Improvement of Toluene-Sensing Performance of SnO2 Nanofibers by Pt Functionalization
Abstract
:1. Introduction
2. Materials and Methods
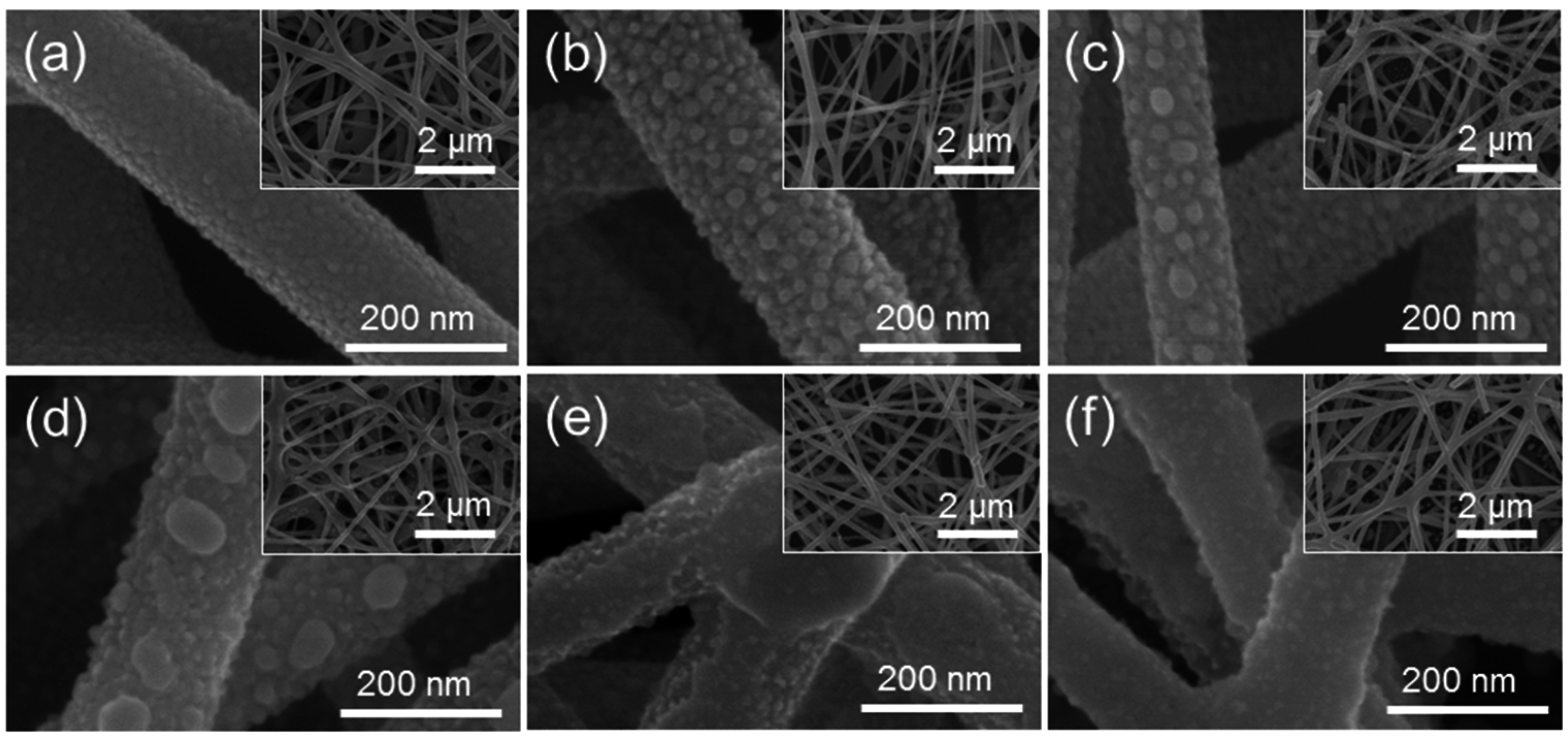
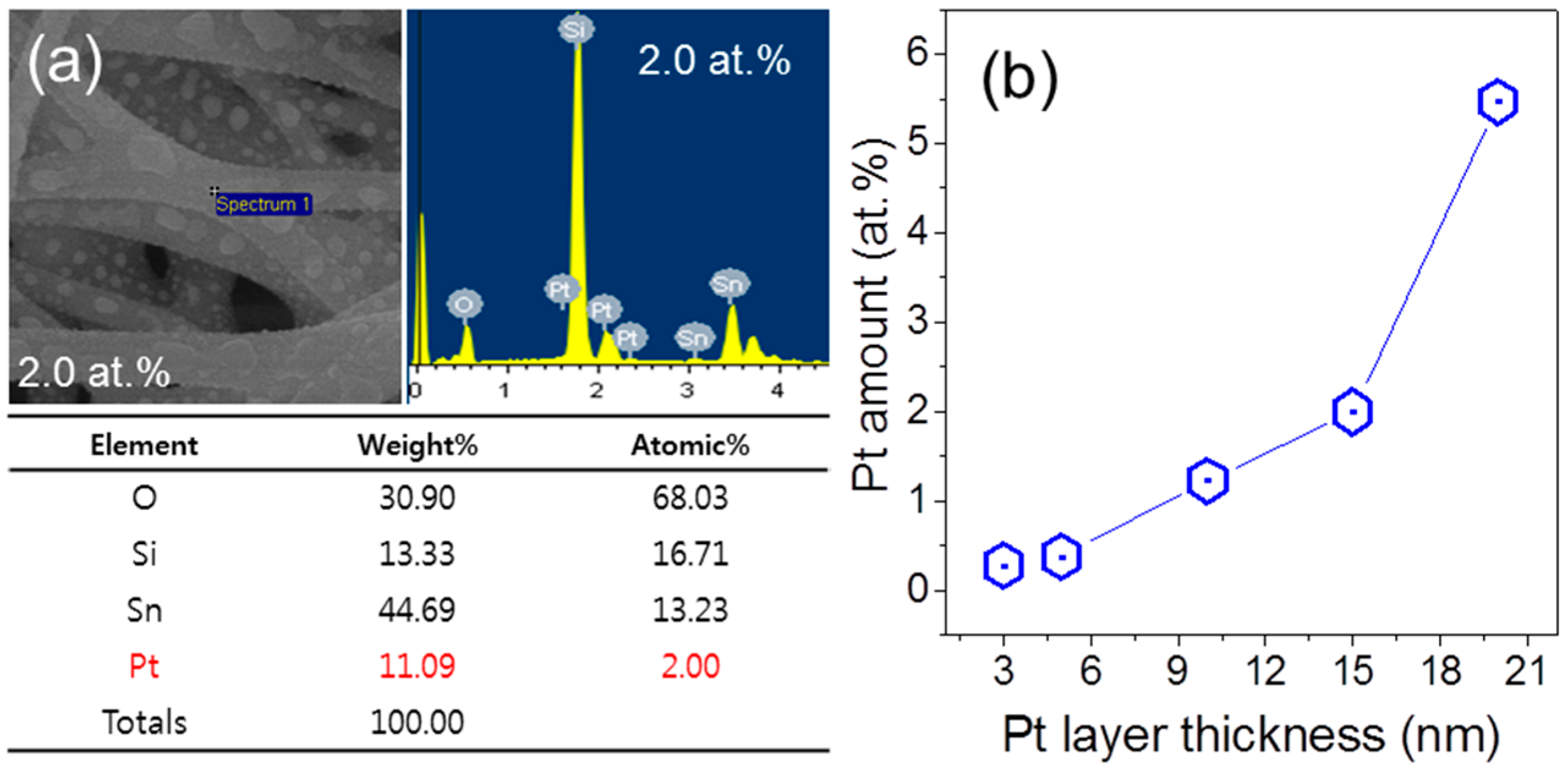
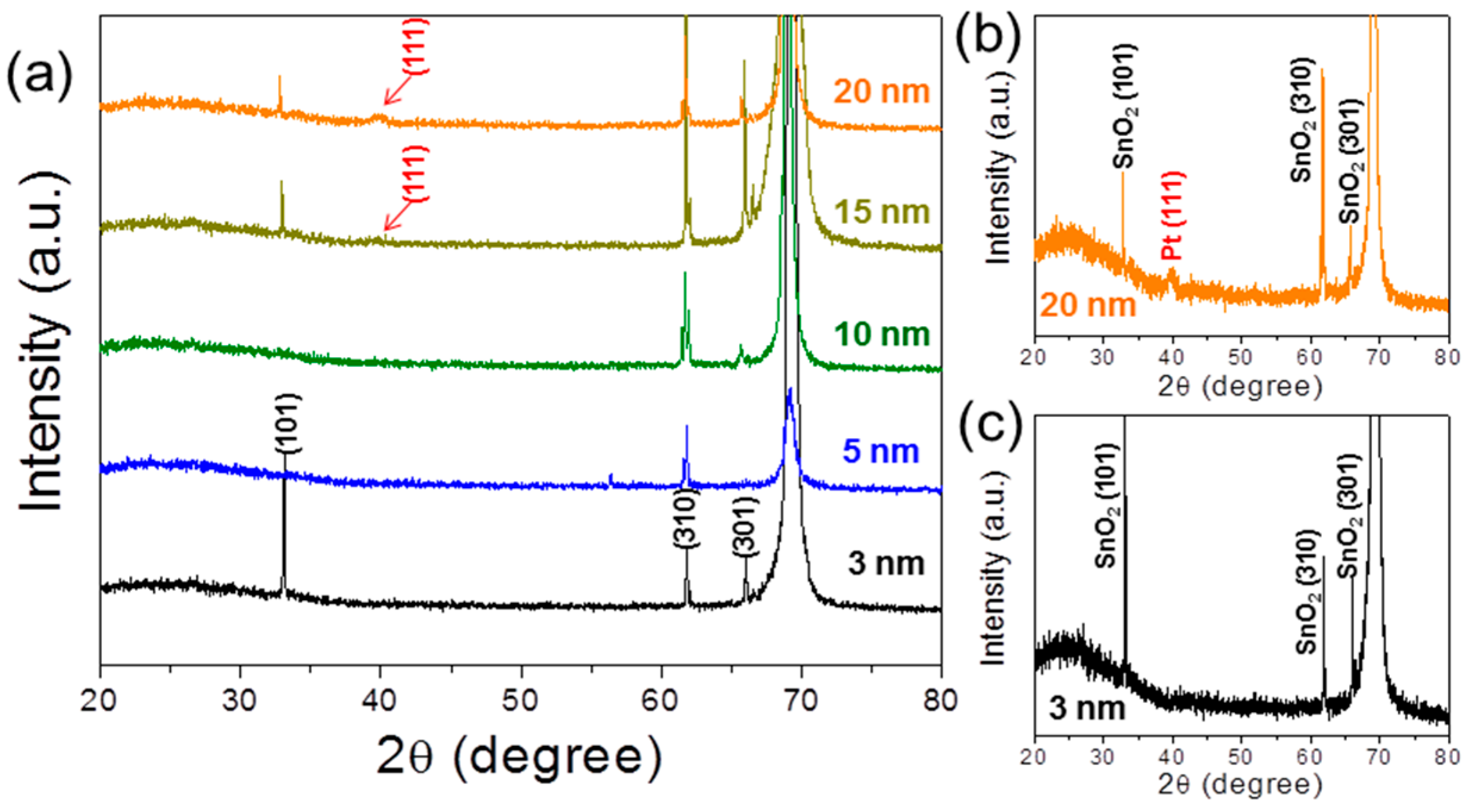
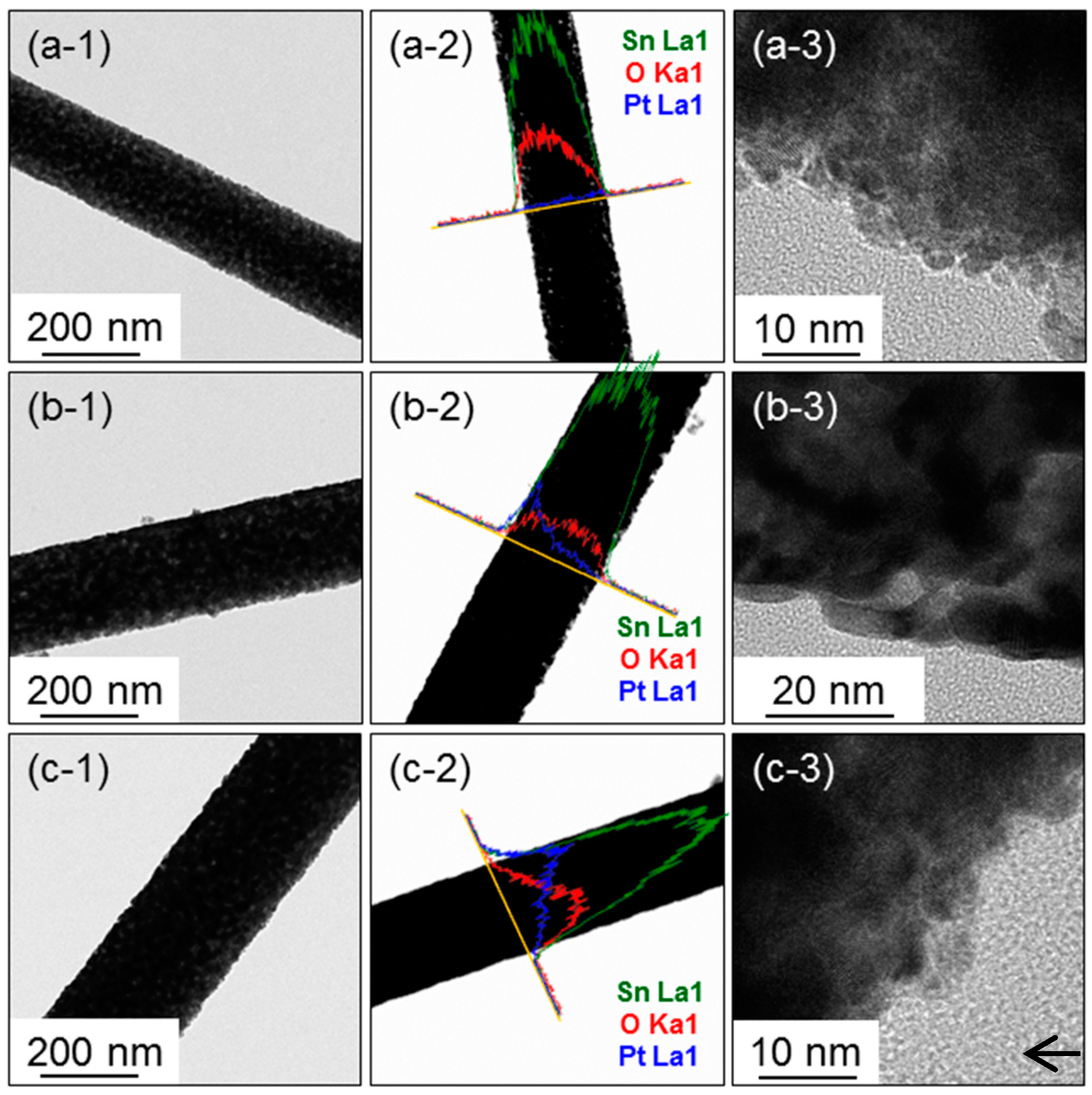
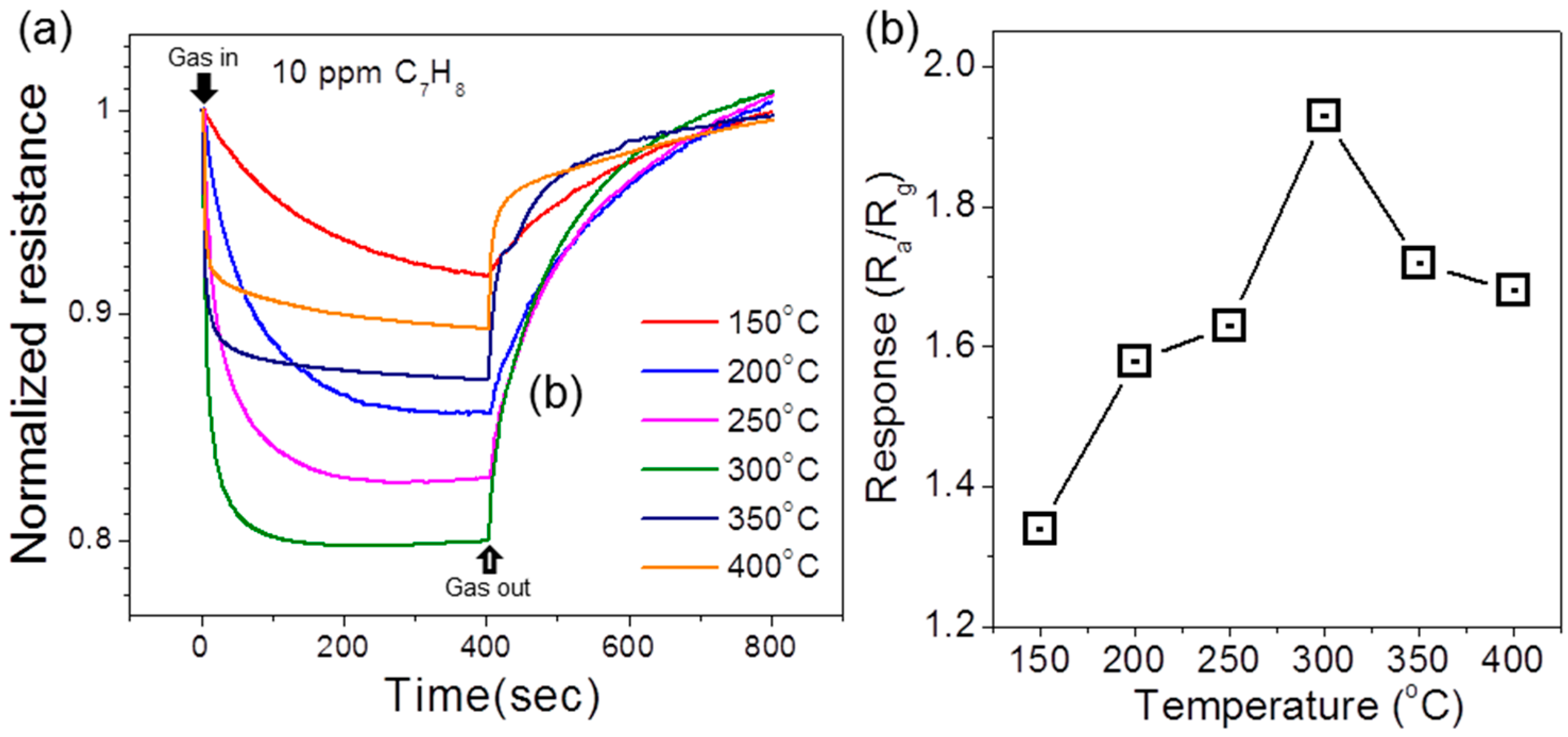
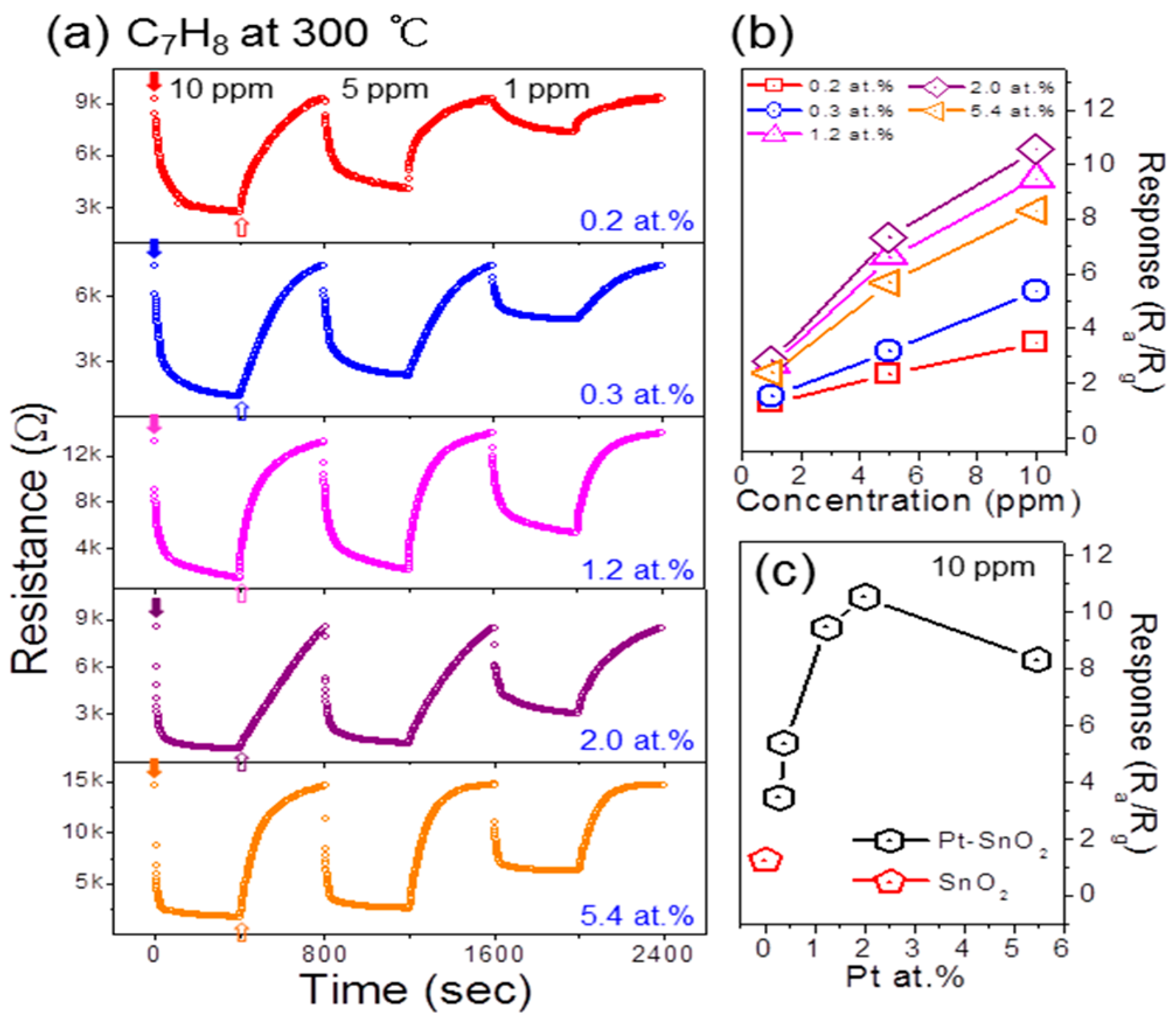
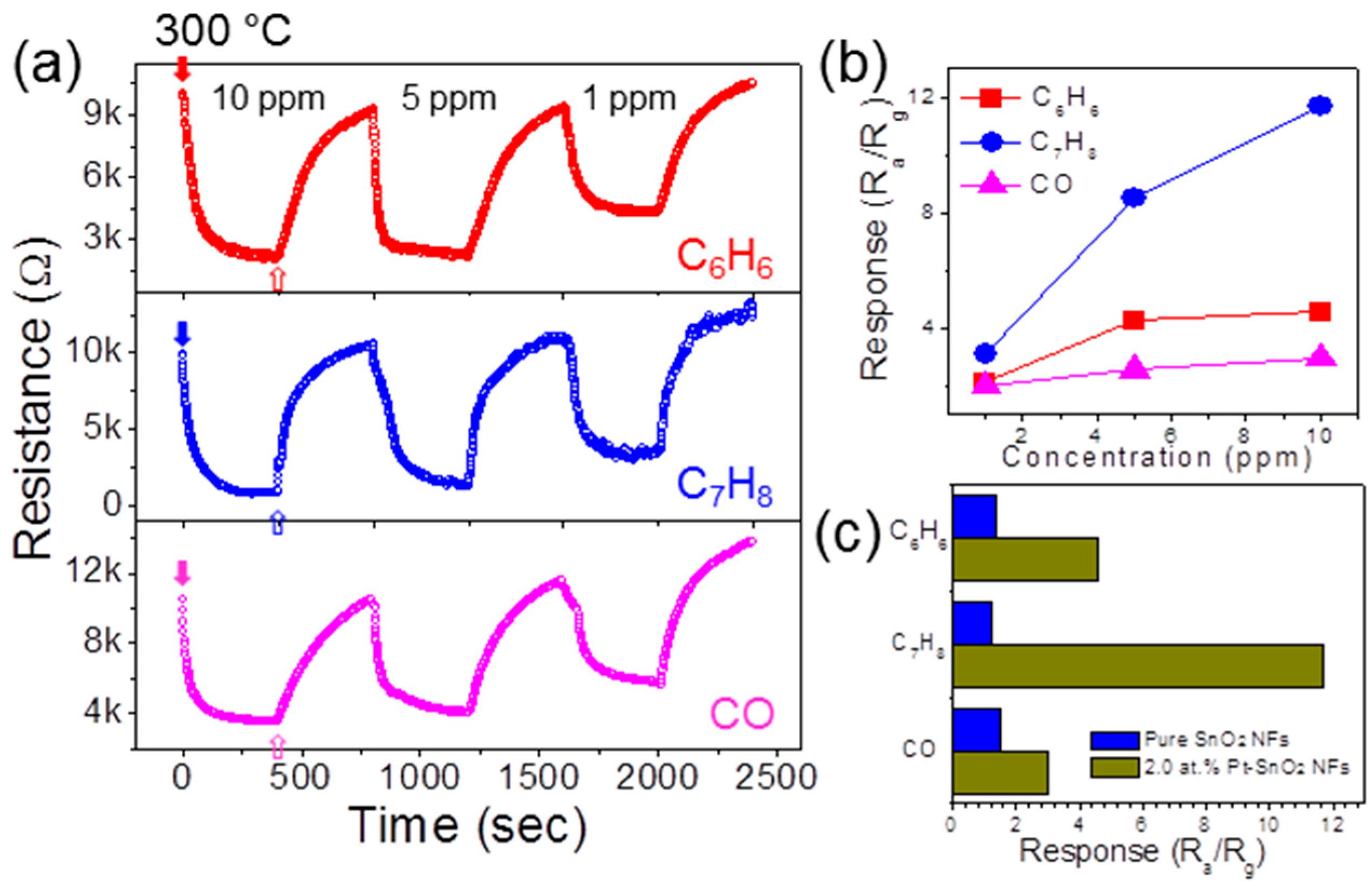
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kong, J.; Franklin, N.R.; Zhou, C.W.; Chapline, M.G.; Peng, S.; Cho, K.J.; Dai, H. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Liu, S.B.; Meng, F.L.; Liu, J.Y.; Jin, Z.; Kong, L.T.; Liu, J. Metal oxide nanostructures and their gas sensing properties: A review. Sensors 2012, 12, 2610–2631. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Choi, S.W.; Kim, S.S. A synthesis and sensing application of hollow ZnO nanofibers with uniform wall thicknesses grown using polymer templates. Nanotechnology 2010, 21, 475601–475610. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Katoch, A.; Sun, G.J.; Kim, S.S. Synthesis and gas sensing performance of ZnO-SnO2 nanofiber-nanowire stem-branch heterostructure. Sens. Actuators B Chem. 2013, 181, 787–794. [Google Scholar] [CrossRef]
- Katoch, A.; Choi, S.W.; Sun, G.J.; Kim, S.S. An approach to detecting a reducing gas by radial modulation of electron-depleted shells in core-shell nanofibers. J. Mater. Chem. A 2013, 1, 13588–13596. [Google Scholar] [CrossRef]
- Park, J.Y.; Asokan, K.; Choi, S.W.; Kim, S.S. Growth kinetics of nanograins in SnO2 fibers and size dependent sensing properties. Sens. Actuators B Chem. 2011, 152, 254–260. [Google Scholar] [CrossRef]
- Katoch, A.; Choi, S.W.; Kim, S.S. Nanograins in electrospun oxide nanofibers. Met. Mater. Int. 2015, 21, 213–221. [Google Scholar] [CrossRef]
- Choi, S.-W.; Katoch, A.; Zhang, J.; Kim, S.S. Electrospun nanofibers of CuO-SnO2 nanocomposite as semiconductor gas sensors for H2S detection. Sens. Actuators B Chem. 2013, 176, 585–591. [Google Scholar] [CrossRef]
- Choi, S.W.; Zhang, J.; Katoch, A.; Kim, S.S. H2S sensing performance of electrospun CuO-loaded SnO2 nanofibers. Sens. Actuators B Chem. 2012, 169, 54–60. [Google Scholar] [CrossRef]
- Byun, J.-H.; Katoch, A.; Choi, S.-W.; Kim, J.-H.; Kim, S.S. A novel synthesis route for Pt-loaded SnO2 nanofibers and their sensing properties. J. Nanosci. Nanotechnol. 2014, 14, 8253–8257. [Google Scholar] [CrossRef] [PubMed]
- Kolmakov, A.; Klenov, D.O.; Lilach, Y.; Stemmer, S.; Moskovits, M. Enhanced gas sensing by individual SnO2 nanowires and nanobelts functionalized with Pd catalyst particles. Nano Lett. 2005, 5, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Romanovskaya, V.; Ivanovskaya, M.; Bogdanov, P. A study of sensing properties of Pt- and Au-loaded In2O3 ceramics. Sens. Actuators B Chem. 1999, 56, 31–36. [Google Scholar] [CrossRef]
- Franke, M.E.; Koplin, T.J.; Simon, U. Metal and metal oxide nanoparticles in chemiresistors: Does the nanoscale matter? Small 2006, 2, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Wang, M.; Yu, J.; Sun, G. Gas sensors based on electrospun nanofibers. Sensors 2009, 9, 1609–1624. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kuang, Q.; Xie, Z.; Zheng, L. The effect of noble metal (Au, Pd and Pt) nanoparticles on the gas sensing performance of SnO2-based sensors: A case study on the {221} high-index faceted SnO2 octahedra. CrystEngComm 2015, 17, 6308–6313. [Google Scholar] [CrossRef]
- Hwang, I.S.; Choi, J.K.; Woo, H.S.; Kim, S.J.; Jung, S.Y.; Seong, T.Y.; Kim, I.; Lee, J. Facile control of C2H5OH sensing characteristics by decorating discrete Ag nanoclusters on SnO2 nanowire networks. ACS Appl. Mater. Interfaces 2011, 3, 3140–3145. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Choi, S.-J.; Lee, I.; Youn, D.-Y.; Park, C.O.; Lee, J.-H.; Tuller, H.; Kim, I. Thin-wall assembled SnO2 fibers functionalized by catalytic Pt nanoparticles and their superior exhaled-breath-sensing properties for the diagnosis of diabetes. Adv. Funct. Mater. 2013, 23, 2357–2367. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Lee, J.H.; Katoch, A.; Choi, S.-W.; Kim, J.-H.; Kim, H.W.; Kim, S.S. Extraordinary improvement of gas-sensing performances in SnO2 nanofibers due to creation of local p-n heterojunctions by loading reduced graphene oxide nanosheets. ACS Appl. Mater. Interfaces 2015, 7, 3101–3109. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zeng, W.; Luo, L.; Zhang, P.; Wang, Z. Gas-sensing properties and mechanisms of Cu-doped SnO2 spheres towards H2S. Ceram. Int. 2016, 42, 10006–10013. [Google Scholar] [CrossRef]
- Kocemba, I.; Rynkowski, J. The influence of catalytic activity on the response of Pt/SnO2 gas sensors to carbon monoxide and hydrogen. Sens. Actuators B Chem. 2011, 155, 659–666. [Google Scholar] [CrossRef]
- Wang, Q.J.; Wang, C.; Sun, H.B.; Sun, P.; Wang, Y.Z.; Lin, J.; Lu, G. Microwave assisted synthesis of hierarchical Pd/SnO2 nanostructures for CO gas sensor. Sens. Actuators B Chem. 2016, 222, 257–263. [Google Scholar] [CrossRef]
- Yin, X.T.; Guo, X.M. Selectivity and sensitivity of Pd-loaded and Fe-doped SnO2 sensor for CO detection. Sens. Actuators B Chem. 2014, 200, 213–218. [Google Scholar] [CrossRef]
- Ren, F.M.; Gao, L.P.; Yuan, Y.W.; Zhang, Y.; Alqrni, A.; Al-Dossary, O.M.; Xu, J. Enhanced BTEX gas-sensing performance of CuO/SnO2 composite. Sens. Actuators B Chem. 2016, 223, 914–920. [Google Scholar] [CrossRef]
- Scott, R.W.J.; Coombs, N.; Ozin, G.A. Non-aqueous synthesis of mesostructured tin dioxide. J. Mater. Chem. 2003, 13, 969–974. [Google Scholar] [CrossRef]
- Xu, H.Y.; Ju, J.X.; Li, W.R.; Zhang, J.; Wang, J.Q.; Cao, B.Q. Superior triethylamine-sensing properties based on TiO2/SnO2 n-n heterojunction nanosheets directly grown on ceramic tubes. Sens. Actuators B Chem. 2016, 228, 634–642. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Yu, K.; Wang, S.; Zhang, Y.; Wei, C. A novel low temperature gas sensor based on Pt-decorated hierarchical 3D SnO2 nanocomposites. Sens. Actuators B Chem. 2016, 232, 91–101. [Google Scholar] [CrossRef]
- Haick, H.; Broza, Y.Y.; Mochalski, P.; Ruzsanyi, V.; Amann, A. Assessment, origin, and implementation of breath volatile cancer markers. Chem. Soc. Rev. 2014, 43, 1423–1449. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.; Porto-Figueira, P.; Cavaco, C.; Taunk, K.; Rapole, S.; Dhakne, R.; Nagarajaram, H.; Câmara, J.S. Breath analysis as a potential and non-invasive frontier in disease diagnosis: An overview. Metabolites 2015, 5, 3–55. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Podmore, I. Current challenges in volatile organic compounds analysis as potential biomarkers of cancer. J. Biomark. 2015, 2015, 981458. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Lee, K.Y.; Min, S.J.; Yoo, Y.K.; Hwang, K.S.; Kim, S.K.; Yi, H. Single-carbon discrimination by selected peptides for individual detection of volatile organic compounds. Sci. Rep. 2015, 5, 9196. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Xu, L.; Song, J.; Zhou, C.; Li, Q.; Liu, D.; Song, H.W. Preparation and gas sensing properties of In2O3/Au nanorods for detection of volatile organic compounds in exhaled breath. Sci. Rep. 2015, 5, 10717. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, S.S. Realization of ppb-scale toluene-sensing abilities with Pt-functionalized SnO2-ZnO core-shell nanowires. ACS Appl. Mater. Interfaces 2015, 7, 17199–17208. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Wu, P.; Kim, H.W.; Kim, S.S. Highly selective sensing of CO, C6H6, and C7H8 gases by catalytic functionalization with metal nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 7173–7183. [Google Scholar] [CrossRef] [PubMed]
- Abideen, Z.U.; Katoch, A.; Kim, J.-H.; Kwon, Y.J.; Kim, H.W.; Kim, S.S. Excellent gas detection of ZnO nanofibers by loading with reduced graphene oxide nanosheets. Sens. Actuators B Chem. 2015, 221, 1499–1507. [Google Scholar] [CrossRef]
- Pavelko, R.G. Material science of SnO2-based oxide systems: Chemical ways to improve sensor performance. In Gas Sensors: Developments, Efficacy and Safety; Nova Science Publishers, Inc.: New York, NY, USA, 2011; pp. 93–138. [Google Scholar]
- Hubner, M.; Koziej, D.; Grunwaldt, J.D.; Weimar, U.; Barsan, N. An Au clusters related spill-over sensitization mechanism in SnO2-based gas sensors identified by operando HERFD-XAS, work function changes, DC resistance and catalytic conversion studies. Phys. Chem. Chem. Phys. 2012, 14, 13249–13254. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Wagner, T. Interaction of nanostructured metal overlayers with oxide surfaces. Surf. Sci. Rep. 2007, 62, 431–498. [Google Scholar] [CrossRef]
- Conner, W.C.; Falconer, J.L. Spillover in heterogeneous catalysis. Chem. Rev. 1995, 95, 759–788. [Google Scholar] [CrossRef]
- Fu, J.; Zhao, C.; Zhang, J.; Peng, Y.; Xie, E. Enhanced gas sensing performance of electrospun Pt-functionalized NiO nanotubes with chemical and electronic sensitization. ACS Appl. Mater. Interfaces 2013, 5, 7410–7416. [Google Scholar] [CrossRef] [PubMed]
- Wongrat, E.; Hongsith, N.; Wongratanaphisan, D.; Gardchareon, A.; Choopun, S. Control of depletion layer width via amount of Au NPs for sensor response enhancement in ZnO nanostructure sensor. Sens. Actuators B Chem. 2012, 171–172, 230–237. [Google Scholar] [CrossRef]
- Li, H.; Xu, J.; Zhu, Y.; Chen, X.; Xiang, Q. Enhanced gas sensing by assembling Pd nanoparticles onto the surface of SnO2 nanowires. Talanta 2010, 82, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Xu, P.; Zhu, Y.; Chen, X.; Yu, W. Decoration of ZnO nanowires with Pt nanoparticles and their improved gas sensing and photocatalytic performance. Nanotechnology 2010, 21, 285501–285508. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhu, J.W.; Yao, P.C.; Li, J.; Bi, H.P.; Wang, X. Synthesis of ZnO-Ag hybrids and their gas-sensing performance toward ethanol. Ind. Eng. Chem. Res. 2015, 54, 8947–8953. [Google Scholar] [CrossRef]
- Abideen, Z.U.; Kim, J.-H.; Kim, S.S. Optimization of metal nanoparticle amount on SnO2 nanowires to achieve superior gas sensing properties. Sens. Actuators B Chem. 2017, 238, 374–380. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Abideen, Z.U.; Zheng, Y.; Kim, S.S. Improvement of Toluene-Sensing Performance of SnO2 Nanofibers by Pt Functionalization. Sensors 2016, 16, 1857. https://doi.org/10.3390/s16111857
Kim J-H, Abideen ZU, Zheng Y, Kim SS. Improvement of Toluene-Sensing Performance of SnO2 Nanofibers by Pt Functionalization. Sensors. 2016; 16(11):1857. https://doi.org/10.3390/s16111857
Chicago/Turabian StyleKim, Jae-Hun, Zain Ul Abideen, Yifang Zheng, and Sang Sub Kim. 2016. "Improvement of Toluene-Sensing Performance of SnO2 Nanofibers by Pt Functionalization" Sensors 16, no. 11: 1857. https://doi.org/10.3390/s16111857





