Lead-Free Piezoelectric Diaphragm Biosensors Based on Micro-Machining Technology and Chemical Solution Deposition
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the 0.82KNN-0.18AN Piezoelectric Layer
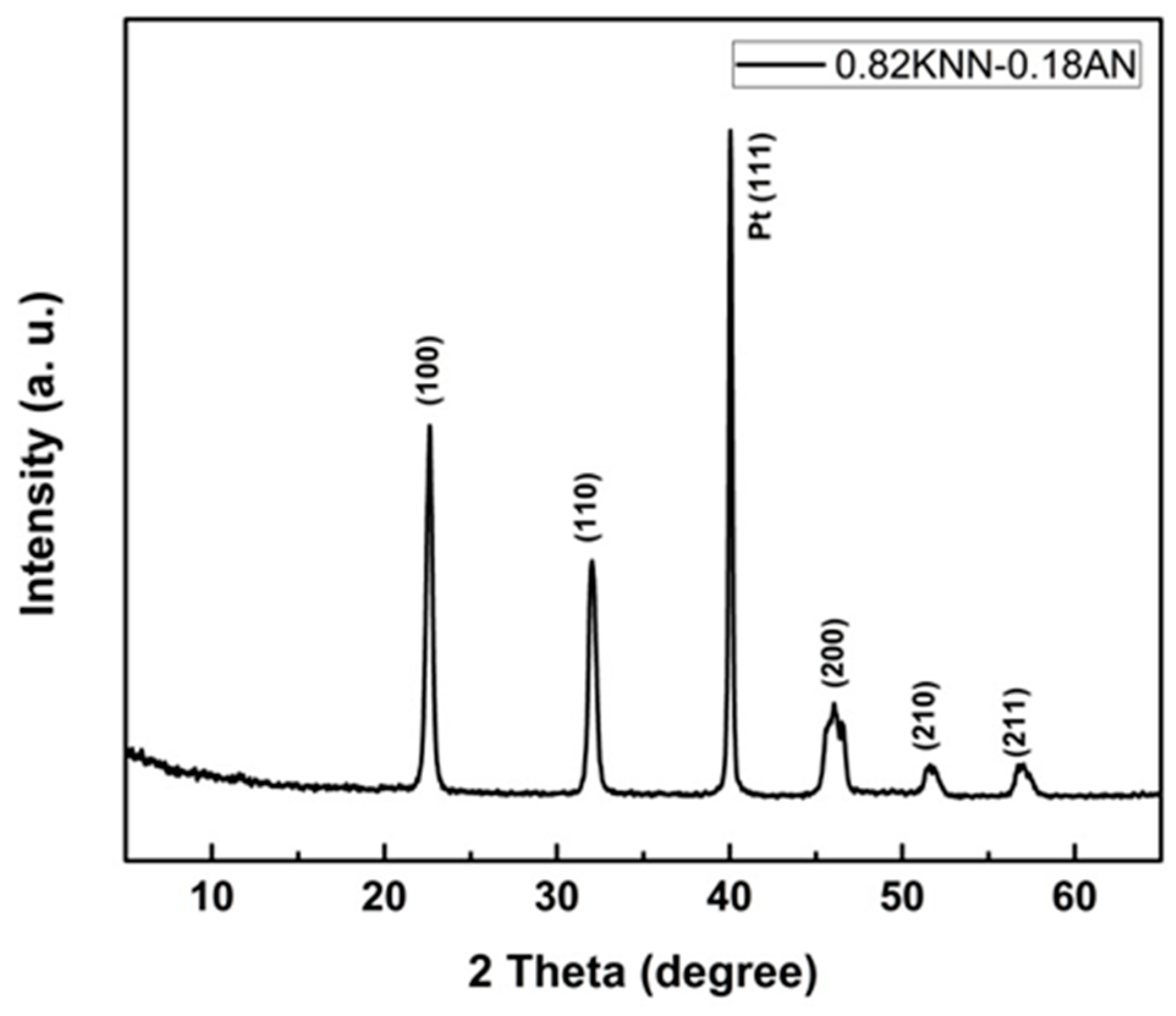

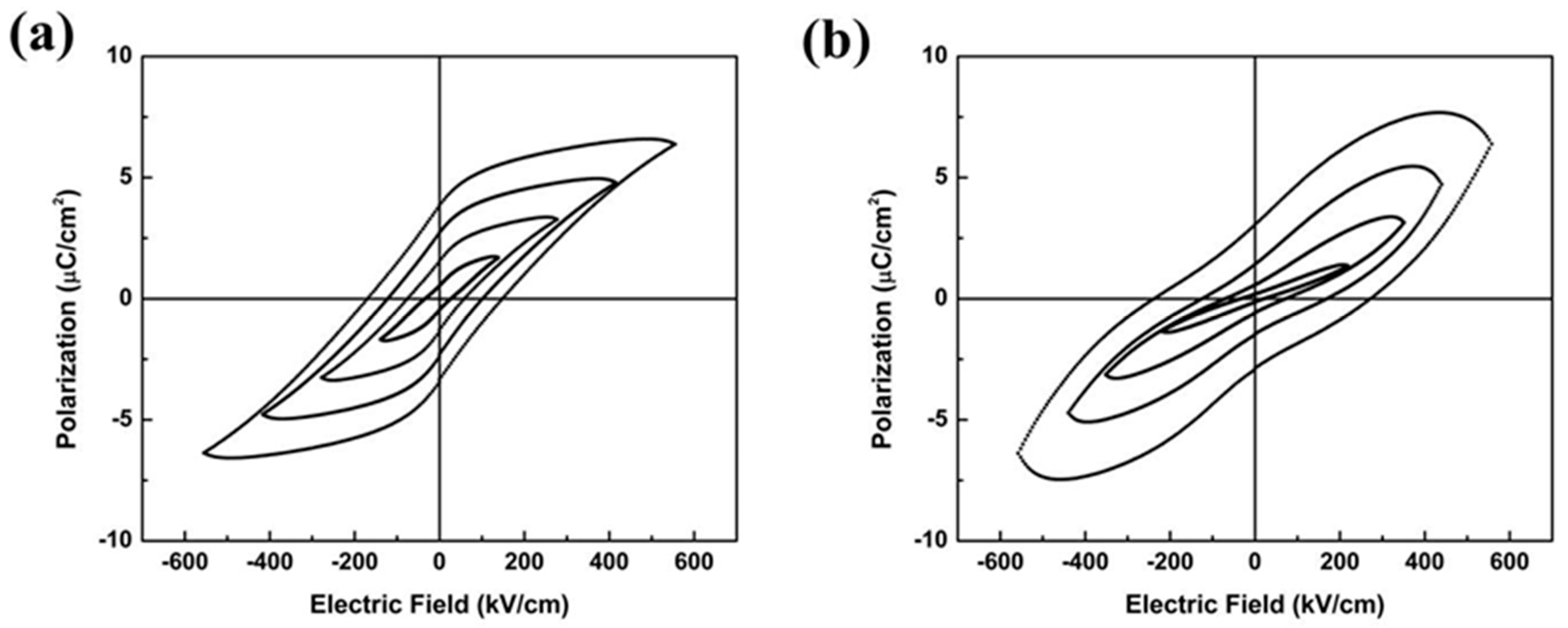
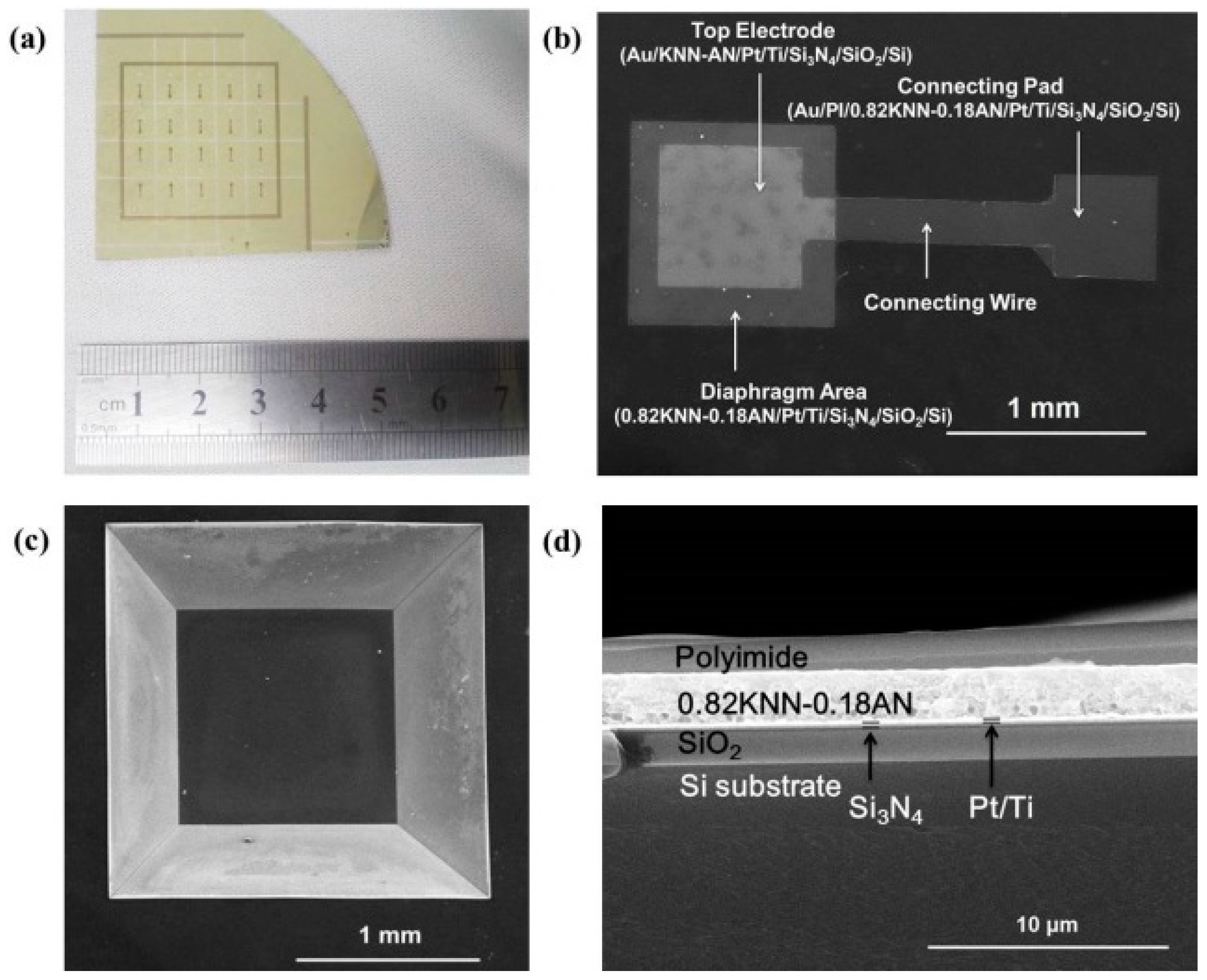
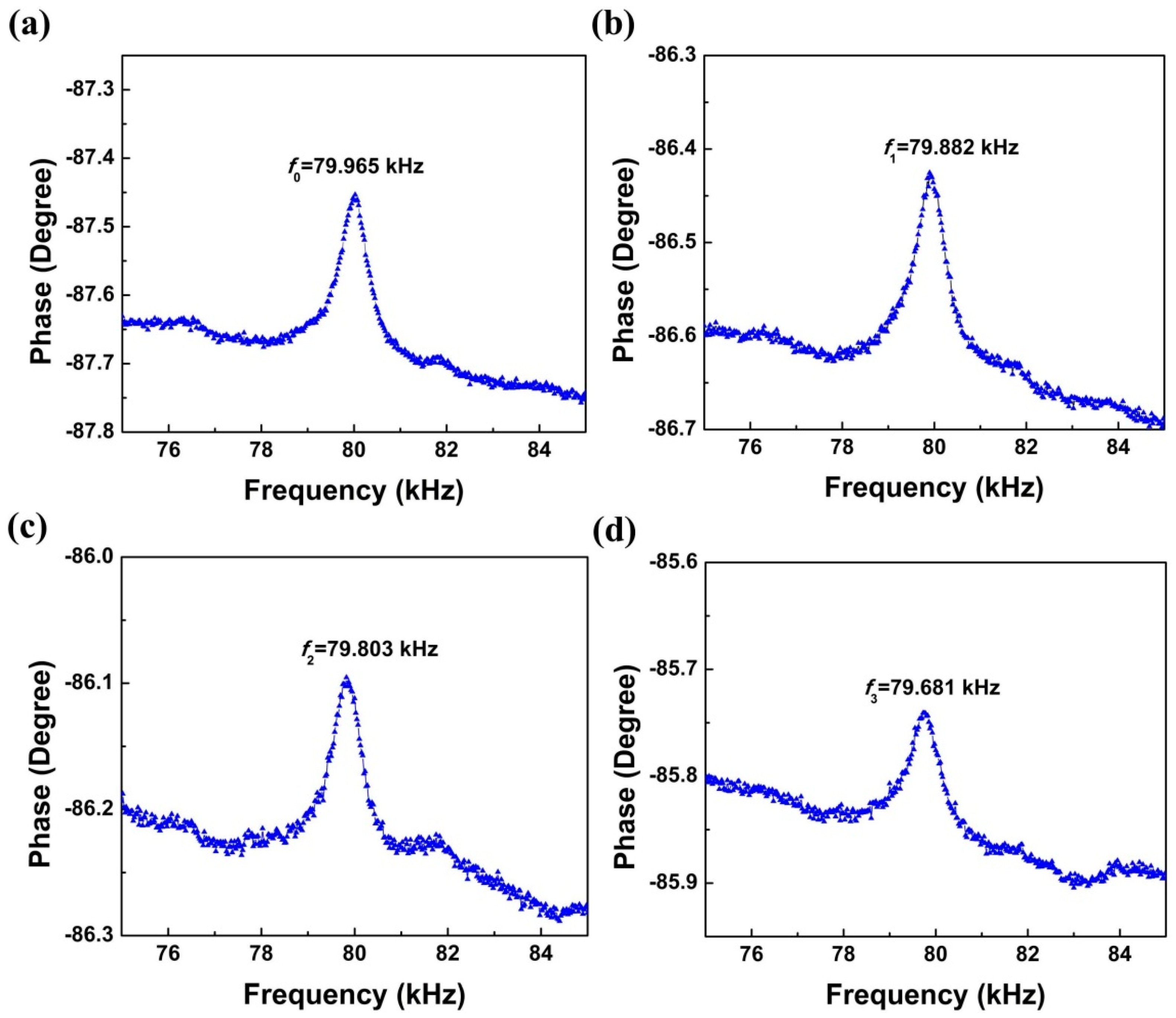
2.2. Characterization of the Piezoelectric Diaphragm Biosensor
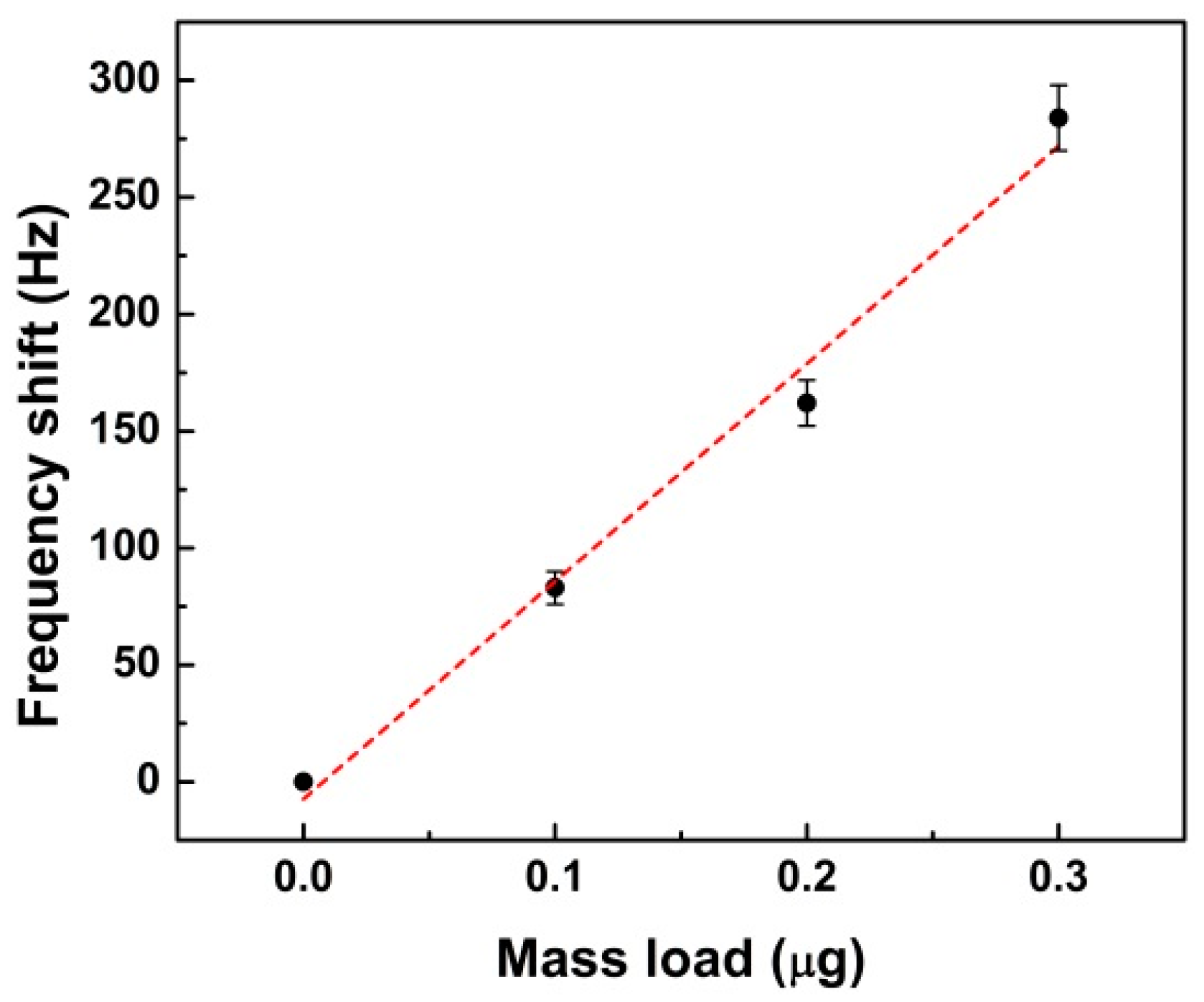
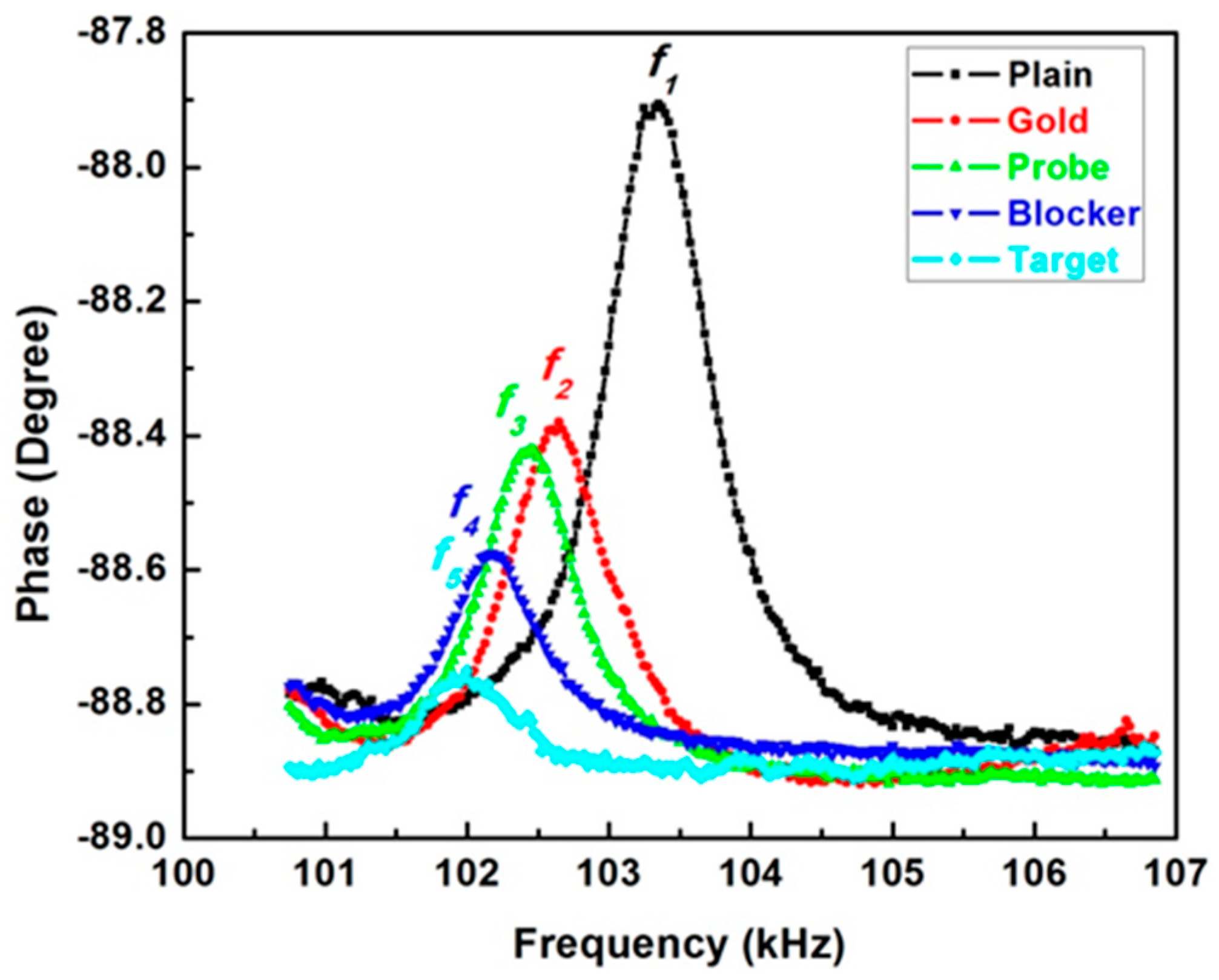

3. Materials and Methods
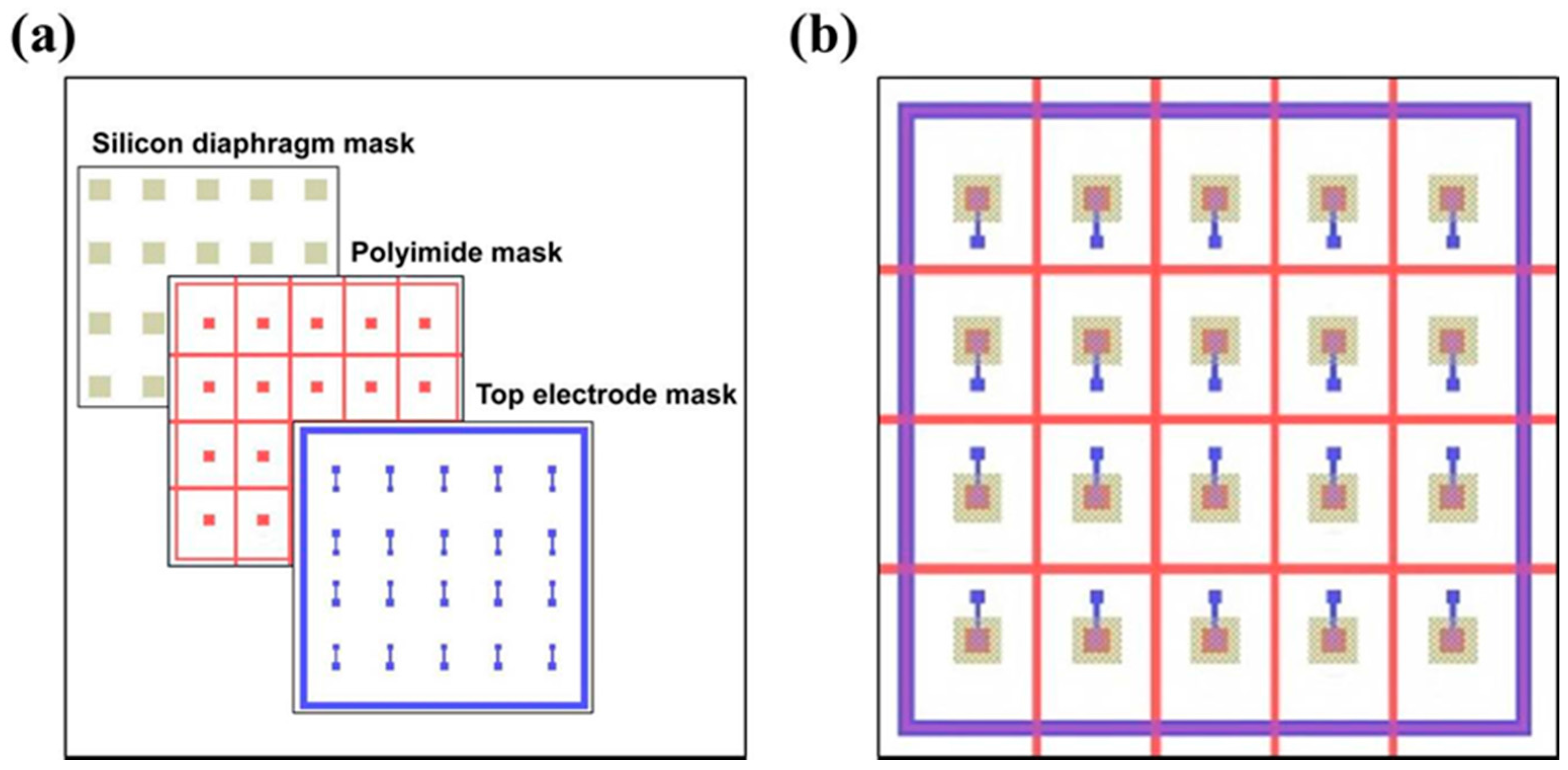
3.1. Design of Device Structure and Sensing Principle
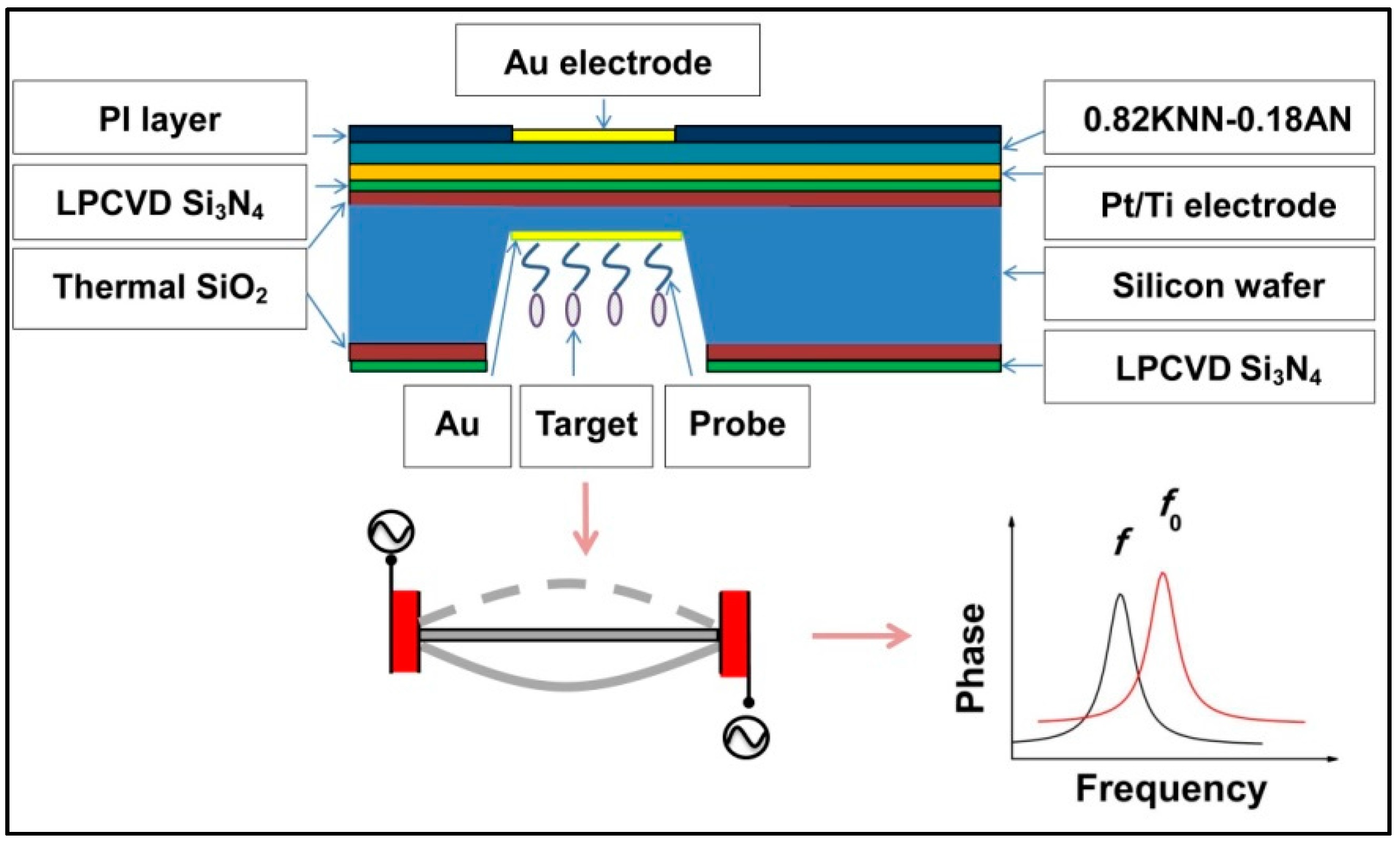
3.2. Fabrication of the Piezoelectric Diaphragm Biosensor
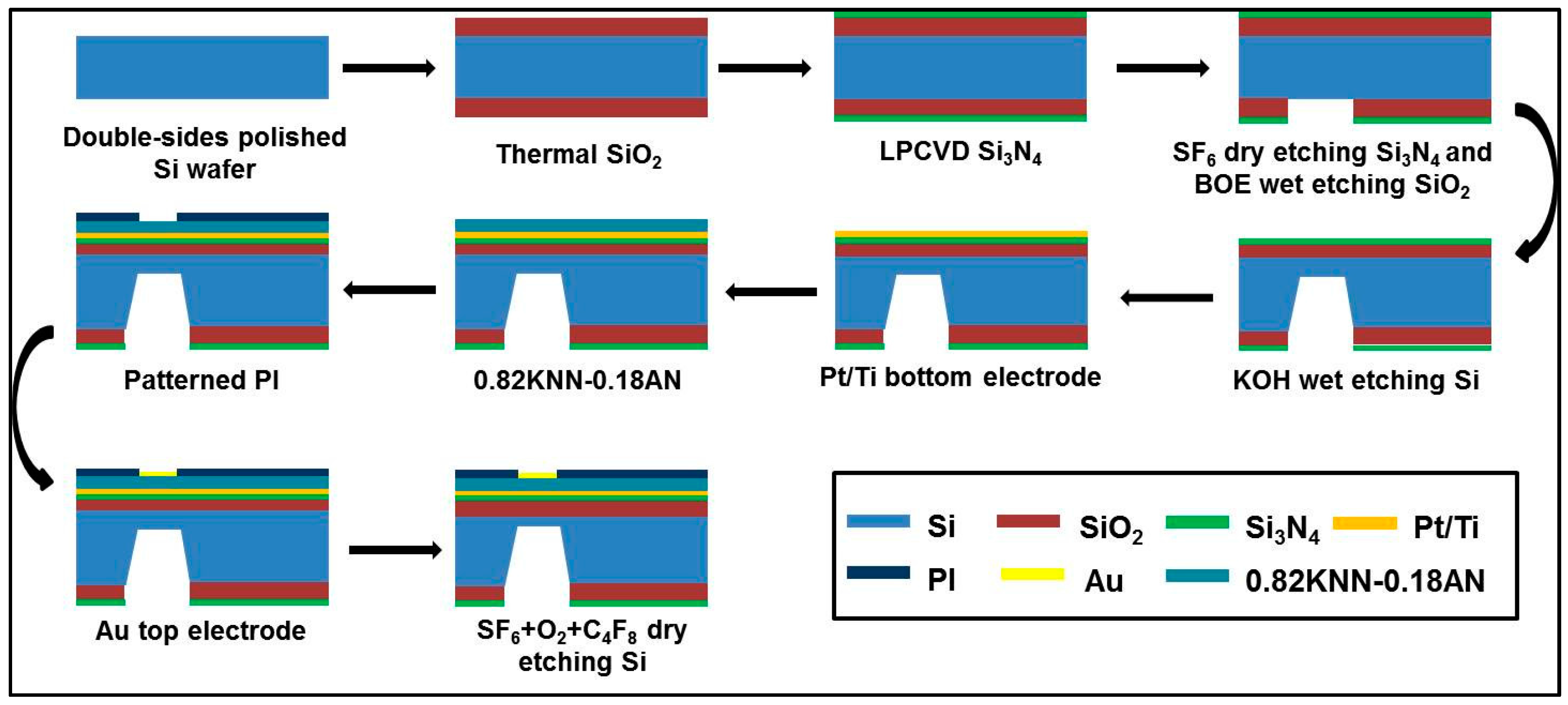
3.3. Measurements of the Resonant Frequencies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mehrabani, S.; Maker, A.J.; Armani, A.M. Hybrid Integrated Label-Free Chemical and Biological Sensors. Sensors 2014, 14, 5890–5928. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Cho, H.; Park, H.J.; Kwon, D.; Lee, J.M.; Chung, B.H. Nanogap biosensors for electrical and label-free detection of biomolecular interactions. Nanotechnology 2009, 20, 455502. [Google Scholar] [CrossRef] [PubMed]
- Esfandyarpour, R.; Javanmard, M.; Koochak, Z.; Esfandyarpour, H.; Harris, J.S.; Davis, R.W. Label-free electronic probing of nucleic acids and proteins at the nanoscale using the nanoneedle biosensor. Biomicrofluidics 2013, 7, 044114. [Google Scholar] [CrossRef] [PubMed]
- Esfandyarpour, R.; Esfandyarpour, H.; Javanmard, M.; Harris, J.S.; Davis, R.W. Microneedle biosensor: A method for direct label-free real time protein detection. Sens. Actuators B Chem. 2013, 177, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Goda, T.; Miyahara, Y. Label-free and reagent-less protein biosensing using aptamer-modified extended-gate field-effect transistors. Biosens. Bioelectron. 2013, 45, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Goda, T.; Tabata, M.; Sanjoh, M.; Uchimura, M.; Iwasaki, Y.; Miyahara, Y. Thiolated 2-methacryloyloxyethyl phosphorylcholine for an antifouling biosensor platform. Chem. Commun. 2013, 49, 8683–8685. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.R.; Skoog, S.A.; Edwards, T.L.; Lopez, D.M.; Wheeler, D.R.; Arango, D.C.; Xiao, X.; Brozik, S.M.; Wang, J.; Polsky, R.; et al. Multiplexed microneedle-based biosensor array for characterization of metabolic acidosis. Talanta 2012, 88, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, M.T.; Yang, Y.C.; Shih, M.H.; Chang, Y.C. Optimization of High-Q Coupled Nanobeam Cavity for Label-Free Sensing. Sensors 2015, 15, 25868–25881. [Google Scholar] [CrossRef]
- Yang, Z.P.; Liu, X.; Zhang, C.J.; Liu, B.Z. A high-performance nonenzymatic piezoelectric sensor based on molecularly imprinted transparent TiO2 film for detection of urea. Biosens. Bioelectron. 2015, 74, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.A.; Blaszykowski, C.; Bokhari, S.; Thompson, S. Ultra-high frequency piezoelectric aptasensor for the label-free detection of cocaine. Biosens. Bioelectron. 2015, 72, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, S.J. A lipid-based method for the preparation of a piezoelectric DNA biosensor. Anal. Biochem. 2014, 458, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.Z.; Chen, C.J.; Shie, D.T.; Liu, J.T. Resonant efficiency improvement design of piezoelectric biosensor for bacteria gravimetric sensing. Biomed. Mater. Eng. 2014, 24, 3597–3604. [Google Scholar] [PubMed]
- Su, L.; Zou, L.; Fong, C.C.; Wong, W.L.; Wei, F.; Wong, K.Y.; Wu, R.S.; Yang, M. Detection of cancer biomarkers by piezoelectric biosensor using PZT ceramic resonator as the transducer. Biosens. Bioelectron. 2013, 46, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Alava, T.; Mathieu, F.; Rameil, P.; Morel, Y.; Soyer, C.; Remiens, D.; Nicu, L. Piezoelectric-actuated, piezoresistive-sensed circular micromembranes for label-free biosensing applications. Appl. Phys. Lett. 2010, 97, 093703. [Google Scholar] [CrossRef]
- Wang, Z.; Miao, J.; Zhu, W. Micromachined ultrasonic transducers and arrays based on piezoelectric thick film. Appl. Phys. A 2008, 91, 107–117. [Google Scholar] [CrossRef]
- Wang, Z.; Miao, J.; Zhu, W. Piezoelectric thick films and their application in MEMS. J. Eur. Ceram. Soc. 2007, 27, 3759–3764. [Google Scholar] [CrossRef]
- Laser, D.J.; Santiago, J.G. A review of micropumps. J. Micromech. Microeng. 2004, 14, R35–R64. [Google Scholar] [CrossRef]
- Perçin, G.K.; Yakub, K.; Butrus, T. Micromachined droplet ejector arrays for controlled ink-jet printing and deposition. Rev. Sci. Instrum. 2002, 73, 2193. [Google Scholar] [CrossRef]
- Hao, R.Z.; Song, H.B.; Zuo, G.M.; Yang, R.F.; Wei, H.P.; Wang, D.B.; Cui, Z.Q.; Zhang, Z.; Cheng, Z.X.; Zhang, X.E. DNA probe functionalized QCM biosensor based on gold nanoparticle amplification for Bacillus anthracis detection. Biosens. Bioelectron. 2011, 26, 3398–3404. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Zhu, T.; Tang, J.; Wu, R.; Chen, Q.; Chen, M.; Zhang, B.; Huang, J.; Fu, W. Hybridization assay of hepatitis B virus by QCM peptide nucleic acid biosensor. Biosens. Bioelectron. 2008, 23, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, S.; Panigrahi, S.; Mallik, S. Olfactory receptor based piezoelectric biosensors for detection of alcohols related to food safety applications. Sens. Actuators B Chem. 2011, 155, 8–18. [Google Scholar] [CrossRef]
- Johnson, B.N.; Mutharasan, R. Biosensing using dynamic-mode cantilever sensors: A review. Biosens. Bioelectron. 2012, 32, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Sindhu, A.; Dilbaghi, N.; Chaudhury, A. Biosensors as innovative tools for the detection of food borne pathogens. Biosens. Bioelectron. 2011, 28, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, Y.; Borg, G.; Li, C.M. Design of a novel disposable piezoelectric co-polymer diaphragm based biosensor unit. Mater. Sci. Eng. C 2011, 31, 95–98. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Z.; Miao, J.; Yu, L.; Li, C.M. Micro-machined piezoelectric membrane-based immunosensor array. Biosens. Bioelectron. 2008, 24, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.H.; Lee, J.H. Self-actuating biosensor using a piezoelectric cantilever and its optimization. J. Phys. Conf. Ser. 2006, 34, 362–367. [Google Scholar] [CrossRef]
- Nicu, L.; Guirardel, M.; Chambosse, F.; Rougerie, P.; Hinh, S.; Trevisiol, E.; Francois, J.; Majoral, J.; Caminade, A.; Cattan, E.; et al. Resonating piezoelectric membranes for microelectromechanically based bioassay: Detection of streptavidin-gold nanoparticles interaction with biotinylated DNA. Sens. Actuators B Chem. 2005, 110, 125–136. [Google Scholar] [CrossRef]
- Olfatnia, M.; Singh, V.R.; Xu, T.; Miao, J.M.; Ong, L.S. Analysis of the vibration modes of piezoelectric circular microdiaphragms. J. Micromech. Microeng. 2010, 20, 085013. [Google Scholar] [CrossRef]
- Zhao, B.; Hu, J.; Ren, W.; Xu, F.; Wu, X.; Shi, P.; Ye, Z. A new biosensor based on PVDF film for detection of nucleic acids. Ceram. Int. 2015, 41, S602–S606. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Ren, W.; Shi, P.; Ye, Z. Preparation and characterization of sodium potassium niobate-silver niobate lead-free films by chemical solution deposition. Ceram. Int. 2015, 41, S228–S233. [Google Scholar] [CrossRef]
- Xu, T.; Miao, J.; Wang, Z.; Yu, L.; Li, C.M. Micro-piezoelectric immunoassay chip for simultaneous detection of Hepatitis B virus and α-fetoprotein. Sens. Actuators B Chem. 2011, 151, 370–376. [Google Scholar] [CrossRef]
- Olfatnia, M.; Xu, T.; Ong, L.S.; Miao, J.M.; Wang, Z.H. Investigation of residual stress and its effects on the vibrational characteristics of piezoelectric-based multilayered microdiaphragms. J. Micromech. Microeng. 2010, 20, 015007. [Google Scholar] [CrossRef]
- Xin, Y.; Li, Z.; Odum, L.; Cheng, Z.Y.; Xu, Z. Piezoelectric diaphragm as a high performance biosensor platform. Appl. Phys. Lett. 2006, 89, 223508. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wu, X.; Shi, P.; Ye, Z.-G. Lead-Free Piezoelectric Diaphragm Biosensors Based on Micro-Machining Technology and Chemical Solution Deposition. Sensors 2016, 16, 69. https://doi.org/10.3390/s16010069
Li X, Wu X, Shi P, Ye Z-G. Lead-Free Piezoelectric Diaphragm Biosensors Based on Micro-Machining Technology and Chemical Solution Deposition. Sensors. 2016; 16(1):69. https://doi.org/10.3390/s16010069
Chicago/Turabian StyleLi, Xiaomeng, Xiaoqing Wu, Peng Shi, and Zuo-Guang Ye. 2016. "Lead-Free Piezoelectric Diaphragm Biosensors Based on Micro-Machining Technology and Chemical Solution Deposition" Sensors 16, no. 1: 69. https://doi.org/10.3390/s16010069





