diabetes mellitus on skeletal muscle progenitor cells. Front. Physiol. 2013, 4, 379. [Google Scholar] [PubMed]
- Giaever, I.; Keese, C.R. Monitoring fibroblast behavior in tissue culture with an applied electric field. Proc. Natl. Acad. Sci. USA 1984, 81, 3761–3764. [Google Scholar] [CrossRef] [PubMed]
- Giaever, I.; Keese, C.R. Micromotion of mammalian cells measured electrically. Proc. Natl. Acad. Sci. USA 1991, 88, 7896–7900. [Google Scholar] [CrossRef] [PubMed]
- Wegener, J.; Keese, C.R.; Giaever, I. Electric cell–substrate impedance sensing (ECIS) as a noninvasive means to monitor the kinetics of cell spreading to artificial surfaces. Exp. Cell Res. 2000, 259, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H.; Wang, L.; Mitchelson, K.; Yu, Z.Y.; Cheng, J. Analysis of the sensitivity and frequency characteristics of coplanar electrical cell–substrate impedance sensors. Biosens. Bioelectron. 2008, 24, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.P.G.; de Florio, D.Z.; Brochsztain, S. Characterization of a Perylenediimide Self-Assembled Monolayer on Indium Tin Oxide Electrodes Using Electrochemical Impedance Spectroscopy. J. Phys. Chem. C 2014, 118, 4103–4112. [Google Scholar] [CrossRef]
- Ceriotti, L.; Ponti, J.; Broggi, F.; Kob, A.; Drechsler, S.; Thedinga, E.; Colpo, P.; Sabbioni, E.; Ehret, R.; Rossi, F. Real-time assessment of cytotoxicity by impedance measurement on a 96-well plate. Sens. Actuators B Chem. 2007, 123, 769–778. [Google Scholar] [CrossRef]
- Daza, P.; Olmo, A.; Canete, D.; Yufera, A. Monitoring living cell assays with bio-impedance sensors. Sens. Actuators B Chem. 2013, 176, 605–610. [Google Scholar] [CrossRef]
- Chen, S.W.; Yang, J.M.; Yang, J.H.; Yang, S.J.; Wang, J.S. A computational modeling and analysis in cell biological dynamics using electric cell–substrate impedance sensing (ECIS). Biosens. Bioelectron. 2012, 33, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.L.; Liao, R.L.; Zhang, X. Real-time monitoring primary cardiomyocyte adhesion based on electrochemical impedance spectroscopy and electrical cell–substrate impedance sensing. Anal. Chem. 2008, 80, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Lachance, B.; Sunahara, G.; Luong, J.H.T. An in-depth analysis of electric cell–substrate impedance sensing to study the attachment and spreading mammalian cells. Anal. Chem. 2002, 74, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.J.; Yu, J.J.; Xiao, L.; Tang, J.C.O.; Zhang, Y.; Wang, P.; Yang, M. Impedance studies of bio-behavior and chemosensitivity of cancer cells by micro-electrode arrays. Biosens. Bioelectron. 2009, 24, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.; Yin, T.I.; Reyes, D.; Urban, G.A. Microfluidic Chip with Integrated Electrical Cell-Impedance Sensing for Monitoring Single Cancer Cell Migration in Three-Dimensional Matrixes. Anal. Chem. 2013, 85, 11068–11076. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.L.; Liao, R.L.; Zhang, X. Impedance-Based Monitoring of Ongoing Cardiomyocyte Death Induced by Tumor Necrosis Factor-alpha. Biophys. J. 2009, 96, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Seebach, J.; Psathaki, K.; Galla, H.J.; Wegener, J. Bioelectrical impedance assay to monitor changes in cell shape during apoptosis. Biosens. Bioelectron. 2004, 19, 583–594. [Google Scholar] [CrossRef]
- Yin, H.Y.; Wang, F.L.; Wang, A.L.; Cheng, J.; Zhou, Y.X. Bioelectrical impedance assay to monitor changes in aspirin-treated human colon cancer HT-29 cell shape during apoptosis. Anal. Lett. 2007, 40, 85–94. [Google Scholar] [CrossRef]
- Keese, C.R.; Wegener, J.; Walker, S.R.; Giaever, L. Electrical wound-healing assay for cells in vitro. Proc. Natl. Acad. Sci. USA 2004, 101, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Chen, S.W.; Yang, J.H.; Hsu, C.C.; Wang, J.S. A quantitative cell modeling and wound-healing analysis based on the Electric Cell–substrate Impedance Sensing (ECIS) method. Comput. Biol. Med. 2016, 69, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.H.; Joos-Vandewalle, J.; Edkins, A.L.; Frost, C.L.; Prinsloo, E. Real-time monitoring of 3T3-L1 preadipocyte differentiation using a commercially available electric cell–substrate impedance sensor system. Biochem. Biophys. Res. Commun. 2014, 443, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Bagnaninchi, P.O.; Drummond, N. Real-time label-free monitoring of adipose-derived stem cell differentiation with electric cell–substrate impedance sensing. Proc. Natl. Acad. Sci. USA 2011, 108, 6462–6467. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.B.; Thielecke, H. Electrical characterization of human mesenchymal stem cell growth on microelectrode. Microelectron. Eng. 2008, 85, 1272–1274. [Google Scholar] [CrossRef]
- Cho, S.; Gorjup, E.; Thielecke, H. Chip-based time-continuous monitoring of toxic effects on stem cell differentiation. Ann. Anat. 2009, 191, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, C.; Buth, H.; Cho, S.B.; Impidjati; Thielecke, H. Detection of the osteogenic differentiation of mesenchymal stem cells in 2D and 3D cultures by electrochemical impedance spectroscopy. J. Biotechnol. 2010, 148, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Park, H.E.; Kim, D.; Koh, H.S.; Cho, S.; Sung, J.S.; Kim, J.Y. Real-Time Monitoring of Neural Differentiation of Human Mesenchymal Stem Cells by Electric Cell–substrate Impedance Sensing. J. Biomed. Biotechnol. 2011, 2011, 485173. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.S.; Choi, W.; Kim, J.Y.; Cho, S. Electrical Impedance Characterization of Adipose Tissue-Derived Stem Cells Cultured on Indium Tin Oxide Electrodes. J. Biomed. Nanotechnol. 2013, 9, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.S.; Dao, L.T.M.; Pyun, J.C.; Cho, S. Effect of cell senescence on the impedance measurement of adipose tissue-derived stem cells. Enzyme Microb. Technol. 2013, 53, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Yea, C.H.; Chueng, S.T.D.; Yin, P.T.T.; Conley, B.; Dardir, K.; Pak, Y.; Jung, G.Y.; Choi, J.W.; Lee, K.B. Large-Scale Nanoelectrode Arrays to Monitor the Dopaminergic Differentiation of Human Neural Stem Cells. Adv. Mater. 2015, 27, 6356–6362. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Shah, S.; Yang, L.T.; Yin, P.T.; Hossain, M.K.; Conley, B.; Choi, J.W.; Lee, K.B. Controlling Differentiation of Adipose-Derived Stem Cells Using Combinatorial Graphene Hybrid-Pattern Arrays. ACS Nano 2015, 9, 3780–3790. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, K.B.; Choi, J.W. 3D graphene oxide-encapsulated gold nanoparticles to detect neural stem cell differentiation. Biomaterials 2013, 34, 8660–8670. [Google Scholar] [CrossRef] [PubMed]
- Yea, C.H.; Jeong, H.C.; Moon, S.H.; Lee, M.O.; Kim, K.J.; Choi, J.W.; Cha, H.J. In situ label-free quantification of human pluripotent stem cells with electrochemical potential. Biomaterials 2016, 75, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Yea, C.H.; An, J.H.; Kim, J.; Choi, J.W. In situ electrochemical detection of embryonic stem cell differentiation. J. Biotechnol. 2013, 166, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rakhilin, S.; Turner, G.; Katz, M.; Warden, R.; Irelan, J.; Abassi, Y.A.; Glass, D.J. Electrical Impedance as a Novel Biomarker of Myotube Atrophy and Hypertrophy. J. Biomol. Screen. 2011, 16, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.M.; Kiely, M.; Jakeman, P.M.; Kiely, P.A.; Carson, B.P. Optimization of an in vitro bioassay to monitor growth and formation of myotubes in real time. Biosci. Rep. 2016, 36, e00330. [Google Scholar] [CrossRef] [PubMed]
- Cho, S. Electrical Impedance Simulation and Characterization of Cell Growth Using the Fricke Model. J. Nanosci. Nanotechnol. 2012, 12, 5228–5232. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Yagati, A.K.; Cho, S. Electrochemical Characterization of Poly-L-Lysine Coating on Indium Tin Oxide Electrode for Enhancing Cell Adhesion. J. Nanosci. Nanotechnol. 2015, 15, 7881–7885. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Min, J.; Cho, S. Indium tin oxide based chip for optical and electrochemical characterization of protein-cell interaction. Jpn. J. Appl. Phys. 2015, 54, 06FN03. [Google Scholar] [CrossRef]
- Lee, G.H.; Pyun, J.C.; Cho, S. Electrical Impedance Characterization of Cell Growth on Interdigitated Microelectrode Array. J. Nanosci. Nanotechnol. 2014, 14, 8342–8346. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Hwang, K.S.; Cho, S. Dependence of Impedance Measurement Sensitivity of Cell Growth on Sensing Area of Circular Interdigitated Electrode. J. Nanosci. Nanotechnol. 2015, 15, 7886–7890. [Google Scholar] [CrossRef] [PubMed]
- Aas, V.; Torbla, S.; Andersen, M.H.; Jensen, J.; Rustan, A.C. Electrical stimulation improves insulin responses in a human skeletal muscle cell model of hyperglycemia. Ann. N. Y. Acad. Sci. 2002, 967, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yoo, M.; Go, G.Y.; Kim, D.H.; Choi, H.; Leem, Y.E.; Kim, Y.K.; Seo, D.W.; Ryu, J.H.; Kang, J.S.; et al. Bakuchiol augments MyoD activation leading to enhanced myoblast differentiation. Chem. Biol. Interact. 2016, 248, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, H.; Kim, Y.S.; Bidwell, C.A.; Kuang, S.H. Myostatin facilitates slow and inhibits fast myosin heavy chain expression during myogenic differentiation. Biochem. Biophys. Res. Commun. 2012, 426, 83–88. [Google Scholar] [CrossRef] [PubMed]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, I.; Hong, Y.; Jun, Y.-H.; Lee, G.-Y.; Jun, H.-S.; Pyun, J.-C.; Choi, J.-W.; Cho, S. Electrical Impedance Monitoring of C2C12 Myoblast Differentiation on an Indium Tin Oxide Electrode. Sensors 2016, 16, 2068. https://doi.org/10.3390/s16122068
Park I, Hong Y, Jun Y-H, Lee G-Y, Jun H-S, Pyun J-C, Choi J-W, Cho S. Electrical Impedance Monitoring of C2C12 Myoblast Differentiation on an Indium Tin Oxide Electrode. Sensors. 2016; 16(12):2068. https://doi.org/10.3390/s16122068
Chicago/Turabian StylePark, Ilhwan, Yeonhee Hong, Young-Hoo Jun, Ga-Yeon Lee, Hee-Sook Jun, Jae-Chul Pyun, Jeong-Woo Choi, and Sungbo Cho. 2016. "Electrical Impedance Monitoring of C2C12 Myoblast Differentiation on an Indium Tin Oxide Electrode" Sensors 16, no. 12: 2068. https://doi.org/10.3390/s16122068





