Development of an Exhaled Breath Monitoring System with Semiconductive Gas Sensors, a Gas Condenser Unit, and Gas Chromatograph Columns
Abstract
:1. Introduction
2. Experimental Section
2.1. Selection of Gas Bags
2.2. Collection of Exhaled Breaths and Gas Condenser-GC/MS Analysis
2.3. Statistical Analysis
2.4. Preparation of Sensor Elements
2.5. Prototype System
2.6. Collection of Exhaled Breaths and Prototype Analysis
2.7. Evaluation of the Performance of the Prototype
3. Results and Discussion
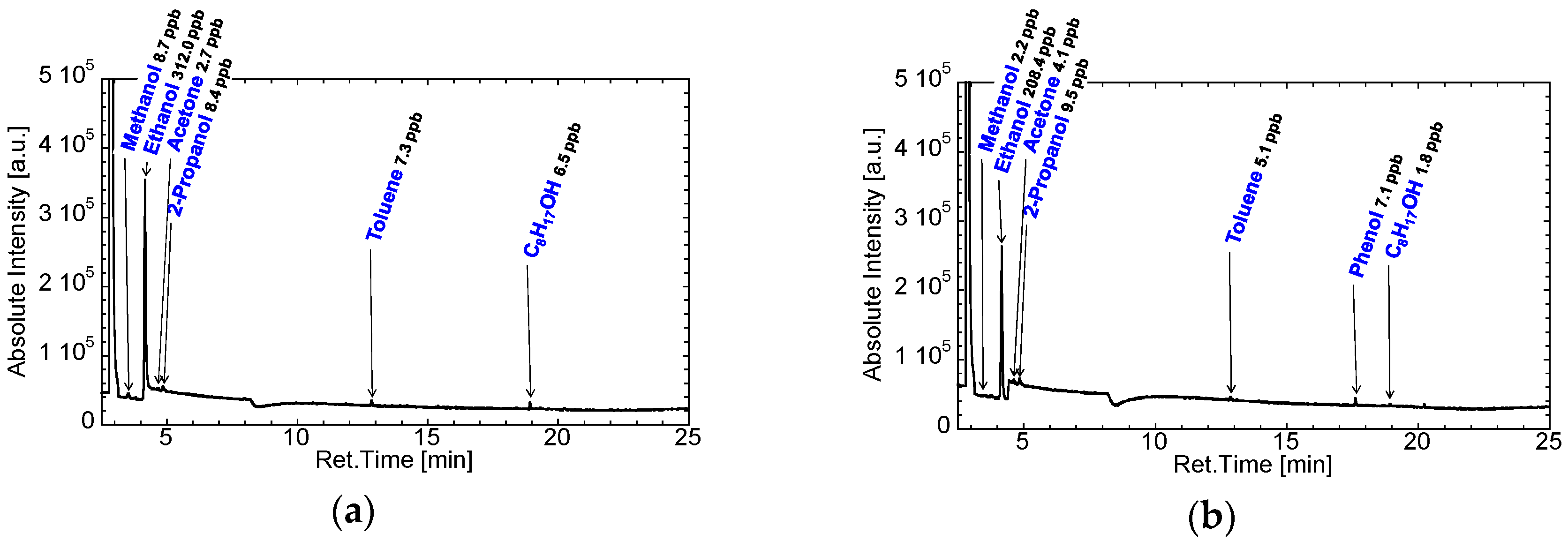
3.1. Selection of Gas Bags and Contamination of Room Air
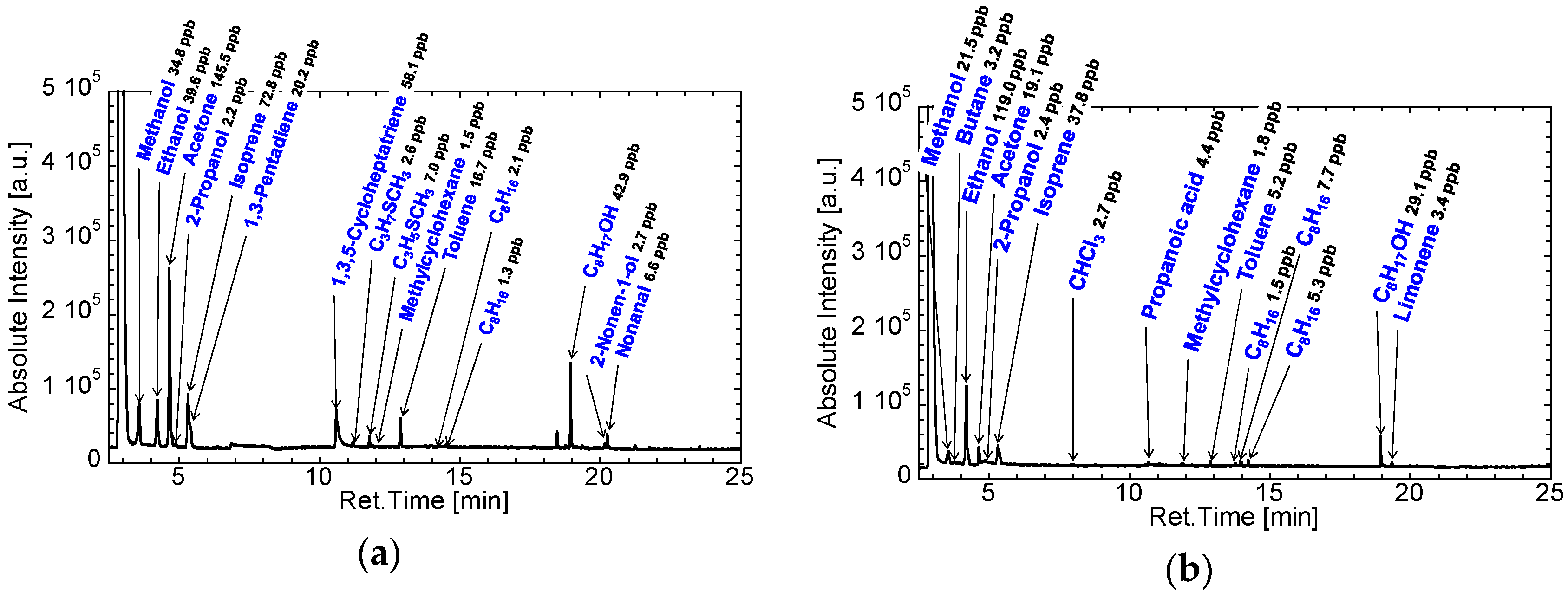
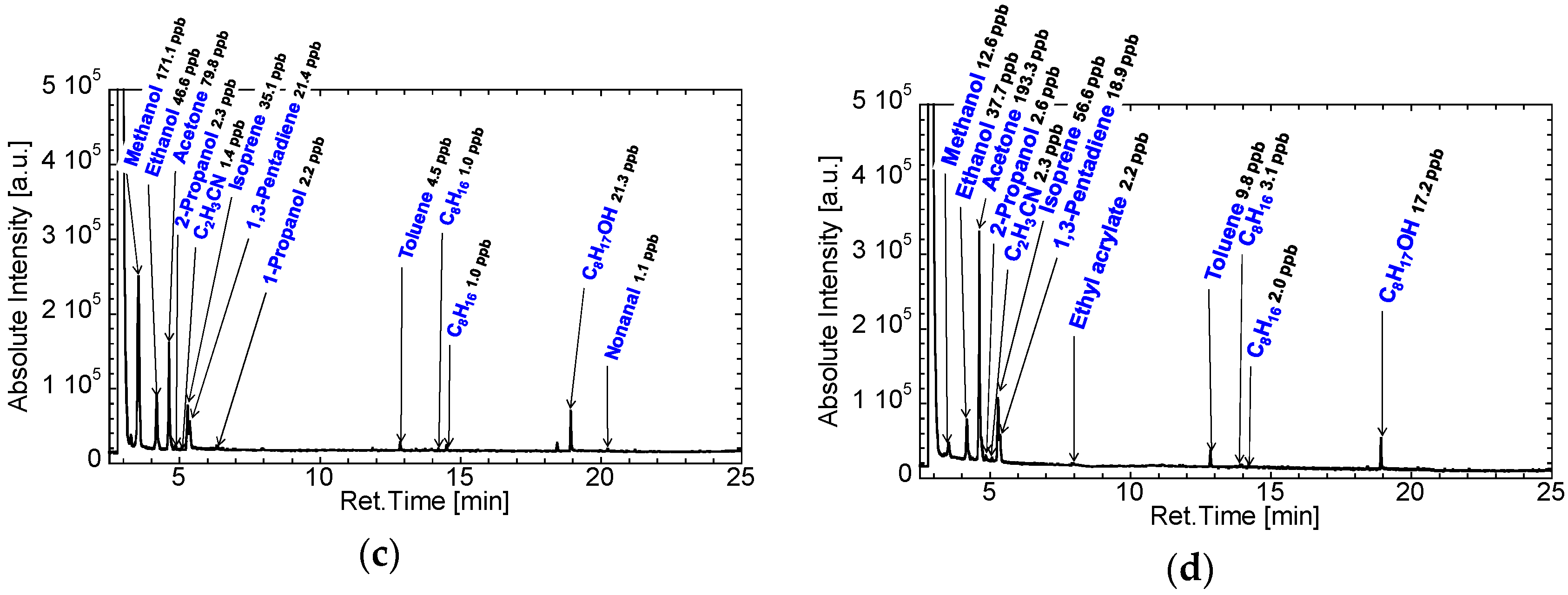
3.2. Gas Condenser-GC/MS Analysis of Exhaled Breaths for Development of the GC-Gas Sensor-Gas Condenser-Type Prototype
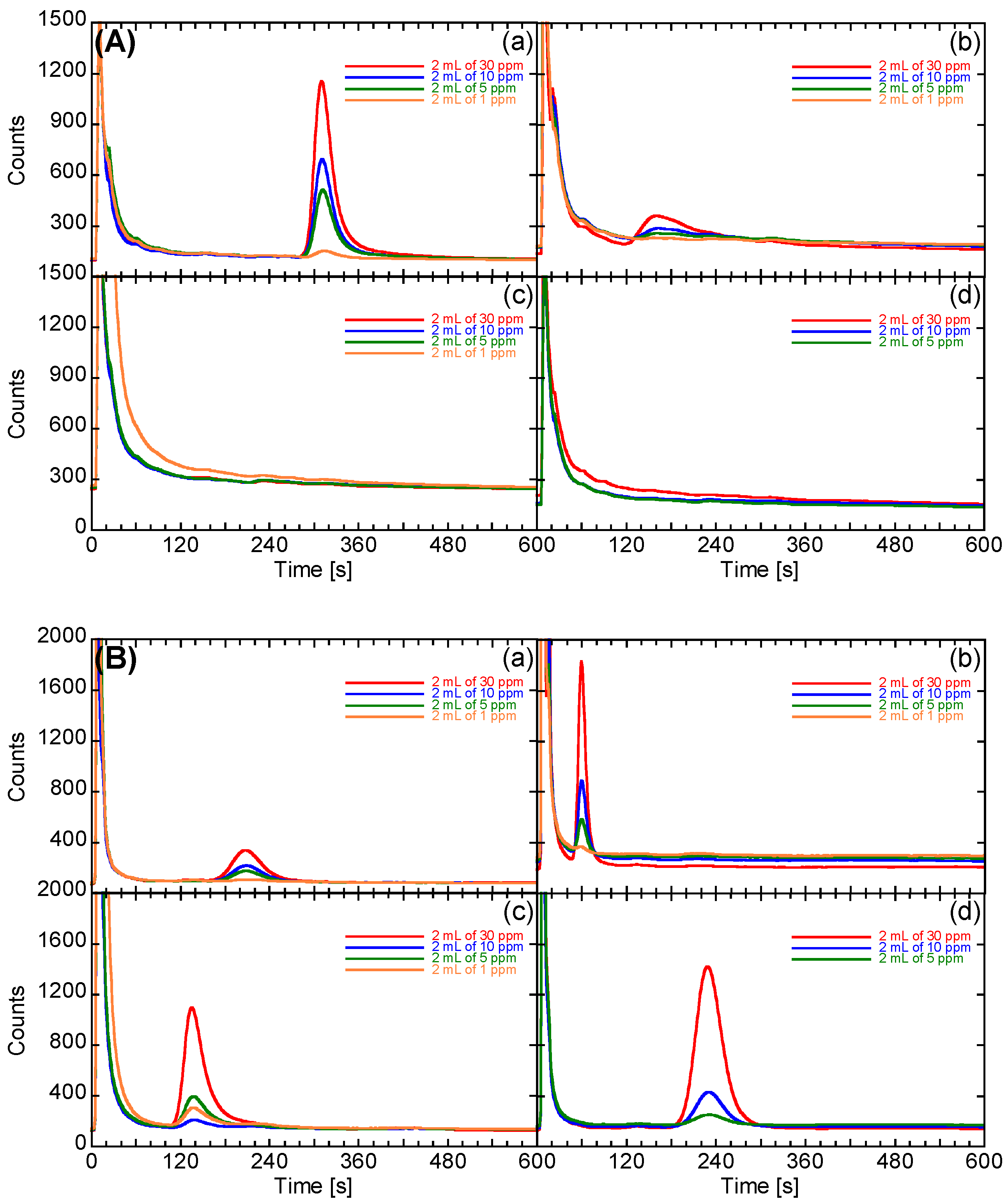
3.3. Development of the Prototype System
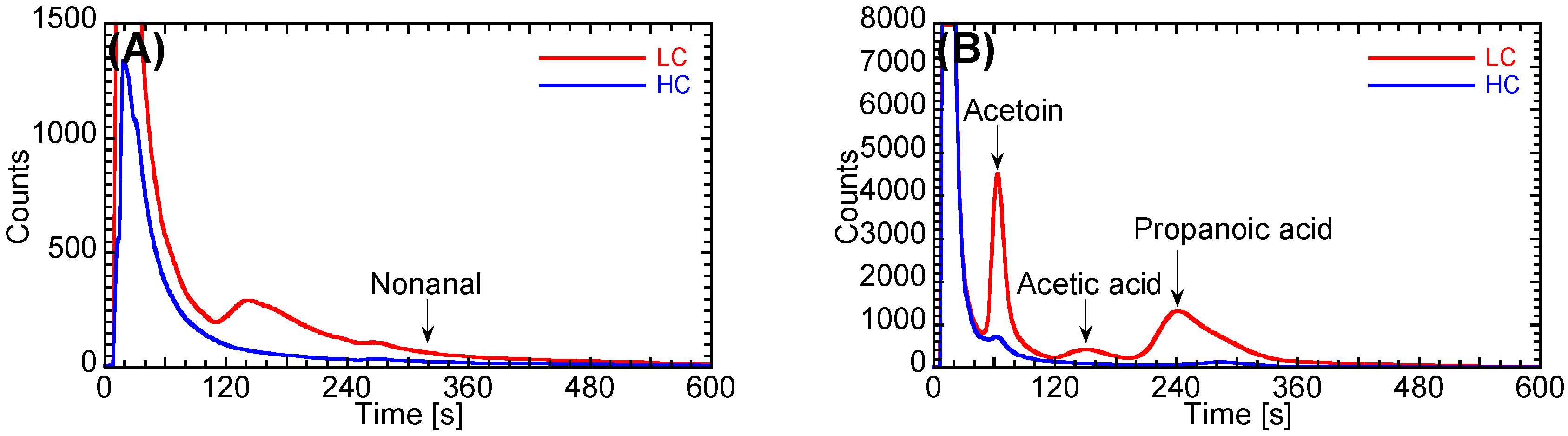
3.4. Evaluation of the Performance of the Prototype
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gelmont, D.; Stein, R.A.; Mead, J.F. Isoprene—The main hydrocarbon in human breath. Biochem. Biophys. Res. Commun. 1981, 99, 1456–1460. [Google Scholar] [CrossRef]
- King, J.; Koc, H.; Unterkofler, K.; Mochalski, P.; Kupferthaler, A.; Teschl, G.; Teschl, S.; Hinterhuber, H.; Amann, A. Physiological modeling of isoprene dynamics in exhaled breath. J. Theor. Biol. 2010, 267, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Yasuno, Y.; Iwakura, M.; Shimada, Y. Relation between volatile sulfur compounds in mouth air and some symptoms in patients complaining of bad breath. J. Dent. Health. 1989, 39, 663–674. [Google Scholar] [CrossRef]
- Kharitonov, S.A.; Barnes, P.J. Biomarkers of some pulmonary diseases in exhaled breath. Biomarkers 2002, 7, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Bach, P.B.; Kelly, M.J.; Tate, R.C.; McCrory, D.C. Screening for lung cancer. Chest 2003, 123, 72S–82S. [Google Scholar] [CrossRef] [PubMed]
- Aberle, D.R.; Abtin, F.; Brown, K. Computed tomography screening for lung cancer: Has it finally arrived? Implications of the national lung screening trial. J. Clin. Oncol. 2013, 31, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar]
- Patz, E.F., Jr.; Goodman, P.C.; Bepler, G. Screening for lung cancer. N. Engl. J. Med. 2000, 343, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Tanner, N.T.; Mehta, H.; Silvestri, G.A. New testing for lung cancer screening. Oncology 2012, 26, 176–182. [Google Scholar] [PubMed]
- Yu, J.-B.; Byun, H.-G.; Zhang, S.; Do, S.-H.; Lim, J.-O.; Huh, J.-S. Exhaled breath analysis of lung cancer patients using a metal oxide sensor. J. Sens. Sci. Technol. 2011, 20, 300–304. [Google Scholar] [CrossRef]
- Hakim, M.; Billan, S.; Tisch, U.; Peng, G.; Dvrokind, I.; Marom, O.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosis of head-and-neck cancer from exhaled breath. Br. J. Cancer 2011, 104, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Hakim, M.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br. J. Cancer 2010, 103, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P.J.; Hammel, J.; Dweik, R.; Na, J.; Czich, C.; Laskowski, D.; Mekhail, T. Diagnosis of lung cancer by the analysis of exhaled breath with a colorimetric sensor array. Thorax 2007, 62, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Tisch, U.; Adams, O.; Hakim, M.; Shehada, N.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nat. Nanotechnol. 2009, 4, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Broza, Y.Y.; Kremer, R.; Tisch, U.; Gevorkyan, A.; Shiban, A.; Best, L.A.; Haick, H. A nanomaterial-based breath test for short-term follow-up after lung tumor resection. Nanomedicine 2013, 9, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Broza, Y.Y.; Haick, H. Nanomaterial-based sensors for detection of disease by volatile organic compounds. Nanomedicine 2013, 8, 785–806. [Google Scholar] [CrossRef] [PubMed]
- Konvalina, G.; Haick, H. Sensors for breath testing: From nanomaterials to comprehensive disease detection. Acc. Chem. Res. 2014, 47, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Tisch, U.; Haick, H. Arrays of chemosensitive monolayer-capped metallic nanoparticles for diagnostic breath testing. Rev. Chem. Eng. 2010, 26, 171–179. [Google Scholar] [CrossRef]
- Peled, N.; Barash, O.; Tisch, U.; Ionescu, R.; Broza, Y.Y.; Ilouze, M.; Mattei, J.; Bunn, P.A., Jr.; Hirsch, F.R.; Haick, H. Volatile fingerprints of cancer specific genetic mutations. Nanomedicine 2013, 9, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P.; Mores, N.; Trové, A.; Mondino, C.; Barnes, P.J. The electronic nose in respiratory medicine. Respiration 2013, 85, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Peled, N.; Hakim, M.; Bunn, P.A., Jr.; Miller, Y.E.; Kennedy, T.C.; Mattei, J.; Mitchell, J.D.; Hirsch, F.R.; Haick, H. Non-invasive breath analysis of pulmonary nodules. J. Thorac. Oncol. 2012, 7, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Santonico, M.; Lucantoni, G.; Pennazza, G.; Capuano, R.; Galluccio, G.; Roscioni, C.; la Delfa, G.; Consoli, D.; Martinelli, E.; Paolesse, R.; et al. In situ detection of lung cancer volatile fingerprints using bronchoscopic air-sampling. Lung Cancer 2012, 77, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P.J.; Wang, X.F.; Xu, Y.; Mekhail, T.; Beukemann, M.C.; Na, J.; Kemling, J.W.; Suslick, K.S.; Sasidhar, M. Exhaled breath analysis with a colorimetric sensor array for the identification and characterization of lung cancer. J. Thorac. Oncol. 2012, 7, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Barash, O.; Peled, N.; Tisch, U.; Bunn, P.A., Jr.; Hirsch, F.R.; Haick, H. Classification of lung cancer histology by gold nanoparticle sensors. Nanomedicine 2012, 8, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Natale, C.D.; Macagnano, A.; Martinelli, E.; Paolesse, R.; D’Arcangelo, G.; Roscioni, C.; Finazzi-Agrò, A.; D’Amico, A. Lung cancer identification by the analysis of breath by means of an array of non-selective gas sensors. Biosens. Bioelectron. 2003, 18, 1209–1218. [Google Scholar] [CrossRef]
- Güntner, A.T.; Koren, V.; Chikkadi, K.; Righettoni, M.; Pratsinis, S.E. E-Nose Sensing of low-ppb formaldehyde in gas mixtures at high relative humidity for breath screening of lung cancer? ACS Sens. 2016, 1, 528–535. [Google Scholar] [CrossRef]
- Westhoff, M.; Litterst, P.; Freitag, L.; Urfer, W.; Bader, S.; Baumbach, J.-I. Ion mobility spectrometry for the detection of volatile organic compounds in exhaled breath of patients with lung cancer: Results of a pilot study. Thorax 2009, 64, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Ligor, T.; Jezierski, T.; Wenda-Piesik, A.; Walczak, M.; Rudnicka, J. Identification of volatile lung cancer markers by gas chromatography-mass spectrometry: Comparison with discrimination by canines. Anal. Bioanal. Chem. 2012, 404, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Boedeker, E.; Friedel, G.; Walles, T. Sniffer dogs as part of a bimodal bionic research approach to develop a lung cancer screening. Interact. Cardiovasc. Thorac. Surg. 2012, 14, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Wu, P.; Kim, H.W.; Kim, S.S. Highly Selective Sensing of CO, C6H6, and C7H8 Gases by Catalytic Functionalization with Metal Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 7173–7183. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Choi, S.-J.; Lee, I.; Youn, D.-Y.; Park, C.O.; Lee, J.-H.; Tuller, H.L.; Kim, I.-D. Thin-wall assembled SnO2 fibers functionalized by catalytic Pt nanoparticles and their superior exhaled-breath-sensing properties for the diagnosis of diabetes. Adv. Funct. Mater. 2013, 23, 2357–2367. [Google Scholar] [CrossRef]
- Kadosaki, M.; Sakai, Y.; Tamura, I.; Matsubara, I.; Itoh, T. Development of an oxide semiconductor thick film gas sensor for the detection of total volatile organic compounds. Electron. Commun. Jpn. 2008, 93, 34–41. [Google Scholar] [CrossRef]
- Sakai, Y.; Kadosaki, M.; Matsubara, I.; Itoh, T. Preparation of total VOC sensor with sensor-response stability for humidity by noble metal addition to SnO2. J. Ceram. Soc. Jpn. 2009, 117, 1297–1301. [Google Scholar] [CrossRef]
- Itoh, T.; Matsubara, I.; Kadosaki, M.; Sakai, Y.; Shin, W.; Izu, N.; Nishibori, M. Gas-sensing properties of tin oxide-based volatile organic compound sensors for total volatile organic compound gases. Sens. Mater. 2009, 21, 251–258. [Google Scholar]
- Itoh, T.; Matsubara, I.; Kadosaki, M.; Sakai, Y.; Shin, W.; Izu, N.; Nishibori, M. Effects of high-humidity aging on platinum, palladium, and gold loaded tin oxide—Volatile organic compound sensors. Sensors 2010, 10, 6513–6521. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Nakashima, T.; Akamatsu, T.; Izu, N.; Shin, W. Nonanal gas sensing properties of platinum, palladium, and gold-loaded tin oxide VOCs sensors. Sens. Actuators B Chem. 2013, 187, 135–141. [Google Scholar] [CrossRef]
- Fuchs, P.; Loeseken, C.; Schubert, J.K.; Miekisch, W. Breath gas aldehydes as biomarkers of lung cancer. Int. J. Cancer 2010, 126, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Gleeson, K.; Hughes, J.; Greenberg, J.; Cataneo, R.N.; Baker, L.; McVay, W.P. Volatile organic compounds in breath as markers of lung cancer: A cross sectional study. Lancet 1999, 353, 1930–1933. [Google Scholar] [CrossRef]
- Bajtarevic, A.; Ager, C.; Pienz, M.; Klieber, M.; Schwarz, K.; Ligor, M.; Ligor, T.; Filipiak, W.; Denz, H.; Fiegl, M.; et al. Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer 2009, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Thakur, R.K.; Yadav, V.K.; Nanda, R.; Chowdhury, S.; Agrawal, A. Lung cancer biomarkers: State of the art. J. Carcinog. 2013, 12, 3. [Google Scholar] [PubMed]
- Wang, Y.; Hu, Y.; Wang, D.; Yu, K.; Wang, L.; Zou, Y.; Zhao, C.; Zhang, X.; Wang, P.; Ying, K. The analysis of volatile organic compounds biomarkers for lung cancer in exhaled breath, tissues and cell lines. Cancer Biomark. 2012, 11, 129–137. [Google Scholar] [PubMed]
- Buszewski, B.; Ulanowska, A.; Kowalkowski, T.; Cieśliński, K. Investigation of lung cancer biomarkers by hyphenated separation techniques and chemometrics. Clin. Chem. Lab. Med. 2011, 50, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, J.; Kowalkowski, T.; Ligor, T.; Buszewski, B. Determination of volatile organic compounds as biomarkers of lung cancer by SPME-GC-TOF/MS and chemometrics. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 3360–3366. [Google Scholar] [CrossRef] [PubMed]
- Poli, D.; Goldoni, M.; Caglieri, A.; Ceresa, G.; Acampa, O.; Carbognani, P.; Rusca, M.; Corradi, M. Breath analysis in non small cell lung cancer patients after surgical tumour resection. Acta Biomed. 2008, 79, 64–72. [Google Scholar] [PubMed]
- Kischkel, S.; Miekisch, W.; Fuchs, P.; Schubert, J.K. Breath analysis during one-lung ventilation in cancer patients. Eur. Respir. J. 2012, 40, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Filipiak, W.; Sponring, A.; Mikoviny, T.; Ager, C.; Schubert, J.; Miekisch, W.; Amann, A.; Troppmair, J. Release of volatile organic compounds (VOCs) from the lung cancer cell line CALU-I in vitro. Cancer Cell Int. 2008, 8, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, N.; Phillips, M.; Amann, A.; Haick, H. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef] [PubMed]
- Poli, D.; Carbognani, P.; Corradi, M.; Goldoni, M.; Acampa, O.; Balbi, B.; Bianchi, L.; Rusca, M.; Mutti, A. Exhaled volatile organic compounds in patients with non-small cell lung cancer: Cross sectional and nested short-term follow-up study. Respir. Res. 2005, 6, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Kant, K.D.; van der Sande, L.J.; Jöbsis, Q.; van Schayck, O.C.; Dompeling, E. Clinical use of exhaled volatile organic compounds in pulmonary diseases: Asystematic review. Respir. Res. 2012, 13, 117–139. [Google Scholar] [CrossRef] [PubMed]
- Pennazza, G.; Santonico, M.; Martinelli, E.; D’Amico, A.; di Natale, C. Interpretation of exhaled volatile organic compounds. Eur. Respir. Mon. 2010, 49, 115–129. [Google Scholar]


| Number of Volunteers, n | Stage of Lung Cancer | Histology | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IA | IB | IIA | IIB | IIIA | IIIB | IV | Ad | Sq | SCLC | Lg | ||
| LC | 107 | 38 | 17 | 9 | 6 | 17 | 11 | 9 | 78 | 21 | 5 | 3 |
| HC | 29 | – | – | – | – | – | – | – | – | – | – | – |
| LC-S | 44 | 18 | 9 | 5 | 3 | 8 | 1 | 0 | 31 | 9 | 3 | 1 |
| VOCs | LC (n = 107) | HC (n = 29) | LC-S (n = 44) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. (1) | Ave. (ppb) | Std. (ppb) | No. | Ave. (ppb) | Std. (ppb) | No. | Ave. (ppb) | Std. (ppb) | |
| Hydrogen cyanide | 57 | 1.7 | 2.3 | 20 | 1.6 | 1.0 | 19 | 0.8 | 0.9 |
| Dimethylether | 5 | 0.1 | 0.3 | 0 | - | - | 3 | 0.4 | 0.8 |
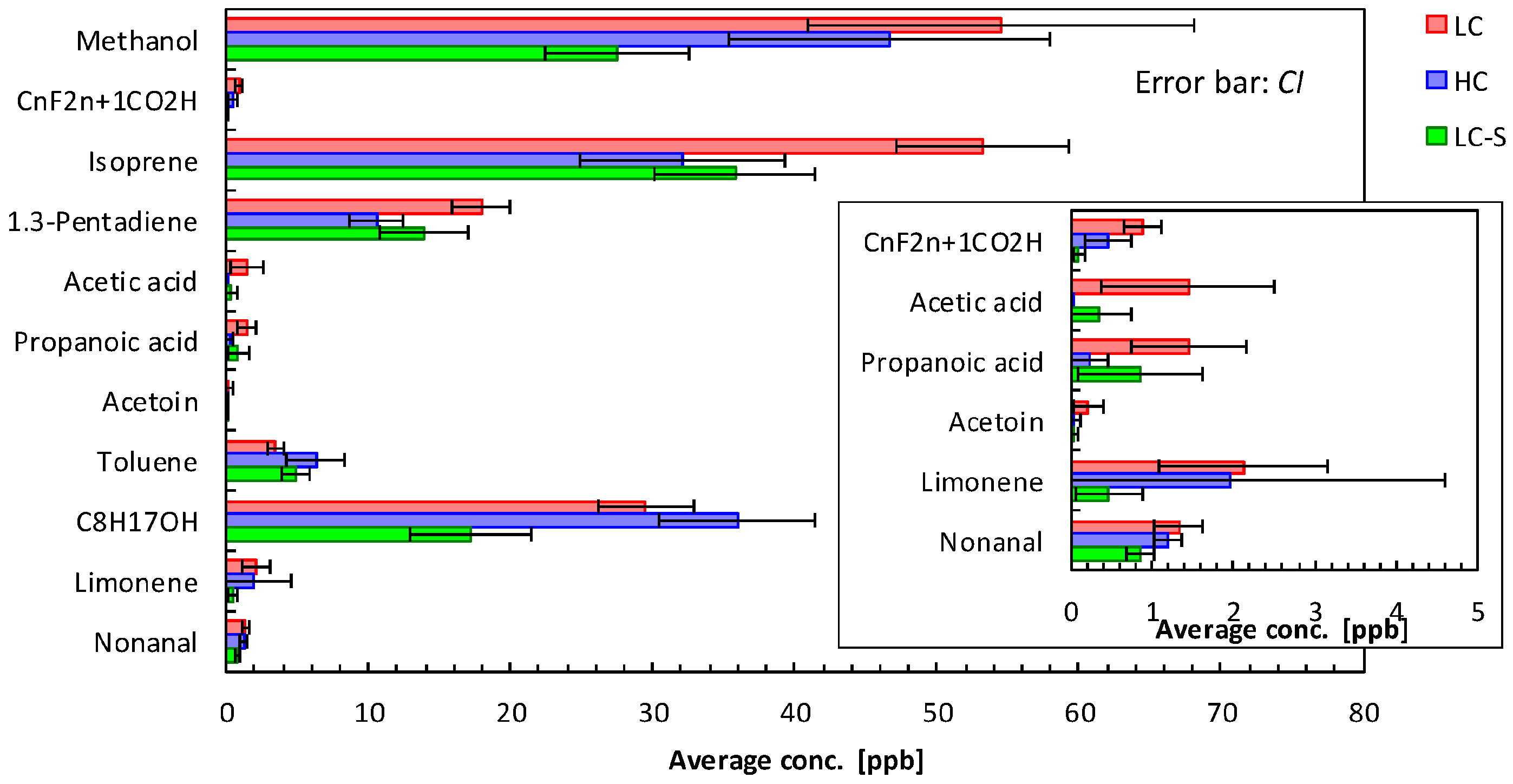
| Methanol | 107 | 54.5 | 71.1 | 29 | 46.6 | 29.6 | 44 | 27.5 | 16.6 |
| Butane | 26 | 0.9 | 2.1 | 14 | 1.3 | 1.3 | 19 | 1.4 | 2.3 |
| CnF2n+1CO2H (2) | 31 | 0.9 | 1.2 | 6 | 0.5 | 0.8 | 1 | 0.1 | 0.2 |
| Ethanol | 107 | 80.3 | 37.3 | 29 | 102.1 | 88.5 | 44 | 75.6 | 47.2 |
| Acetonitrile | 8 | 3.7 | 17.9 | 7 | 6.2 | 29.5 | 1 | 2.3 | 14.2 |
| Acetone | 107 | 81.6 | 185.4 | 29 | 68.7 | 35.4 | 44 | 64.6 | 51.5 |
| 2-Propanol | 97 | 3.2 | 6.5 | 24 | 3.5 | 6.2 | 42 | 3.1 | 1.2 |
| Acrylonitrile | 68 | 2.1 | 1.8 | 19 | 1.8 | 1.3 | 36 | 2.2 | 1.3 |
| Isoprene | 107 | 53.2 | 31.4 | 28 | 32.1 | 18.9 | 43 | 35.8 | 18.5 |
| 1,3-Pentadiene | 106 | 17.9 | 10.7 | 28 | 10.6 | 5.1 | 43 | 13.9 | 10.3 |
| Methyl acetate | 1 | 0.2 | 1.1 | 0 | - | - | 2 | 0.2 | 0.3 |
| C5H6 (2) | 0 | - | - | 1 | 0.1 | 0.3 | 0 | - | - |
| 1-Propanol | 67 | 3.2 | 8.7 | 22 | 3.5 | 4.4 | 21 | 2.6 | 5.1 |
| 3,3-Dimethylpentan-2-one | 2 | 0.1 | 0.4 | 0 | - | - | 0 | - | - |
| Methyl ethyl ketone | 1 | 0.0 | 0.2 | 0 | - | - | 1 | 0.1 | 0.4 |
| 2-Methylfuran | 0 | - | - | 1 | 0.1 | 0.2 | 0 | - | - |
| 2-Propanoic acid | 1 | 0.0 | 0.3 | 0 | - | - | 1 | 0.1 | 0.3 |
| Ethyl acetate | 36 | 0.8 | 1.0 | 8 | 0.7 | 0.9 | 22 | 2.5 | 9.9 |
| Chloroform | 47 | 1.0 | 1.4 | 11 | 1.2 | 1.7 | 20 | 0.9 | 0.6 |
| Furan | 1 | 0.2 | 2.4 | 0 | - | - | 0 | - | - |
| Isopropyl alcohol | 0 | - | - | 0 | - | - | 1 | 0.1 | 0.4 |
| C6H8 (2) | 0 | - | - | 1 | 0.1 | 0.3 | 0 | - | - |
| Acetic acid | 11 | 1.4 | 5.6 | 0 | - | - | 2 | 0.3 | 1.3 |
| Benzene | 1 | 0.0 | 0.2 | 2 | 0.3 | 0.8 | 0 | - | - |
| C4H8O2 (1) | 2 | 0.8 | 7.2 | 0 | - | - | 1 | 0.1 | 0.2 |
| C3H5SCH3 (2) | 1 | 0.0 | 0.2 | 1 | 0.1 | 0.2 | 0 | - | - |
| ((CH3)3Si)2O | 8 | 3.0 | 24.9 | 0 | - | - | 0 | - | - |
| C6H12O3 (2) | 3 | 0.4 | 3.4 | 0 | - | - | 1 | 0.2 | 1.0 |
| Acetoin | 6 | 0.2 | 1.0 | 0 | - | - | 1 | 0.0 | 0.2 |
| Ethyl propanoate | 1 | 0.0 | 0.2 | 0 | - | - | 2 | 0.2 | 0.9 |
| Heptane | 3 | 0.1 | 0.3 | 1 | 0.1 | 0.2 | 1 | 0.1 | 0.2 |
| C3H7SCH3 (2) | 9 | 0.3 | 0.7 | 6 | 0.5 | 1.0 | 4 | 0.1 | 0.3 |
| Propanoic acid | 12 | 1.5 | 3.7 | 2 | 0.2 | 0.6 | 4 | 0.9 | 2.5 |
| C3H5SCH3 (2) | 15 | 0.4 | 0.9 | 4 | 0.5 | 1.2 | 1 | 0.2 | 0.5 |
| C5H11OH (2) | 0 | - | - | 0 | - | - | 2 | 0.1 | 0.3 |
| Methylcyclohexane | 51 | 1.4 | 2.6 | 16 | 1.4 | 1.2 | 25 | 1.7 | 1.5 |
| Toluene | 98 | 3.5 | 2.8 | 29 | 6.3 | 5.4 | 40 | 4.8 | 3.2 |
| C8H16 (2) | 15 | 0.5 | 0.5 | 4 | 0.6 | 0.6 | 0 | - | - |
| C8H16 (2) | 91 | 2.5 | 1.7 | 28 | 2.7 | 1.6 | 30 | 1.5 | 1.0 |
| C8H16 (2) | 69 | 1.6 | 1.1 | 18 | 1.7 | 1.1 | 18 | 0.9 | 0.7 |
| Ethylcyclohexane | 1 | 0.0 | 0.2 | 0 | - | - | 0 | - | - |
| Xylene | 6 | 0.4 | 0.5 | 3 | 0.6 | 0.6 | 0 | - | - |
| Butyl ethyl ketone | 1 | 0.2 | 1.8 | 0 | - | - | 0 | - | - |
| C7H15OH (2) | 1 | 0.0 | 0.2 | 0 | - | - | 0 | - | - |
| Nonane | 1 | 0.0 | 0.2 | 0 | - | - | 0 | - | - |
| α-Pinene | 1 | 0.1 | 0.9 | 0 | - | - | 0 | - | - |
| Dichlorobenzene | 19 | 1.0 | 2.5 | 4 | 0.6 | 1.5 | 5 | 1.4 | 4.5 |
| C8H17OH (2) | 105 | 29.5 | 17.5 | 29 | 36.0 | 14.4 | 43 | 17.2 | 13.8 |
| Limonene | 27 | 2.1 | 5.4 | 7 | 2.0 | 6.9 | 2 | 0.5 | 1.3 |
| C10H18O (2) | 7 | 1.0 | 5.0 | 4 | 2.3 | 7.2 | 2 | 0.1 | 0.4 |
| 1-Methylstyrene | 3 | 0.1 | 0.3 | 0 | - | - | 2 | 0.1 | 0.3 |
| Nonanal | 63 | 1.3 | 1.5 | 18 | 1.2 | 0.5 | 15 | 0.9 | 0.6 |
| Undecane | 33 | 2.2 | 5.0 | 7 | 3.2 | 6.2 | 24 | 5.1 | 7.7 |
| Menthol | 6 | 2.1 | 19.0 | 0 | - | - | 2 | 4.2 | 25.4 |
| Combinations of VOCs | Correlation Coefficient, r | |||
|---|---|---|---|---|
| LC | HC | LC-S | ||
| Acetic acid | Propanoic acid | 0.60 | - | 0.19 |
| Propanoic acid | Acetoin | 0.73 | - | 0.90 |
| Propanoic acid | Nonanal | 0.64 | 0.15 | −0.01 |
| Acetoin | Nonanal | 0.66 | −0.10 | −0.02 |
| Combinations of Target VOCs | From Prototype | From Gas Condenser-GC/MS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ccutoff (ppb) | Number of Samples Classified | Ccutoff * (ppb) | Number of Samples Classified | ||||||
| VOC A | VOC B | VOC A | VOC B | LC (n = 74) | HC (n = 27) | VOC A | VOC B | LC (n = 107) | HC (n = 29) |
| Nonanal | Acetoin | 27.4 | 64.1 | 4 | 1 | 2.0 | 1.0 | 1 | 0 |
| Nonanal | Acetic acid | 27.4 | 420 | 2 | 1 | 2.0 | 1.0 | 2 | 0 |
| Nonanal | Propanoic acid | 27.4 | 229 | 4 | 1 | 2.0 | 2.5 | 3 | 0 |
| Acetoin | Acetic acid | 64.1 | 420 | 5 | 1 | 1.0 | 1.0 | 1 | 0 |
| Acetoin | Propanoic acid | 64.1 | 229 | 5 | 1 | 1.0 | 2.5 | 4 | 0 |
| Acetic acid | Propanoic acid | 420 | 229 | 2 | 1 | 1.0 | 2.5 | 8 | 0 |
| Total ** | 10 (13.5%) | 1 (3.7%) | 14 (13.1%) | 0 (0.0%) | |||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itoh, T.; Miwa, T.; Tsuruta, A.; Akamatsu, T.; Izu, N.; Shin, W.; Park, J.; Hida, T.; Eda, T.; Setoguchi, Y. Development of an Exhaled Breath Monitoring System with Semiconductive Gas Sensors, a Gas Condenser Unit, and Gas Chromatograph Columns. Sensors 2016, 16, 1891. https://doi.org/10.3390/s16111891
Itoh T, Miwa T, Tsuruta A, Akamatsu T, Izu N, Shin W, Park J, Hida T, Eda T, Setoguchi Y. Development of an Exhaled Breath Monitoring System with Semiconductive Gas Sensors, a Gas Condenser Unit, and Gas Chromatograph Columns. Sensors. 2016; 16(11):1891. https://doi.org/10.3390/s16111891
Chicago/Turabian StyleItoh, Toshio, Toshio Miwa, Akihiro Tsuruta, Takafumi Akamatsu, Noriya Izu, Woosuck Shin, Jangchul Park, Toyoaki Hida, Takeshi Eda, and Yasuhiro Setoguchi. 2016. "Development of an Exhaled Breath Monitoring System with Semiconductive Gas Sensors, a Gas Condenser Unit, and Gas Chromatograph Columns" Sensors 16, no. 11: 1891. https://doi.org/10.3390/s16111891





