Potentiometric Aptasensing of Vibrio alginolyticus Based on DNA Nanostructure-Modified Magnetic Beads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the DNA Nanostructure-Modified Magnetic Beads
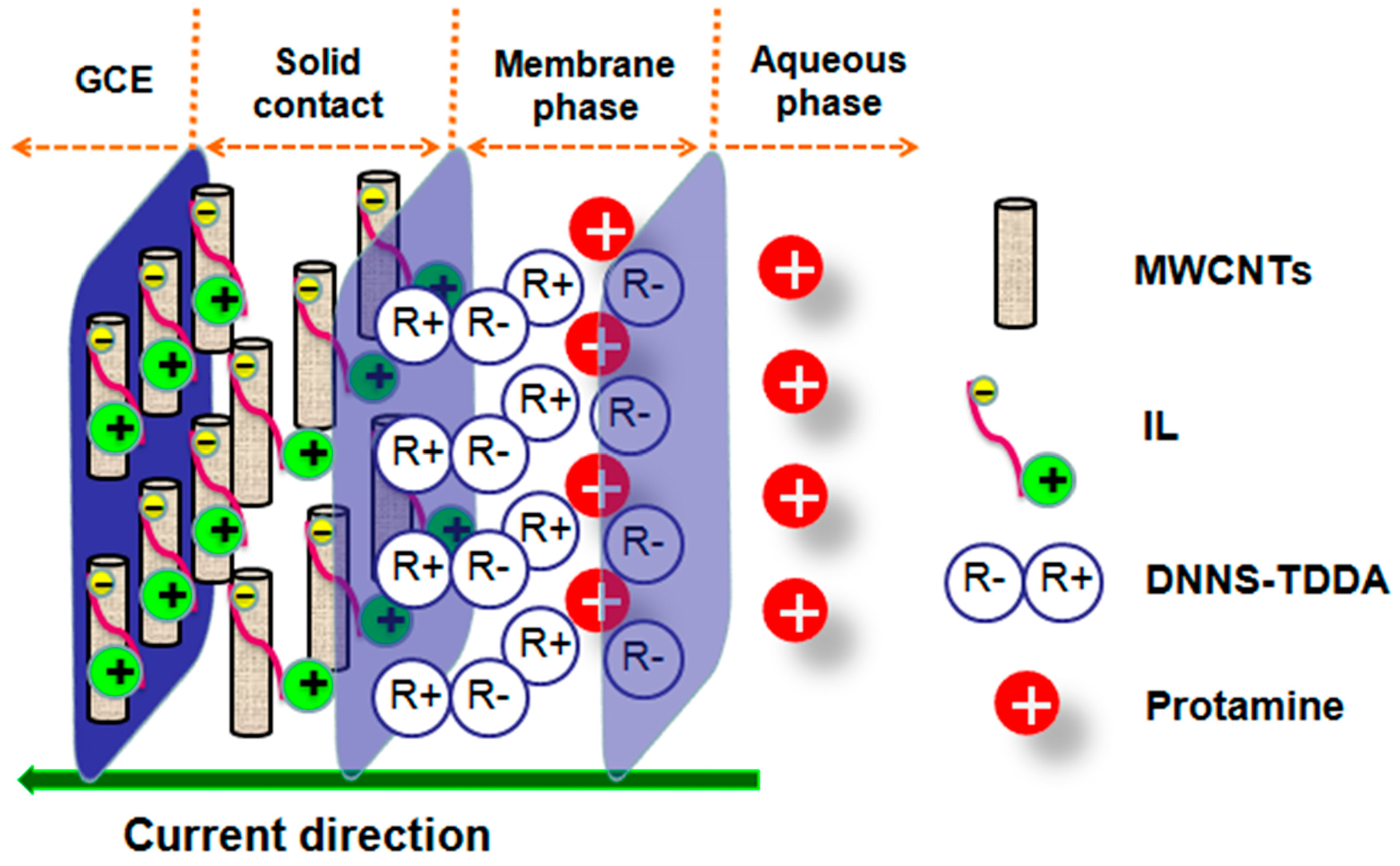
2.3. Preparation of the Solid-Contact Polycation-Sensitive Electrode
2.4. Determination of V. alginolyticus via Chronopotentiometry
3. Results and Discussion
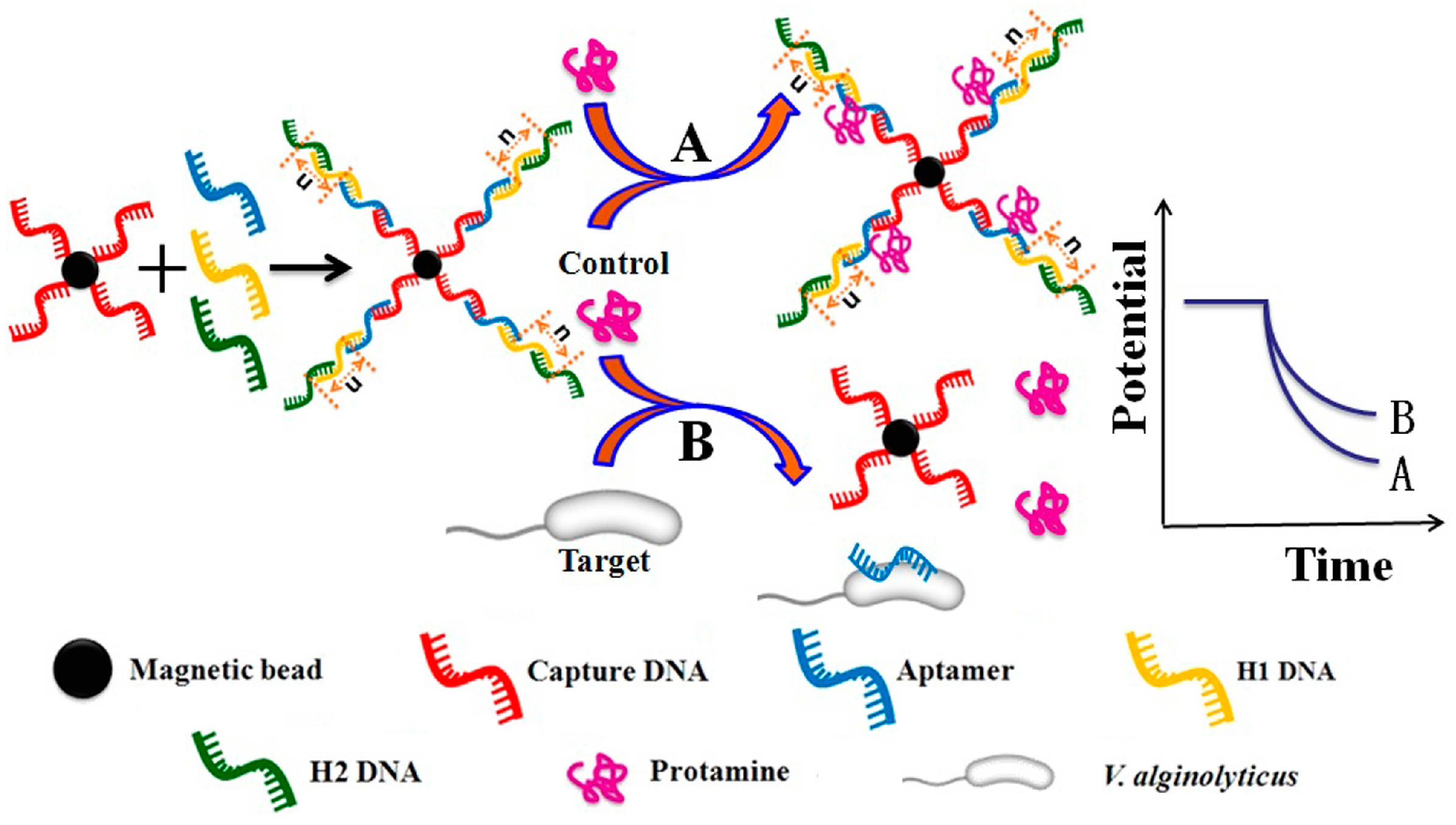
3.1. Sensing Principle
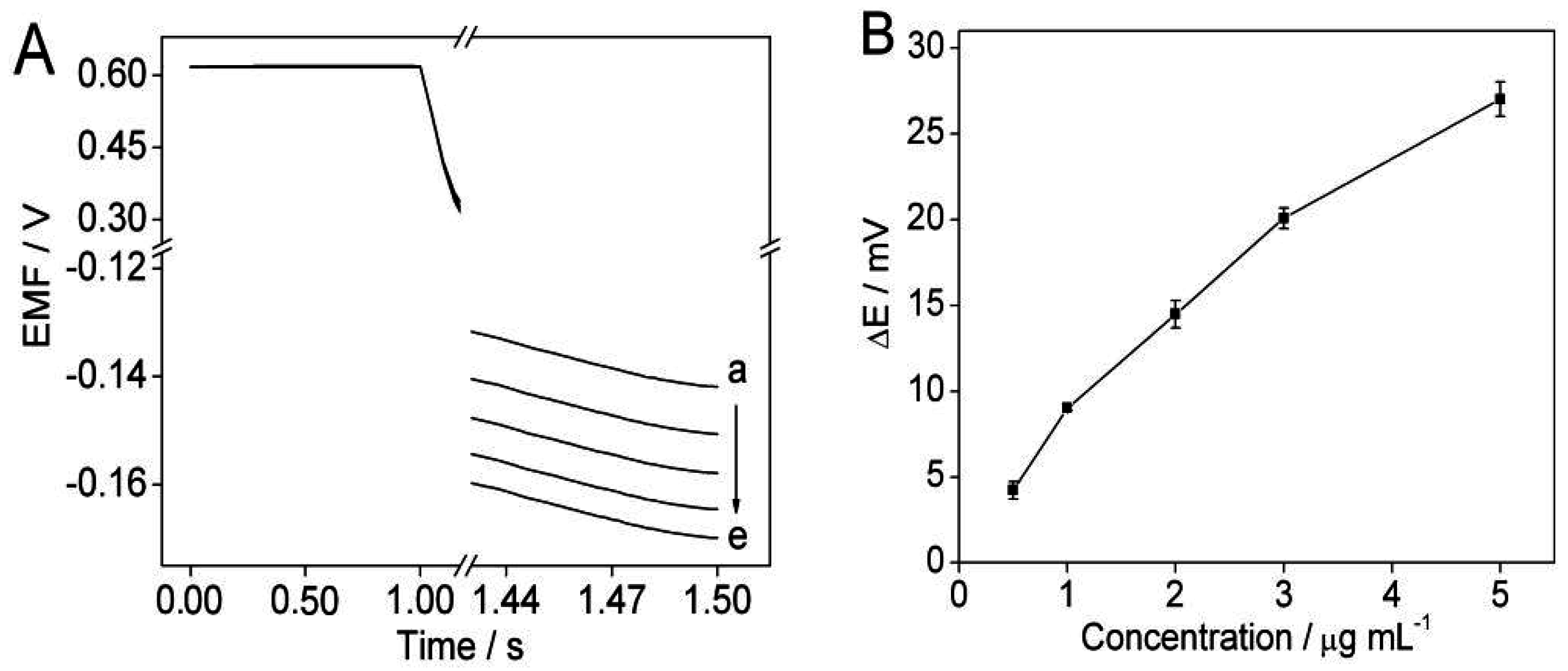
3.2. Optimization of Protamine Concentration
3.3. Optimization of the Amount of the DNA Nanostructure-Modified Magnetic Beads
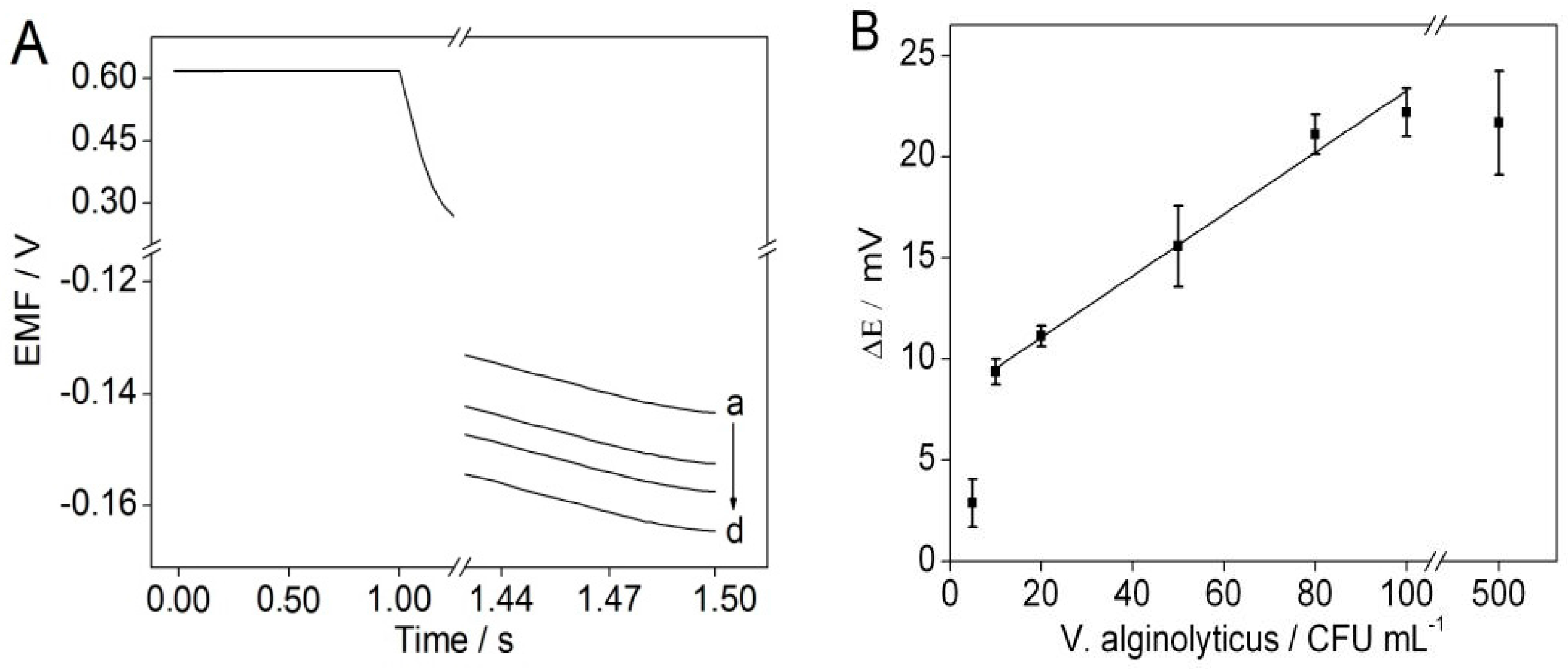
3.4. The Detection of V. alginolyticus via Chronopotentiometry
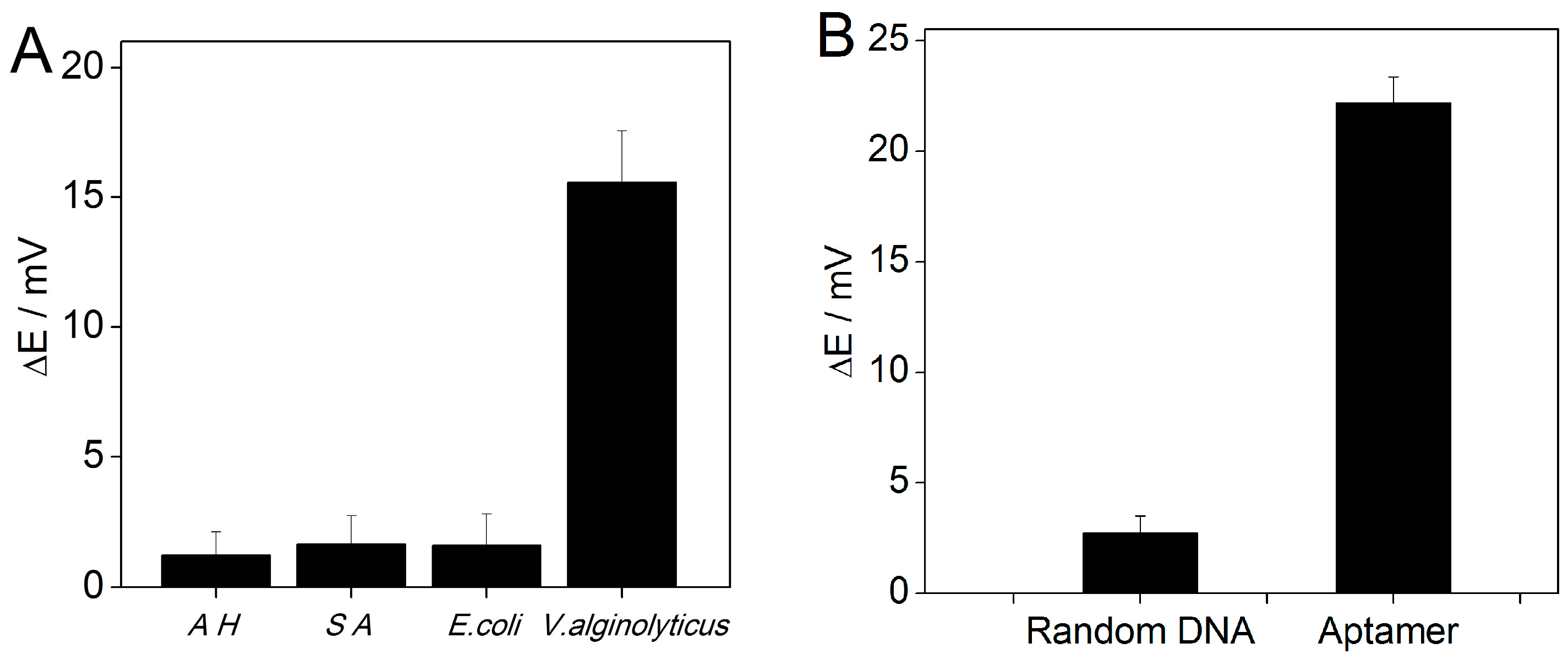
3.5. Specificity Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, Z.; Zhang, L.P.; Ren, C.H.; Zhao, J.J.; Chen, C.; Jiang, X.; Luo, P.; Hu, C.Q. Autophagy is induced by the type III secretion system of Vibrio alginolyticus in several mammalian cell lines. Arch. Microbiol. 2011, 193, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.K.; Qiao, G.; Kim, S.K. Effect of multiple infections with white spot syndrome virus and Vibrio anguillarum on Pacific white shrimp Litopenaeus vannamei (L.): Mortality and viral replication. J. Fish Dis. 2014, 37, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Tang, X.M.; Wu, R.X.; Yan, Q.P.; Tang, H.; Luo, J.W.; Niu, S.F.; Qu, Y.K.; Sun, L.W. Identification and characteristics of aptamers against inactivated Vibrio alginolyticus. LWT–Food. Sci. Technol. 2015, 64, 1138–1142. [Google Scholar] [CrossRef]
- Xu, Y.G.; Cui, L.C.; Liu, Z.M.; Li, D.D.; Li, S.L.; Zhao, M.C. Simultaneous detection of five pathogenic Vibrio species in seafood by a multiplex polymerase chain reaction coupled with high performance liquid chromatography assay. Food Control 2015, 53, 109–116. [Google Scholar] [CrossRef]
- Durai, S.; Pandian, S.K.; Balamurugan, K. Establishment of a Caenorhabditis elegans infection model for Vibrio alginolyticus. J. Basic Microb. 2011, 51, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, J.; Sun, J.S.; Li, Y.; Xue, S.X.; Chen, X.Q.; Li, X.S.; Du, G.X. Facile synthesis of multifunctional multi-walled carbon nanotube for pathogen Vibrio alginolyticus detection in fishery and environmental samples. Talanta 2014, 128, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Fukui, Y.; Sawabe, T. Improved one-step colony PCR detection of Vibrio harveyi. Microbes. Environ. 2007, 1, 1–10. [Google Scholar] [CrossRef]
- Xie, Z.Y.; Ke, S.W.; Hu, C.Q.; Zhu, Z.X.; Wang, S.F.; Zhou, Y.C. First characterization of bacterial pathogen, Vibrio alginolyticus, for porites and rewsi white syndrome in the South China Sea. PLoS ONE 2013, 8, e75425. [Google Scholar]
- Wei, S.; Zhao, H.; Xian, Y.Y.; Hussain, M.; Wu, X.Y. Multiplex PCR assays for the detection of Vibrio alginolyticus, Vibrio parahaemolyticus, Vibrio vulnificus, and Vibrio cholerae with an internal amplification control. Diagn. Micr. Infec. Dis. 2014, 79, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.W.; Zheng, Q.Y.; Fu, J.F.; Xu, J.Y.; Cao, J.J. A raid multiplex PCR-DHPLC method of detection and identification of pathogenic bacteria in aquatic products. J. Food Saf. 2015, 35, 50–58. [Google Scholar] [CrossRef]
- Citartan, M.; Ch’ng, E.S.; Rozhdestvensky, T.S.; Tang, T.H. Aptamers as the ‘capturing’ agents in aptamer-based capture assays. Microchem. J. 2016, 128, 187–197. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Tao, C.C. Rational design of a mismatched aptamer-DNA duplex probe to improve the analytical performance of electrochemical aptamer sensors. Electrochim. Acta 2016, 209, 479–485. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Y.G.; Zhang, Y.; Ma, H.M.; Pang, X.H.; Hu, L.H.; Du, B.; Wei, Q. Facile fabrication of an electrochemical aptasensor based on magnetic electrode by using streptavidin modified magnetic beads for sensitive and specific detection of Hg2+. Biosens. Bioelectron. 2016, 82, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Liu, H.Y.; Tian, T.; Song, X.R.; Yu, J.G.; Yan, M. A simple and rapid detection assay for peptides based on the specific recognition of aptamer and signal amplification of hybridization chain reaction. Biosens. Bioelectron. 2016, 83, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Numnuam, A.; Chumbimuni-Torres, K.Y.; Xiang, Y.; Bash, R.; Thavarungkul, P.; Kanatharana, P.; Pretsch, E.; Wang, J.; Bakker, E. Aptamer-based potentiometric measurements of proteins using ion-selective microelectrodes. Anal. Chem. 2008, 80, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Y.; Kang, Y.J. Bioprobes based on aptamer and silica fluorescent nanoparticles for bacteria Salmonella typhimurium detection. Nanoscale Res. Lett. 2016, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Gupta, R.; Sinha, M.; Kumar, R.; Bhalla, V. MoS2 based digital response platform for aptamer based fluorescent detection of pathogens. Microchim. Acta 2016, 183, 1501–1506. [Google Scholar] [CrossRef]
- Chandola, C.; Kalme, S.; Casteleijn, M.; Urtti, A.; Neerathilin, M. Application of aptamers in diagnostics, drug-delivery and imaging. J. Biosci. 2016, 41, 535–561. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.J.; Wang, J.; Liu, H.Y.; Li, Z.H.; Jiang, F.L.; Wang, F.B.; Yuan, Q. Rapid and selective detection of pathogenic bacteria in bloodstream infections with aptamer-based recognition. ACS Appl. Mater. Interfaces 2016, 8, 19371–19378. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-Gómez, V.; Campuzano, S.; Pedrero, M.; Pingarrón, J.M. Immunosensor for the determination of Staphylococcus aureus using a tyrosinase-mercaptopropionic acid modified electrode as an amperometric transducer. Anal. Bioanal. Chem. 2008, 391, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Labib, M.; Zamay, A.S.; Kolovskaya, O.S.; Reshetneva, I.T.; Zamay, G.S.; Kibbee, R.J.; Sattar, S.A.; Zamay, T.N.; Berezovski, M.V. Aptamer-based impedimetric sensor for bacterial typing. Anal. Chem. 2012, 84, 8114–8117. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Vallés, C.; Benito, A.M.; Maser, W.K.; Rius, F.X.; Riu, J. Graphene-based potentiometric biosensor for the immediate detection of living bacteria. Biosens. Bioelectron. 2014, 54, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.W.; Lei, J.H.; Ma, X.; Gong, J.; Qin, W. Potentiometric aptasensing of Listeria monocytogenes using protamine as an indicator. Anal. Chem. 2014, 86, 9412–9416. [Google Scholar] [CrossRef] [PubMed]
- Shvarev, A.; Bakker, E. Reversible electrochemical detection of non-electroactive polyions. J. Am. Chem. Soc. 2003, 125, 11192–11193. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.C.; Kawde, A.N.; Wang, J. Aptamer biosensor for label-free impedance spectroscopy detection of proteins based on recognition-induced switching of the surface charge. Chem. Commun. 2005, 34, 4267–4269. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.K.H.; Ge, B.X.; Yu, H.Z. Aptamer-based biosensors for label-free voltammetric detection of lysozyme. Anal. Chem. 2007, 79, 5158–5164. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, X.F.; Shao, Y.H.; Le, X.C. Aptamer-based affinity chromatographic assays for thrombin. Anal. Chem. 2008, 80, 7586–7593. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.W.; Gu, Y.; Li, F.; Zhang, H.X.; Qin, W. DNA nanostructure-based magnetic beads for potentiometric aptasensing. Anal. Chem. 2015, 87, 6465–6469. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.W.; Lei, J.P.; Ju, H.X. Functionalization of carbon nanotubes with water-insoluble porphyrin in ionic liquid: Direct electrochemistry and highly sensitive amperometric biosensing for trichloroacetic acid. Chem. Eur. J. 2009, 15, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.S.; Wang, H.; Bi, S.G.; Xue, Y.; Xue, Z.G.; Liao, Y.G.; Zhou, X.P.; Xie, X.L.; Mai, Y.W. Enhanced ion transport in polymer-ionic liquid electrolytes containing ionic liquid-functionalized nanostructured carbon materials. Carbon 2015, 86, 86–97. [Google Scholar] [CrossRef]
- Wardak, C. Solid contact cadmium ion-selective electrode based on ionic liquid and carbon nanotubes. Sens. Actuators B Chem. 2015, 209, 131–137. [Google Scholar] [CrossRef]
- Ramamurthy, N.; Baliga, N.; Wahr, J.A.; Schaller, U.; Yang, V.C.; Meyerhoff, M.E. Improved protamine- sensitive membrane electrode for monitoring heparin concentrations in whole blood via protamine titration. Clin. Chem. 1998, 44, 606–613. [Google Scholar] [PubMed]

| Oligonucleotide | Sequence |
|---|---|
| Aptamer DNA | 5′-TCAGTCGCTTCGCCGTCTCCTTCAGCCGGGGTGGTCAGTAGGAGCAGCACAAGAGGGAGACCCCAGAGGG-3′ [3] |
| Capture DNA | 5′-TTTTTCCCTCTGGGGTCTCCC-3′ (modified with biotin at 5′ terminal end) |
| H 1 DNA | 5′-CGGCGAAGCGACTGACAAAGTCTAGTCGCT-3′ |
| H 2 DNA | 5′-TCAGTCGCTTCGCCGAGCGACTAGACTTTG-3′ |
| Random DNA | 5′-GAGTAGTTCGTG GCCTAG-3′ |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, G.; Ding, J.; Yu, H.; Yin, T.; Qin, W. Potentiometric Aptasensing of Vibrio alginolyticus Based on DNA Nanostructure-Modified Magnetic Beads. Sensors 2016, 16, 2052. https://doi.org/10.3390/s16122052
Zhao G, Ding J, Yu H, Yin T, Qin W. Potentiometric Aptasensing of Vibrio alginolyticus Based on DNA Nanostructure-Modified Magnetic Beads. Sensors. 2016; 16(12):2052. https://doi.org/10.3390/s16122052
Chicago/Turabian StyleZhao, Guangtao, Jiawang Ding, Han Yu, Tanji Yin, and Wei Qin. 2016. "Potentiometric Aptasensing of Vibrio alginolyticus Based on DNA Nanostructure-Modified Magnetic Beads" Sensors 16, no. 12: 2052. https://doi.org/10.3390/s16122052





