A Graphene Oxide-Based Fluorescent Method for the Detection of Human Chorionic Gonadotropin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
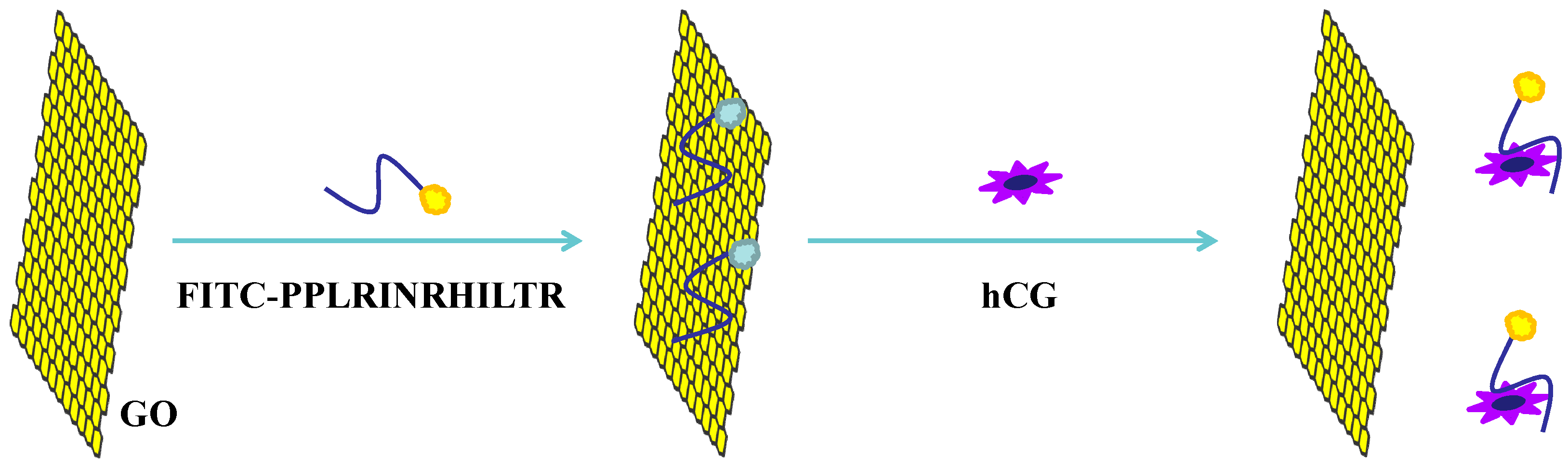
2.2. Quenching Studies
2.3. Detection of hCG
3. Results and Discussion
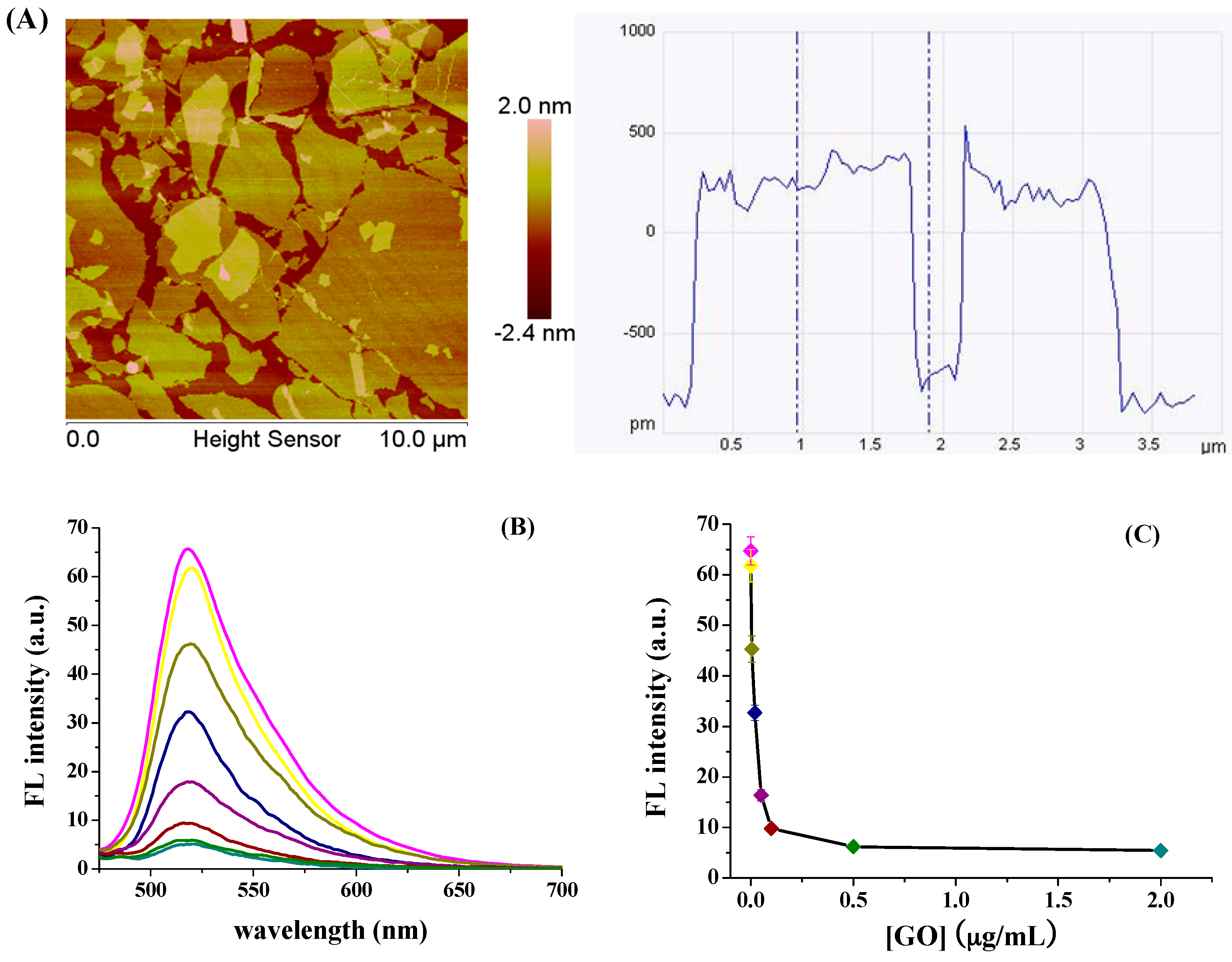
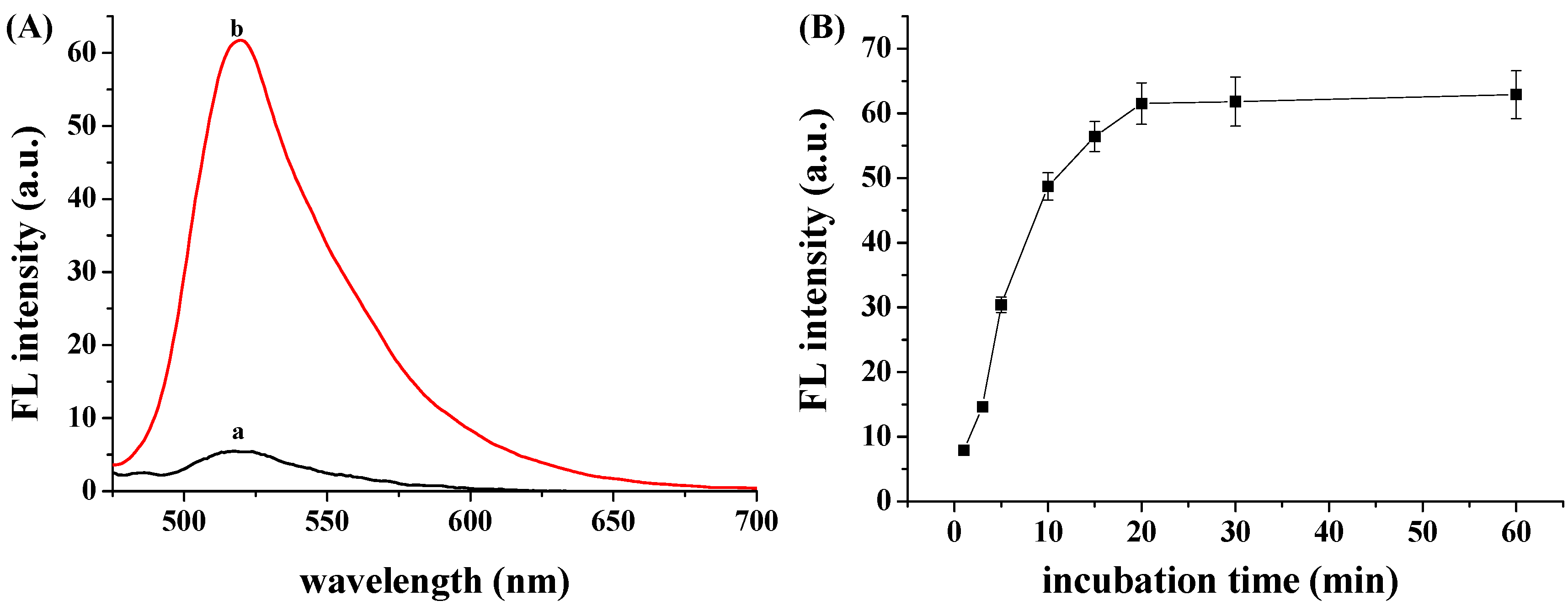
3.1. Quenching Efficiency of GO to FITC-PPLRINRHILTR
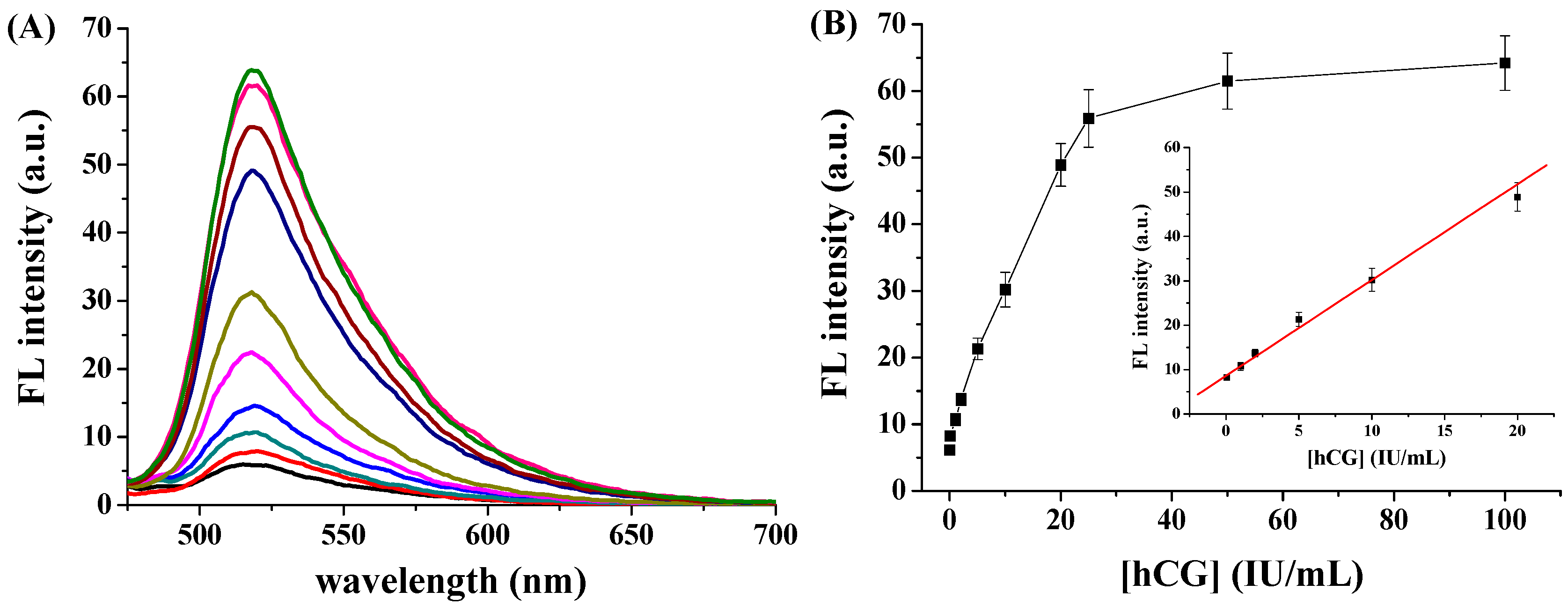
3.2. hCG Detection
3.3. Sensitivity to hCG
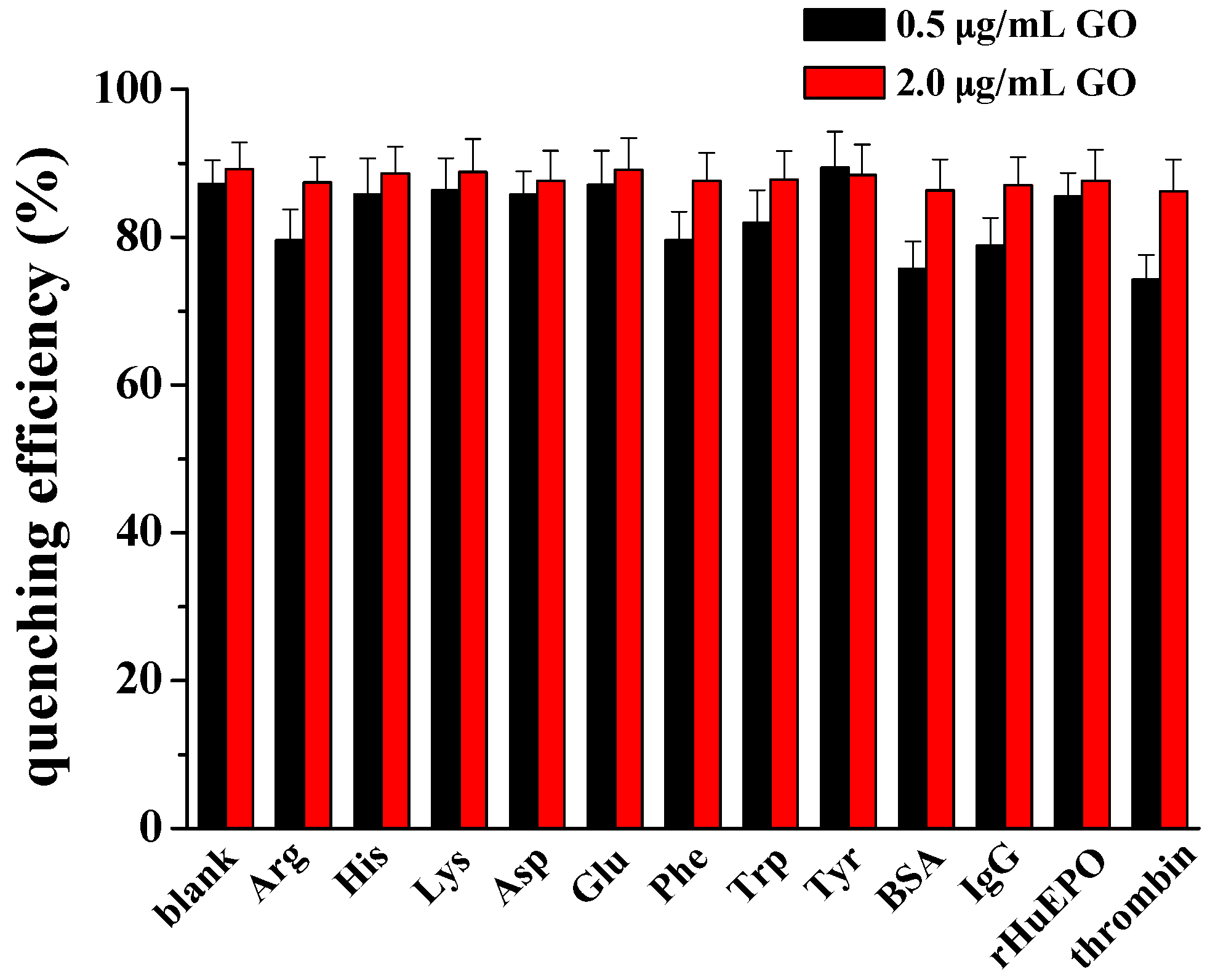
3.4. Selectivity and Real Sample Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yeh, C.-H.; Zhao, Z.-Q.; Shen, P.-L.; Lin, Y.-C. Optimization of an optical inspection system based on the taguchi method for quantitative analysis of point-of-care testing. Sensors 2014, 14, 16148–16158. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Yang, K.-L. Antibody-free detection of human chorionic gonadotropin by use of liquid crystals. Anal. Chem. 2013, 85, 10710–10716. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Xu, H.; Xu, P.; Chen, K.; Mu, R.; Fu, J.; Gu, H. Ultrasensitive ELISA using enzyme-loaded nanospherical brushes as labels. Anal. Chem. 2014, 86, 9367–9371. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Li, L.; Li, S.; Li, M.; Ge, S.; Yu, J.; Yan, M.; Song, X. Fluorescence-based immunoassay for human chorionic gonadotropin based on polyfluorene-coated silica nanoparticles and polyaniline-coated Fe3O4 nanoparticles. Microchim. Acta 2013, 180, 1509–1516. [Google Scholar] [CrossRef]
- Su, J.; Zhou, Z.; Li, H.; Liu, S. Quantitative detection of human chorionic gonadotropin antigen via immunogold chromatographic test strips. Anal. Methods 2014, 6, 450–455. [Google Scholar] [CrossRef]
- Yan, X.; Huang, Z.; He, M.; Liao, X.; Zhang, C.; Yin, G.; Gu, J. Detection of HCG-antigen based on enhanced photoluminescence of hierarchical ZnO arrays. Colloids Surf. B Biointerface 2012, 89, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yuan, H.; Shen, H.; Guo, Y.; Li, X.; Liu, D.; Xu, L.; Ma, L.; Li, L.S. Synthesis of size-tunable photoluminescent aqueous CdSe/ZnS microspheres via a phase transfer method with amphiphilic oligomer and their application for detection of HCG antigen. J. Mater. Chem. 2011, 21, 7393–7400. [Google Scholar] [CrossRef]
- Piliarik, M.; Vaisocherová, H.; Homola, J. A new surface plasmon resonance sensor for high-throughput screening applications. Biosens. Bioelectron. 2005, 20, 2104–2110. [Google Scholar] [CrossRef] [PubMed]
- Chetcuti, A.F.; Wong, D.K.Y. An indirect perfluorosulfonated ionomer-coated electrochemical immunosensor for the detection of the protein human chorionic gonadotrophin. Anal. Chem. 1999, 71, 4088–4094. [Google Scholar] [CrossRef] [PubMed]
- Kerman, K.; Nagatani, N.; Chikae, M.; Yuhi, T.; Takamura, Y.; Tamiya, E. Label-free electrochemical immunoassay for the fetection of human chorionic gonadotropin hormone. Anal. Chem. 2006, 78, 5612–5616. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wu, D.; Li, H.; Xu, C.; Wang, H.; Zhao, Y.; Cai, Y.; Wei, Q.; Du, B. Label-free amperometric immunosensor for the detection of human serum chorionic gonadotropin based on nanoporous gold and graphene. Anal. Biochem. 2011, 414, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.A.; Yoshikaw, H.; Tamiy, E.; Yasin, H.M.; Ahmed, M.U. A highly sensitive gold nanoparticle bioprobe based electrochemical immunosensor using screen printed graphene biochip. RSC Adv. 2014, 4, 58460–58466. [Google Scholar] [CrossRef]
- Lu, J.J.; Liu, S.Q.; Ge, S.G.; Yan, M.; Yu, J.H.; Hu, X.T. Ultrasensitive electrochemical immunosensor based on Au nanoparticles dotted carbon nanotube–graphenecomposite and functionalized mesoporous materials. Biosens. Bioelectron. 2012, 33, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Gao, F.; Chen, J.; Xie, L.; Wang, Q. Application of 2-(4-Formylphenyl) [60]Fulleropyrrolidine as anelectrode matrix for cross linker-free immobilization of HCG-antibodyand the sensing analysis. Sens. Actuators B Chem. 2016, 231, 376–383. [Google Scholar] [CrossRef]
- Roushani, M.; Valipour, A. Voltammetric immunosensor for human chorionic gonadotropin using a glassy carbon electrode modified with silver nanoparticles and a nanocomposite composed of graphene, chitosan and ionic liquid, and using riboflavin as a redox probe. Microchim. Acta 2016, 183, 845–853. [Google Scholar] [CrossRef]
- Roushani, M.; Valipour, A. Using electrochemical oxidation of Rutin in modeling a novel andsensitive immunosensor based on Pt nanoparticle and graphene–ionicliquid–chitosan nanocomposite to detect human chorionicgonadotropin. Sens. Actuators B Chem. 2016, 222, 1103–1111. [Google Scholar] [CrossRef]
- Tan, F.; Yan, F.; Ju, H. Sensitive reagentless electrochemical immunosensor based on an ormosil sol–gel membrane for human chorionic gonadotrophin. Biosens. Bioelectron. 2007, 22, 2945–2951. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.; Conlan, R.S.; Guy, O.J.; Goreti, M.; Sales, F. Label free human chorionic gonadotropin detection at picogram levels using oriented antibodies bound to graphene screen-printed electrodes. J. Mater. Chem. B 2014, 2, 1852–1865. [Google Scholar] [CrossRef]
- Teixeira, S.; Ferreira, N.S.; Conlan, R.S.; Guy, O.J.; Sales, M.G.F. Chitosan/AuNPs modified graphene electrochemical sensor for label-free human chorionic gonadotropin detection. Electroanalysis 2014, 26, 2591–2598. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Y.; Shi, L.; Cai, Y.; Ma, H.; Du, B.; Wei, Q. Electrochemical immunosensor for ultrasensitive detection of human chorionic gonadotropin based on Pd@SBA-15. Electroanalysis 2013, 25, 427–432. [Google Scholar] [CrossRef]
- Cohen, B.A.; Colas, P.; Brent, R. An artificial cell-cycle inhibitor isolated from a combinatorial Library. Proc. Natl. Acad. Sci. USA 1998, 95, 14272–14277. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.J.; Tkac, J.; Laurenson, S.; Ferrigno, P.K. Peptide aptamers in label-free protein detection: 1. Characterization of the immobilized scaffold. Anal. Chem. 2007, 79, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xie, H.; Cao, Y.; Ding, X.; Yin, Y.; Li, G. A general way to assay protein by coupling peptide with signal reporter via supermolecule formation. Anal. Chem. 2013, 85, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.N.; Li, H.; Huang, Y.; Wang, X.Y.; Yin, Y.M.; Li, G.X. Combining peptide and DNA for protein assay: CRIP1 detection for breast cancer staging. ACS Appl. Mater. Interface 2014, 6, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, J.; Liu, X.; Nie, Z.; Qing, M.; Guo, M.; Yao, S. Aptameric peptide for one-step detection of protein kinase. Anal. Chem. 2012, 84, 4746–4753. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Chen, C.-P.; Lee, C.-H.; Chen, C.-Y.; Lin, C.-W. Colorimetric detection of human chorionic gonadotropin using catalytic gold nanoparticles and a peptide aptamer. Chem. Commun. 2014, 50, 14443–14446. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Chen, C.-Y.; Chen, C.-P.; Lin, C.-W. Facile colorimetric detection of human chorionic gonadotropin based on the peptide-induced aggregation of gold nanoparticles. Anal. Methods 2015, 7, 29–33. [Google Scholar] [CrossRef]
- Chen, Y.; Star, A.; Vidal, S. Sweet carbon nanostructures: Carbohydrate conjugates with carbon nanotubes and graphene and their applications. Chem. Soc. Rev. 2012, 42, 4532–4542. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Kim, Y.-K.; Shin, D.; Ryoo, S.-R.; Hong, B.H.; Min, D.-H. Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wu, L.; Qu, X. New horizons for diagnostics and therapeutic applications of graphene and graphene oxide. Adv. Mater. 2013, 25, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yim, Y.; Kim, S.; Choi, M.-H.; Choi, B.-S.; Lee, Y.; Min, D.-H. In-depth investigation of the interaction between DNA and nano-sized graphene oxide. Carbon 2016, 97, 92–98. [Google Scholar] [CrossRef]
- Wick, P.; Louw-Gaume, A.E.; Kucki, M.; Krug, H.F.; Kostarelos, K.; Fadeel, B.; Dawson, K.A.; Salvati, A.; Vzquez, E.; Ballerini, L.; et al. Classification framework for graphene-based materials. Angew. Chem. Int. Ed. 2014, 53, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ding, L.; Wang, Q.; Hu, Y.; Jia, L.; Yu, J.-S.; Ju, H. Folate receptor-targeted and cathepsin B-activatable nanoprobe for in situ therapeutic monitoring of photosensitive cell death. Anal. Chem. 2015, 87, 3841–3848. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zhang, Y.; Feng, T.; Shi, W.; Li, X.; Ma, H. A graphene oxide-peptide fluorescence sensor tailor-made for simple and sensitive detection of matrix metalloproteinase 2. Chem. Commun. 2011, 47, 10680–10682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yin, B.-C.; Wang, X.-F.; Ye, B.-C. Interaction of peptides with graphene oxide and its application for real-time monitoring of protease activity. Chem. Commun. 2011, 47, 2399–2401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, X.; Liu, W.; Liu, X.; Nie, Z.; Qing, M.; Nie, L.; Yao, S. Graphene oxide−peptide nanocomplex as a versatile fluorescence probe of protein kinase activity based on phosphorylation protection against carboxypeptidase digestion. Anal. Chem. 2013, 85, 5746–5754. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xia, N.; Yu, J. A graphene oxide-based fluorescent scheme for the determination of the activity of the β-site amyloid precursor protein (BACE1) and its inhibitors. Microchim. Acta 2016, 183, 265–271. [Google Scholar] [CrossRef]
- Jang, H.; Kim, Y.-K.; Kwon, H.-M.; Yeo, W.-S.; Kim, D.-E.; Min, D.-H. A graphene-based platform for the assay of duplex-DNA unwinding by helicase. Angew. Chem. Int. Ed. 2010, 49, 5703–5707. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Cargill, A.A.; Das, S.R.; Medintz, I.L.; Claussen, J.C. Biosensing with förster resonance energy transfer coupling between fluorophores and nanocarbon allotropes. Sensors 2015, 15, 14766–14787. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Hu, J.; He, Z. A split G-quadruplex and graphene oxide-based low-background platform for fluorescence authentication of pseudostellaria heterophylla. Sensors 2014, 14, 22971–22981. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, B.; Ding, J.; Liu, J. Fluorescent sensors using DNA-functionalized graphene oxide. Anal. Bioanal. Chem. 2014, 406, 6885–6902. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Chen, T.; Xiong, X.; Mao, Y.; Zhu, G.; Yasun, E.; Li, C.; Zhu, Z.; Tan, W. Semiquantification of ATP in live cells using nonspecific desorption of DNA from graphene oxide as the internal reference. Anal. Chem. 2012, 84, 8622–8627. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.J.; An, Z.; Compton, O.C.; Nguyen, S.T. Tunable biomolecular interaction and fluorescence quenching ability of graphene oxide: Application to “turn-on” DNA sensing in biological media. Small 2012, 8, 2469–2476. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.-T.; Yan, X.-P. Fabrication of transferrin functionalized gold nanoclusters/graphene oxide nanocomposite for turn-on near-infrared fluorescent bioimaging of cancer cells and small animals. Anal. Chem. 2013, 85, 2529–2535. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Hu, D.; Lin, C.-T.; Li, J.; Lin, Y. Aptamer/graphene oxide nanocomplex forin situ molecular probing in living cells. J. Am. Chem. Soc. 2010, 132, 9274–9276. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, C.; Qu, K.; Song, Y.; Ren, J.; Miyoshi, D.; Sugimoto, N.; Qu, X. Ultrasensitive and selective detection of a prognostic indicator in early-stage cancer using graphene oxide and carbon nanotubes. Adv. Funct. Mater. 2010, 20, 3967–3971. [Google Scholar] [CrossRef]
- Lu, C.-H.; Li, J.; Zhang, X.-L.; Zheng, A.-X.; Yang, H.-H.; Chen, X.; Chen, G.-N. General approach for monitoring peptide-protein interactions based on graphene-peptide complex. Anal. Chem. 2011, 83, 7276–7282. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Guo, L.; Wang, L.; Li, F.; Lu, J.; Ga, O.J.; Fan, C.; Huang, Q. A graphene oxide-based fluorescent biosensor for the analysis of peptide-receptor interactions and imaging in somatostatin receptor subtype 2 overexpressed tumor cells. Anal. Chem. 2013, 85, 7732–7737. [Google Scholar]
- Long, F.; Zhu, A.; Shi, H.; Wang, H. Hapten-grafted graphene as a transducer for homogeneous competitive immunoassay of small molecules. Anal. Chem. 2014, 86, 2862–2866. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xia, N.; Zhang, J.; Mao, W.; Wu, Y.; Ge, X. A graphene oxide-based fluorescent platform for selective detection of amyloid-β oligomers. Anal. Methods 2015, 7, 8727–8732. [Google Scholar] [CrossRef]
- Gu, X.; Yang, G.; Zhang, G.; Zhang, D.; Zhu, D. A new fluorescence turn-on assay for trypsin and inhibitor screening based on graphene oxide. ACS Appl. Mater. Interface 2011, 3, 1175–1179. [Google Scholar] [CrossRef] [PubMed]

| Materials | Methods | Detection Limit | Linear Range | Reference |
|---|---|---|---|---|
| SPAAB-HRP/anti-hCG | ELISA | 0.012 mIU/mL | - | [3] |
| anti-hCG/ZnO | PL | 2 ng/mL | 2–20 ng/mL | [6] |
| anti-hCG/CdSe-ZnS QDs | PL | 0.5 mIU/mL | - | [7] |
| anti-hCG/AuNPs | IGCA | 5 ng/mL | 10–600 ng/mL | [5] |
| anti-hCG/gold film | SPR | <500 ng/mL | - | [8] |
| peptide aptamer | LC | 1 IU/mL | 12.5–100 mIU/mL | [2] |
| PF@SiO2-Ab2 and Fe3O4@PANI-Ab1 | fluorescence | 3 pg/mL | 0.01–100 ng/mL | [4] |
| peptide aptamer/AuNPs/4-nitrophenol | colorimetry | 15 mIU/mL | 15–750 mIU/mL | [26] |
| peptide aptamer/AuNPs | colorimetry | 25 mIU/mL | 25–1000 mIU/mL | [27] |
| anti-hCG/Au-MWCNTs/GS/GCE | DPV | 0.0026 mIU/mL | 0.005–500 mIU/mL | [13] |
| anti-hCG/AuE | SWSV | 15 pM | 15–300 pM | [10] |
| HRP-Ab2/hCG/Ab1/nafion/GCE | CA | 11.2 mIU/mL | 200 mIU/mL | [9] |
| anti-hCG/CS/graphene-SPE | EIS | 0.016 ng/mL | 0.1–25 ng/mL | [19] |
| anti-HCG/ FPD/GCE | EIS | 0.03 ng/mL | 0.1–10 ng/mL | [14] |
| anti-hCG/Pd@SBA-15/TH/HSO3-GS/GCE | CV | 8.60 pg/mL | 0.01–16.00 ng/mL | [20] |
| GCE/GS/NPG/anti-hCG | CV | 0.034 ng/mL | 0.5–40.00 ng/mL | [11] |
| hCG/HRP-anti-hCG/sol–gel/GE | DPV | 0.3 mIU/mL | 0.5–50 mIU/mL | [17] |
| Peptide aptamer/GO | fluorescence | 20 mIU/mL | 0.05–20 IU/mL | This work |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, N.; Wang, X.; Liu, L. A Graphene Oxide-Based Fluorescent Method for the Detection of Human Chorionic Gonadotropin. Sensors 2016, 16, 1699. https://doi.org/10.3390/s16101699
Xia N, Wang X, Liu L. A Graphene Oxide-Based Fluorescent Method for the Detection of Human Chorionic Gonadotropin. Sensors. 2016; 16(10):1699. https://doi.org/10.3390/s16101699
Chicago/Turabian StyleXia, Ning, Xin Wang, and Lin Liu. 2016. "A Graphene Oxide-Based Fluorescent Method for the Detection of Human Chorionic Gonadotropin" Sensors 16, no. 10: 1699. https://doi.org/10.3390/s16101699





