Detection of Copper(II) Ions Using Glycine on Hydrazine-Adsorbed Gold Nanoparticles via Raman Spectroscopy
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Instrumentations
2.3. Preparation of AuNPs and GLY-Cu2+ Complex
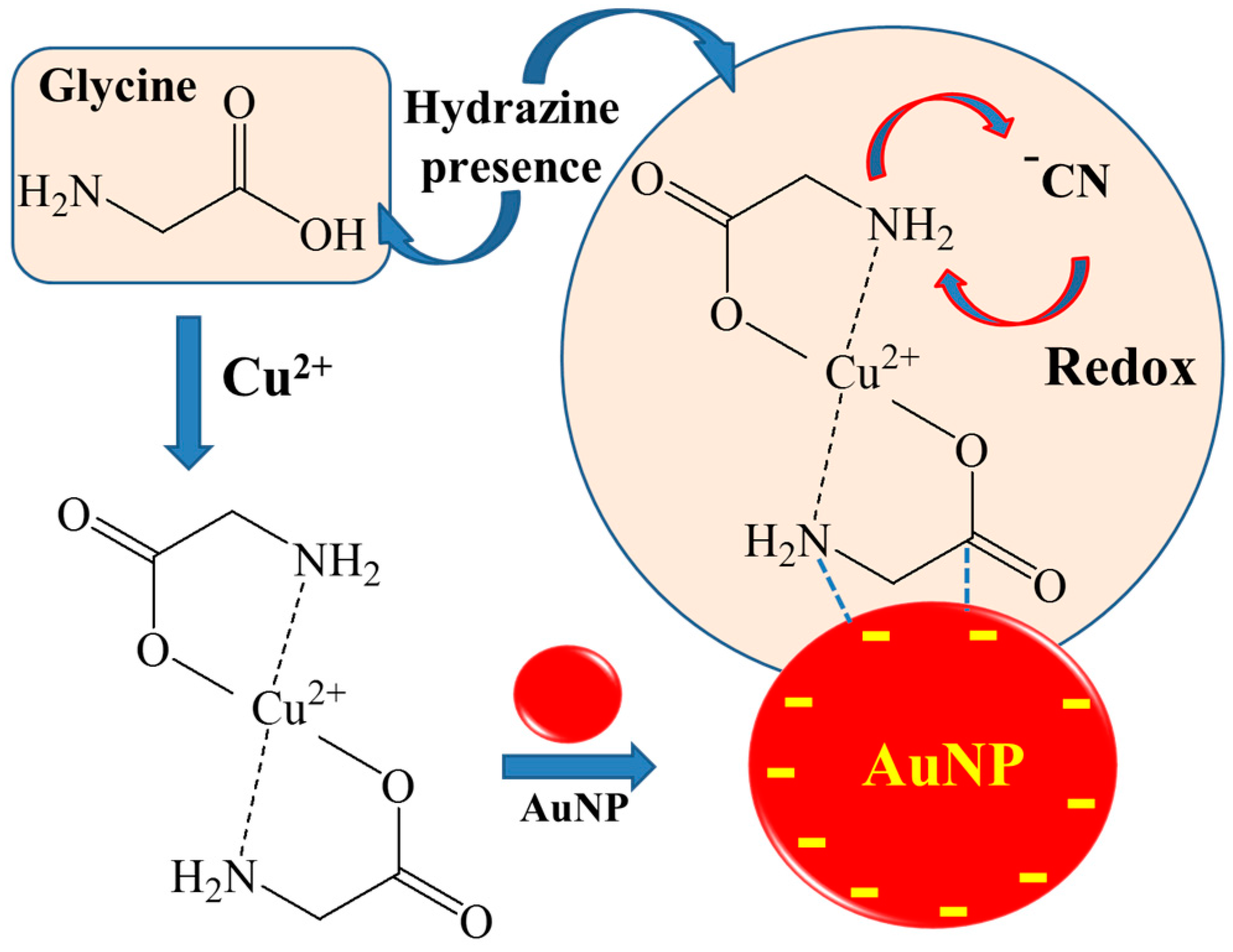
3. Results and Discussion
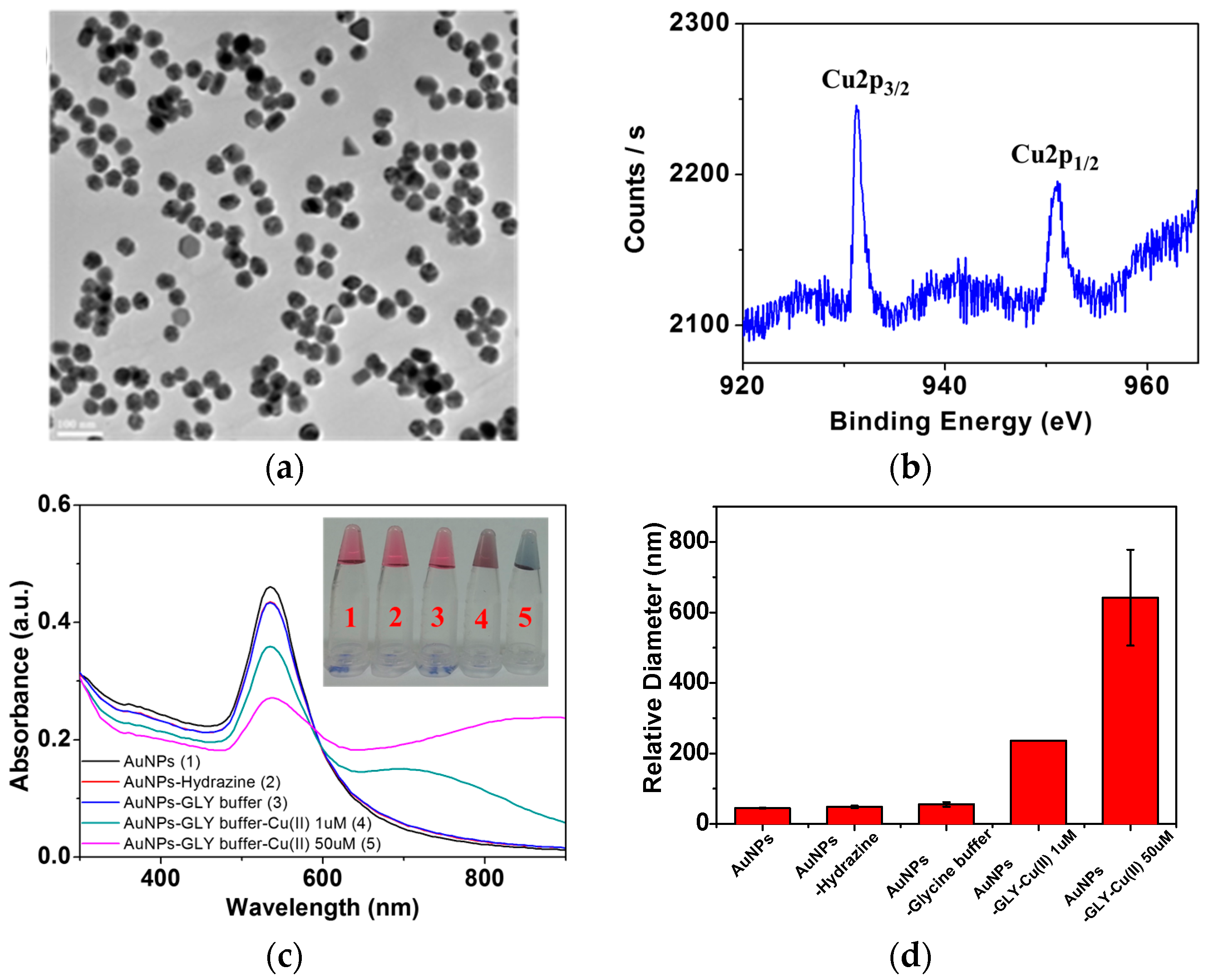
3.1. Physical Characterization
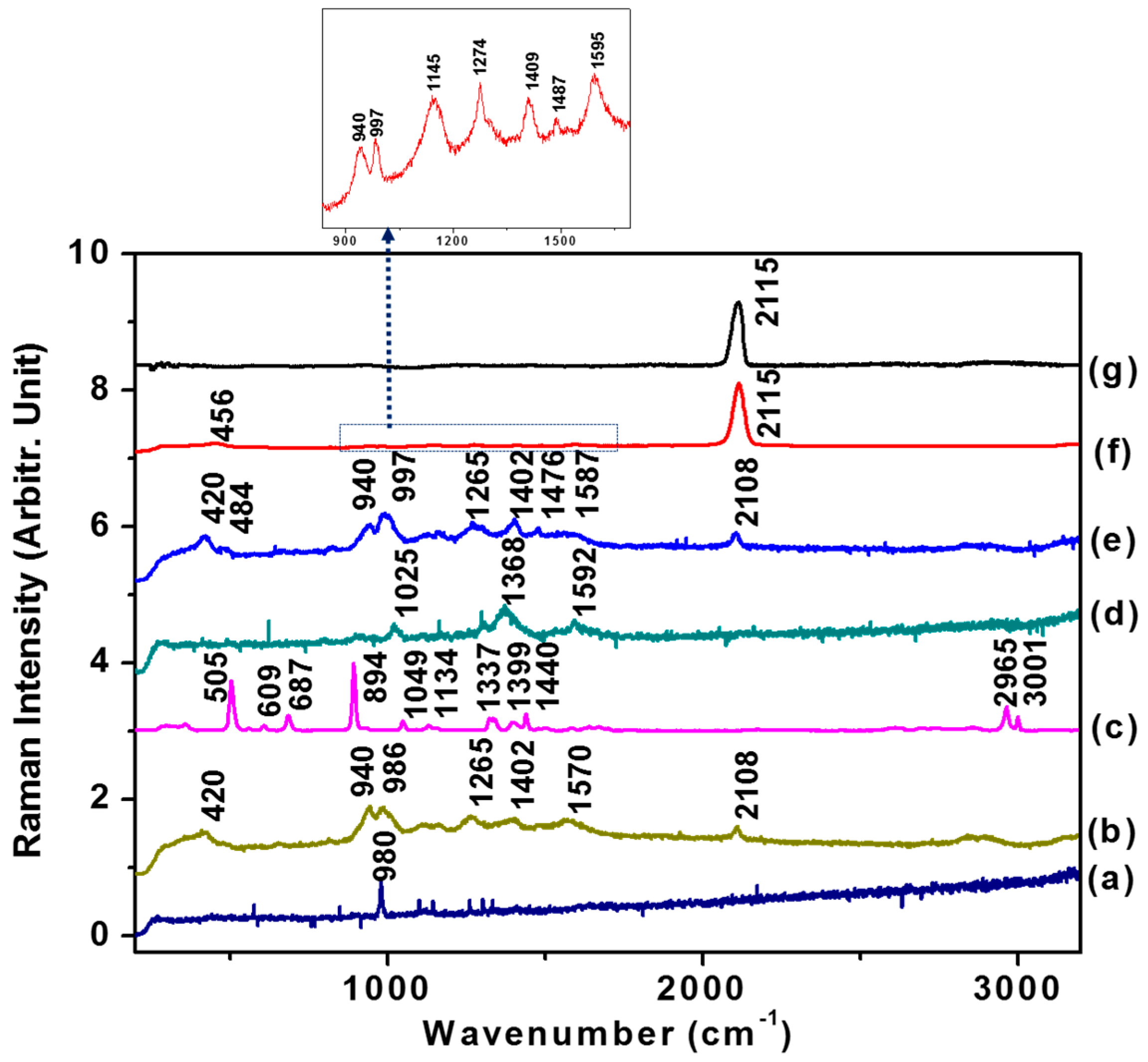
3.2. Raman Spectra of Hydrazine and GLY on AuNPs
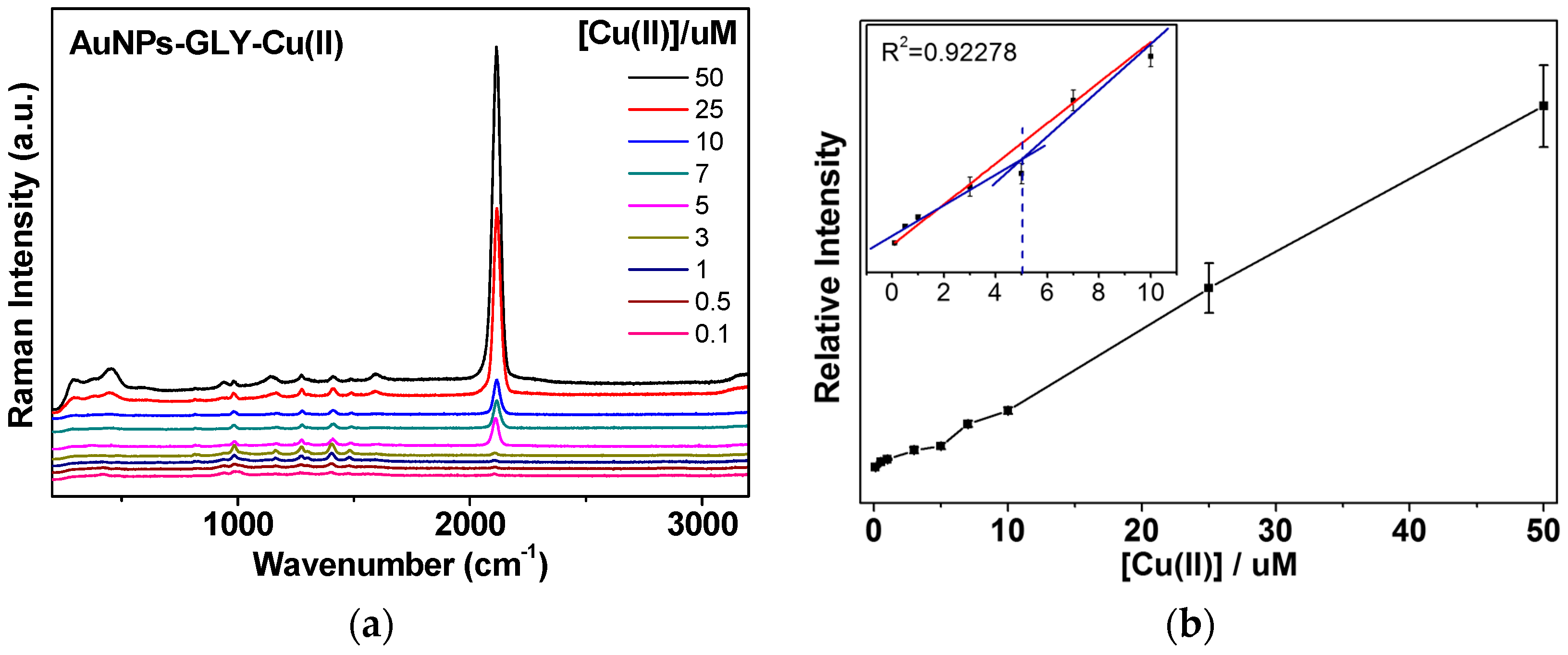
3.3. CN Formation Reactions of GLY-Cu2+ on AuNPs and Selective Test
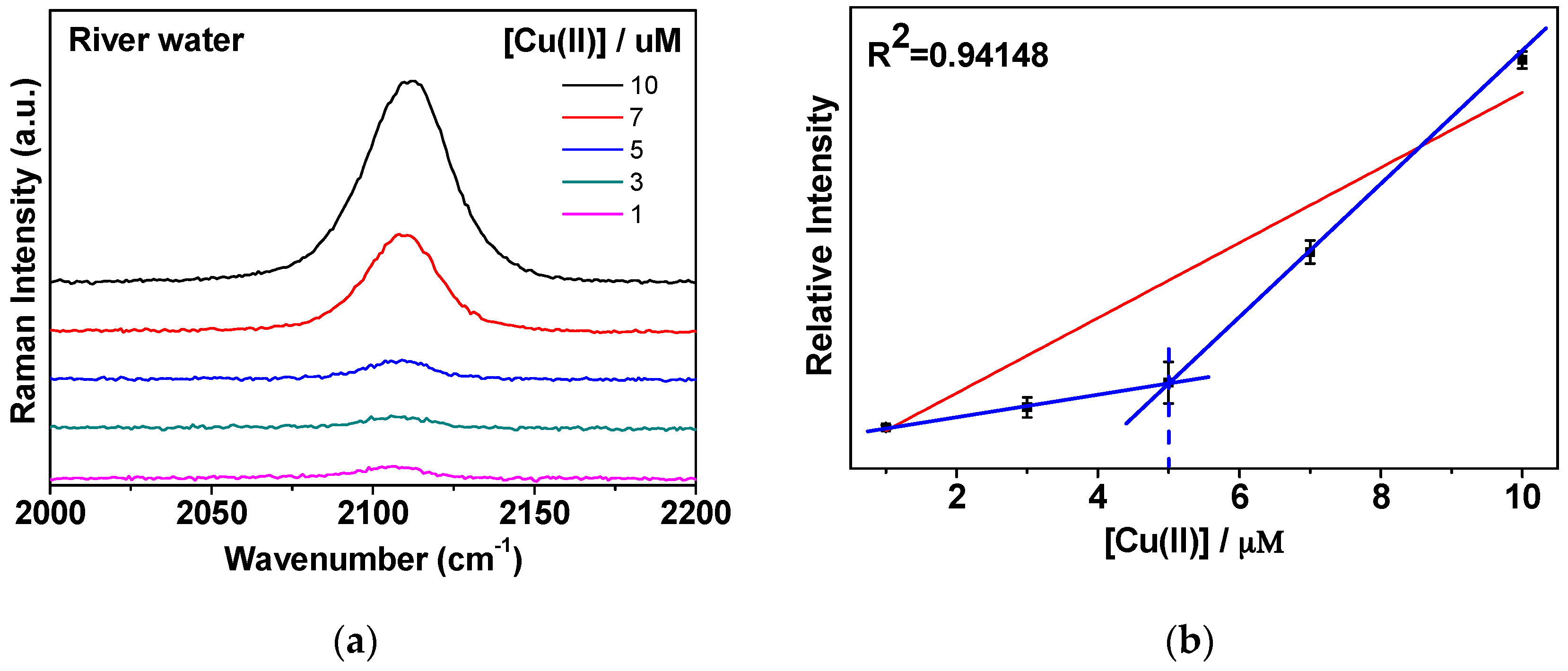
3.4. CN Bands in River Water Samples Contacting Highly Concentrated Na, Ca, Mg, and K Ions
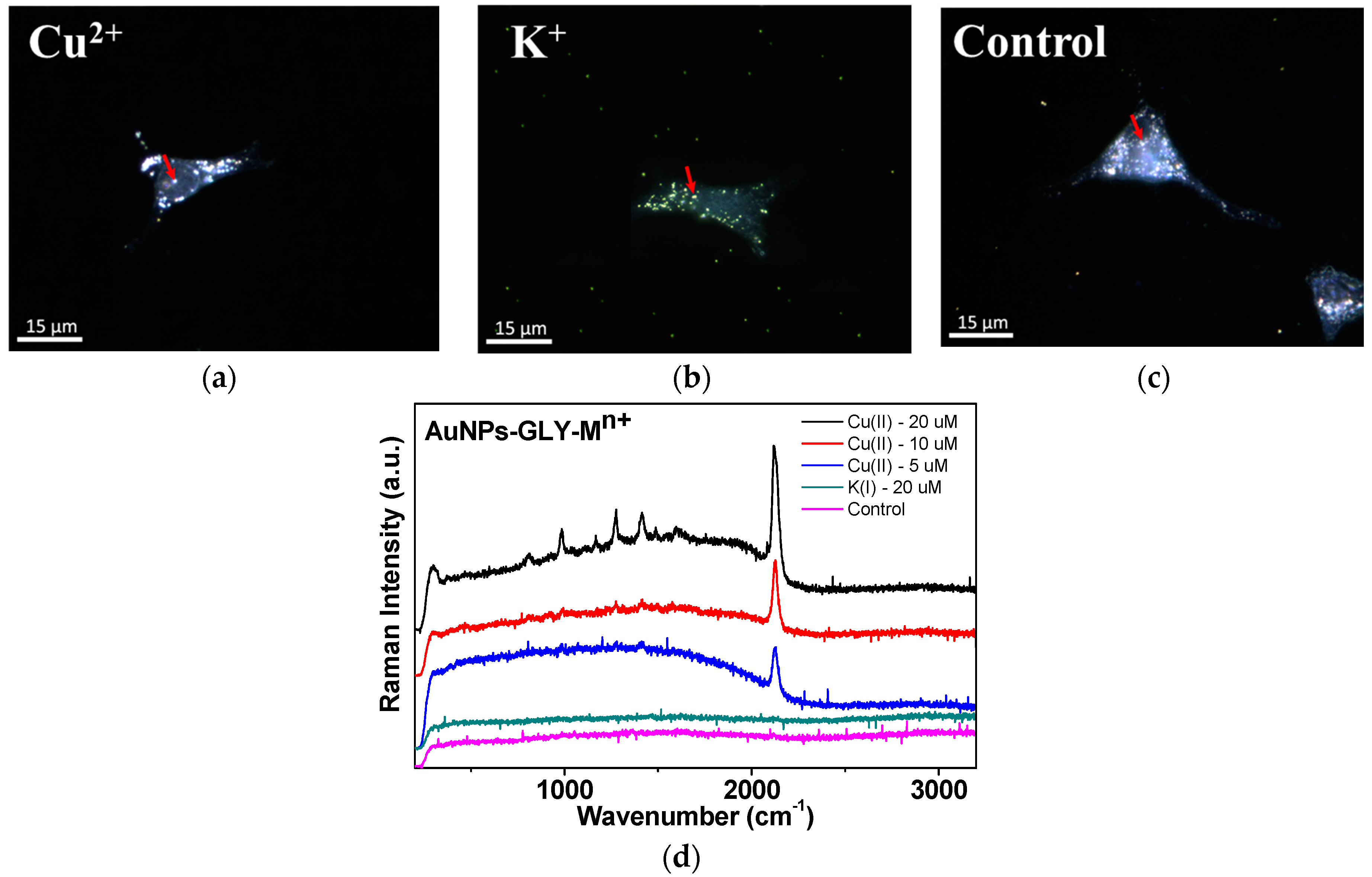
3.5. Cu2+ Ion Detection in Cancer Cells
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- March, G.; Nguyen, T.D.; Piro, B. Modified electrodes used for electrochemical detection of metal ions in environmental analysis. Biosensors 2015, 5, 241–275. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Wei, H.; Zhang, S.; Xu, H. Recent advances in plasmonic sensors. Sensors 2014, 14, 7959–7973. [Google Scholar] [CrossRef] [PubMed]
- Doria, G.; Conde, J.; Veigas, B.; Giestas, L.; Almeida, C.; Assunção, M.; Rosa, J.; Baptista, P.V. Noble metal nanoparticles for biosensing applications. Sensors 2012, 12, 1657–1687. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, P.; Singh, D.K.; Sadhu, S.; Poddar, P. Study of the nucleation and growth of antibiotic labeled Au NPs and blue luminescent Au8 quantum clusters for Hg(2+) ion sensing, cellular imaging and antibacterial applications. Nanoscale 2015, 7, 19985–20002. [Google Scholar] [CrossRef] [PubMed]
- Kassu, A.; Farley, C., III; Sharma, A.; Kim, W.; Guo, J. Effect of pore size and film thickness on gold-coated nanoporous anodic aluminum oxide substrates for surface-enhanced Raman scattering sensor. Sensors 2015, 15, 29924–29937. [Google Scholar] [CrossRef] [PubMed]
- Yaman, Y.T.; Abaci, S. Sensitive adsorptive voltammetric method for determination of bisphenol A by gold nanoparticle/polyvinylpyrrolidone-modified pencil graphite electrode. Sensors 2016, 16, 756. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lv, P.; Zhu, J.; Lu, Y.; Chen, C.; Zhang, X.; Wei, Q. NiCu alloy nanoparticle-loaded carbon nanofibers for phenolic biosensor applications. Sensors 2015, 15, 29419–29433. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Poljak, A.; Marjo, C.; Rutlidge, H.; Rich, A.; Jayasena, T.; Inestrosa, N.C.; Sachdev, P. Metal and complementary molecular bioimaging in Alzheimer’s disease. Front. Aging Neurosci. 2014, 6, 138. [Google Scholar] [CrossRef] [PubMed]
- Majedi, S.M.; Kelly, B.C.; Lee, H.K. Toward a robust analytical method for separating trace levels of nano-materials in natural waters: Cloud point extraction of nano-copper(II) oxide. Environ. Sci. Pollut. Res. Int. 2014, 21, 11811–11822. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J. Copper toxicity in the general population. Clin. Neurophysiol. 2010, 121, 459–460. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Risk Assessment, Management and Communication of Drinking Water Contamination; U.S. EPA 625/4-89/024; Environmental Protection Agency: Washington, DC, USA, 1989.
- Sadollahkhani, A.; Hatamie, A.; Nur, O.; Willander, M.; Zargar, B.; Kazeminezhad, I. Colorimetric disposable paper coated with ZnO@ZnS core-shell nanoparticles for detection of copper ions in aqueous solutions. ACS Appl. Mater. Interfaces 2014, 6, 17694–17701. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Cai, N.; Du, Y.; He, Y.; Yeung, E.S. Sensitive and selective detection of copper ions with highly stable polyethyleneimine-protected silver nanoclusters. Anal. Chem. 2014, 86, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Wang, H.; Vongsvivut, J.; Li, R.; Glushenkov, A.M.; He, J.; Chen, Y.; Barrow, C.J.; Yang, W. Self-assembly of core-satellite gold nanoparticles for colorimetric detection of copper ions. Anal. Chim. Acta 2013, 803, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Tsoutsi, D.; Guerrini, L.; Hermida-Ramon, J.-M.; Giannini, V.; Liz-Marzán, L.M.; Wei, A.; Alvarez-Puebla, R.A. Simultaneous SERS detection of copper and cobalt at ultratrace levels. Nanoscale 2013, 5, 5841–5846. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, J.; Lai, Y.; Wu, C.; Sun, S.; He, Y.; Ma, H. Ultrasensitive and selective detection of copper (II) and mercury (II) ions by dye-coded silver nanoparticle-based SERS probes. Biosens. Bioelectron. 2013, 39, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Ernst, K.H.; Christmann, K. The interaction of glycine with a platinum (111) surface. Surf. Sci. 1989, 224, 277–310. [Google Scholar] [CrossRef]
- Han, J.W.; James, J.N.; Sholl, D.S. Chemical speciation of adsorbed glycine on metal surfaces. J. Chem. Phys. 2011, 135, 034703. [Google Scholar] [CrossRef] [PubMed]
- Colaneri, M.J.; Vitali, J.; Peisach, J. Aspects of structure and bonding in copper-amino acid complexes revealed by single-crystal EPR/ENDOR spectroscopy and density functional calculations. J. Phys. Chem. A 2009, 113, 5700–5709. [Google Scholar] [CrossRef] [PubMed]
- Zhen, C.-H.; Sun, S.-G.; Fan, C.-J.; Chen, S.-P.; Mao, B.-W.; Fan, Y.-J. In situ FTIRS and EQCM studies of glycine adsorption and oxidation on Au(111) electrode in alkaline solutions. Electrochim. Acta 2004, 49, 1249–1255. [Google Scholar] [CrossRef]
- Hu, Q.; Tay, L.L.; Noestheden, M.; Pezacki, J.P. Mammalian cell surface imaging with nitrile-functionalized nanoprobes: Biophysical characterization of aggregation and polarization anisotropy in SERS imaging. J. Am. Chem. Soc. 2007, 129, 14–15. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Tian, X.; Hong, S.; Dai, P.; You, Q.; Wang, R.; Feng, L.; Xie, C.; Tian, Z.Q.; Chen, X. A bioorthogonal Raman reporter strategy for SERS detection of glycans on live cells. Angew. Chem. Int. Ed. Engl. 2013, 52, 7266–7271. [Google Scholar] [CrossRef] [PubMed]
- Cong, V.T.; Ganbold, E.-O.; Saha, J.K.; Jang, J.; Min, J.; Choo, J.; Kim, S.; Song, N.W.; Son, S.J.; Lee, S.B.; et al. Gold nanoparticle silica nanopeapods. J. Am. Chem. Soc. 2014, 136, 3833–3841. [Google Scholar] [CrossRef] [PubMed]
- Ly, N.H.; Oh, C.H.; Joo, S.W. A submicromolar Cr(III) sensor with a complex of methionine using gold nanoparticles. Sens. Actuators B Chem. 2015, 219, 276–282. [Google Scholar] [CrossRef]
- Ly, N.H.; Joo, S.-W. Zn(II)-concentration dependent Raman spectra in the dithizonecomplex on gold nanoparticle surfaces in environmental water samples. Appl. Surf. Sci. 2015, 356, 1005–1011. [Google Scholar] [CrossRef]
- Ly, N.H.; Joo, S.-W. Silver nanoparticle-enhanced resonance Raman sensor of chromium(III) in seawater samples. Sensors 2015, 15, 10088–10099. [Google Scholar] [CrossRef] [PubMed]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics Inc.: Chanhassen, MN, USA, 1995. [Google Scholar]
- Kumar, S.; Rai, A.K.; Singh, V.B.; Rai, S.B. Vibrational spectrum of glycine molecule. Spectrochim. Acta A 2005, 61, 2741–2746. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, L. Collective vibrational spectra of α-and γ-glycine studied by terahertz and Raman spectroscopy. J. Phys. D Appl. Phys. 2005, 38, 3741–3745. [Google Scholar] [CrossRef]
- Podstawka, E.; Ozaki, Y.; Proniewicz, L.M. Part I: Surface-enhanced Raman spectroscopy investigation of amino acids and their homodipeptides adsorbed on colloidal silver. Appl. Spectrosc. 2004, 58, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Durig, J.R.; Griffin, M.G.; Macnamee, R.W. Raman spectra of gases. XV: Hydrazine and hydrazine-d4. J. Raman Spectrosc. 1975, 3, 133–141. [Google Scholar] [CrossRef]
- Braibanti, A.; Dallavalle, F.; Pellinghelli, M.A.; Leporati, E. The nitrogen-nitrogen stretching band in hydrazine derivatives and complexes. Inorg. Chem. 1968, 7, 1430–1433. [Google Scholar] [CrossRef]
- Sheets, R.W.; Blyhokler, G. Isocyanate formation from adsorbed carbon monoxide and ammonia or hydrazine on vanadium, iron, and nickel. J. Phys. Chem. 1975, 79, 1572–1573. [Google Scholar] [CrossRef]
- Ly, N.H.; Joo, S.-W. Surface anchoring mode-dependent hydrolysis reactions of hydrazone groups on gold examined by pH-dependent Raman spectroscopy. J. Raman Spectrosc. 2015, 46, 1082–1086. [Google Scholar] [CrossRef]
- Oraby, E.A.; Eksteen, J.J. The selective leaching of copper from a gold–copper concentrate in glycine solutions. Hydrometallurgy 2014, 150, 14–19. [Google Scholar] [CrossRef]
- Cho, K.; Jang, Y.S.; Gong, M.-S.; Kim, K.; Joo, S.-W. Determination of cyanide species in silver and gold plating solutions by Raman spectroscopy. Appl. Spectrosc. 2002, 56, 1147–1151. [Google Scholar] [CrossRef]
- Li, G.; Luo, Y. Preparation and characterization of dendrimer-templated Ag-Cu bimetallic nanoclusters. Inorg. Chem. 2008, 47, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Bowmaker, G.A.; Kennedy, B.J.; Reid, J.C. Crystal structures of AuCN and AgCN and vibrational spectroscopic studies of AuCN, AgCN, and CuCN. Inorg. Chem. 1998, 37, 3968–3974. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ly, N.H.; Seo, C.; Joo, S.-W. Detection of Copper(II) Ions Using Glycine on Hydrazine-Adsorbed Gold Nanoparticles via Raman Spectroscopy. Sensors 2016, 16, 1785. https://doi.org/10.3390/s16111785
Ly NH, Seo C, Joo S-W. Detection of Copper(II) Ions Using Glycine on Hydrazine-Adsorbed Gold Nanoparticles via Raman Spectroscopy. Sensors. 2016; 16(11):1785. https://doi.org/10.3390/s16111785
Chicago/Turabian StyleLy, Nguyễn Hoàng, Chulhun Seo, and Sang-Woo Joo. 2016. "Detection of Copper(II) Ions Using Glycine on Hydrazine-Adsorbed Gold Nanoparticles via Raman Spectroscopy" Sensors 16, no. 11: 1785. https://doi.org/10.3390/s16111785





