Blood Group Typing: From Classical Strategies to the Application of Synthetic Antibodies Generated by Molecular Imprinting †
Abstract
:1. Introduction
2. Classical Strategies in BG Typing
2.1. Slide Method
2.2. Tube Test
2.3. Microplate Technology
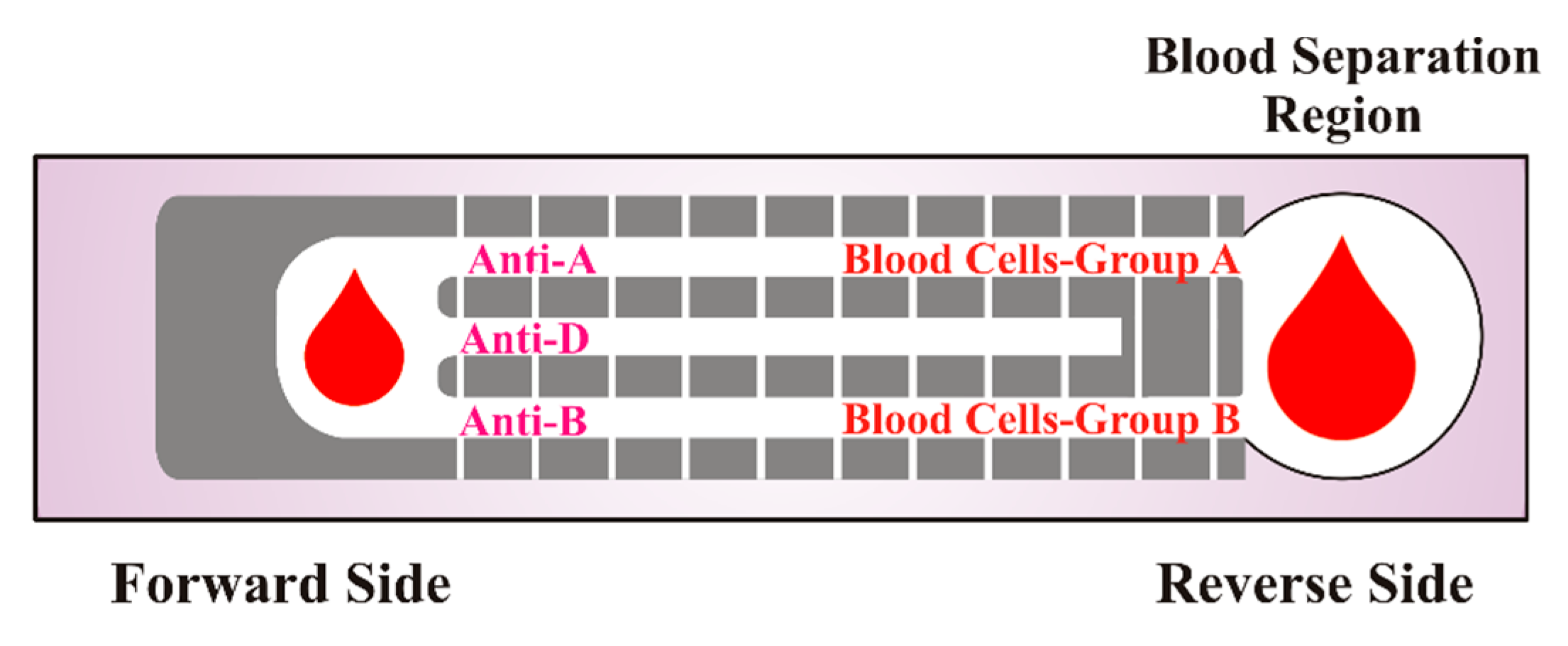
2.4. Column/Gel Centrifugation
3. Synthetic Receptors Generated by Molecular Imprinting for ABO Blood Typing
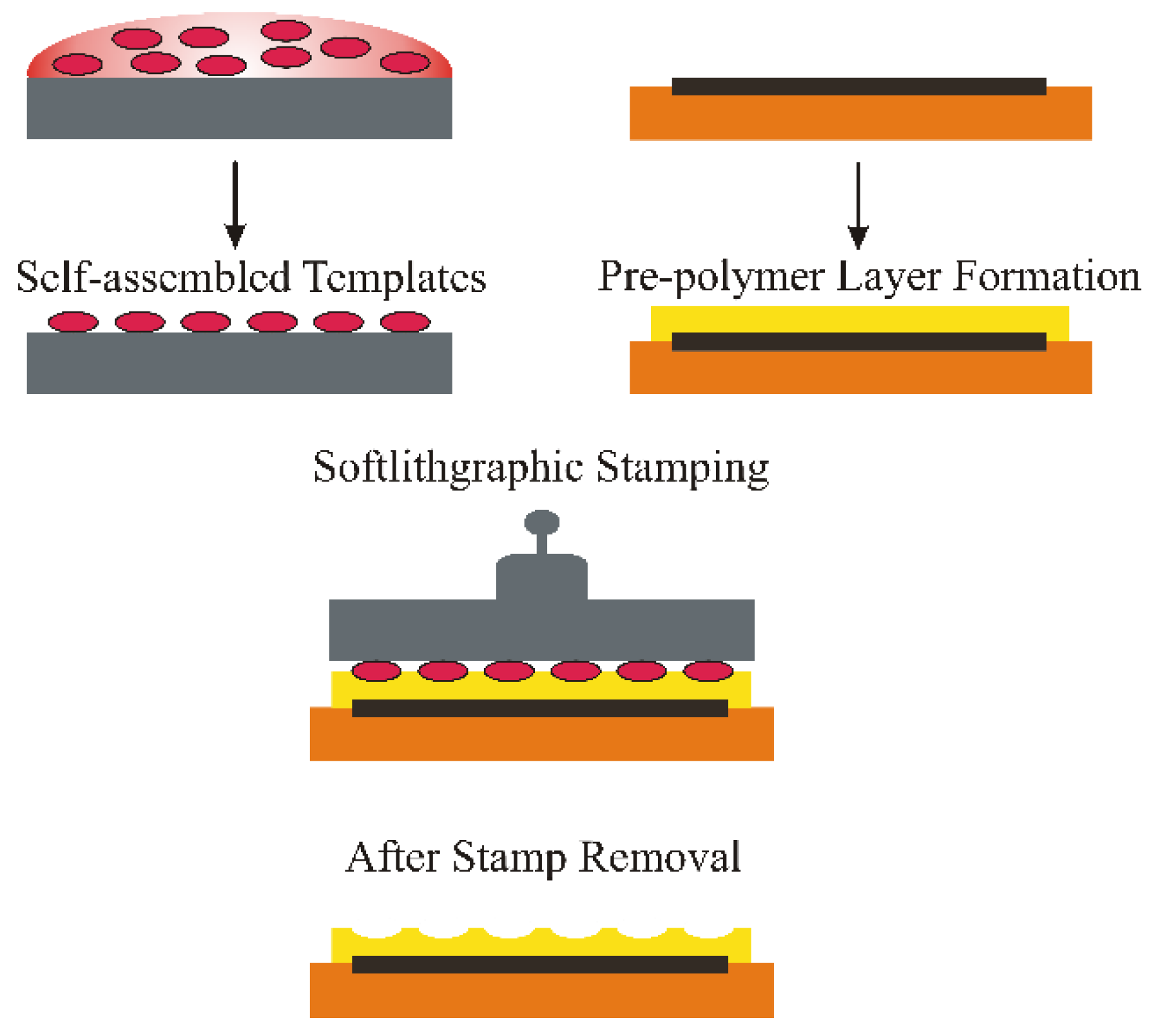
Surface Imprinting of Erythrocytes
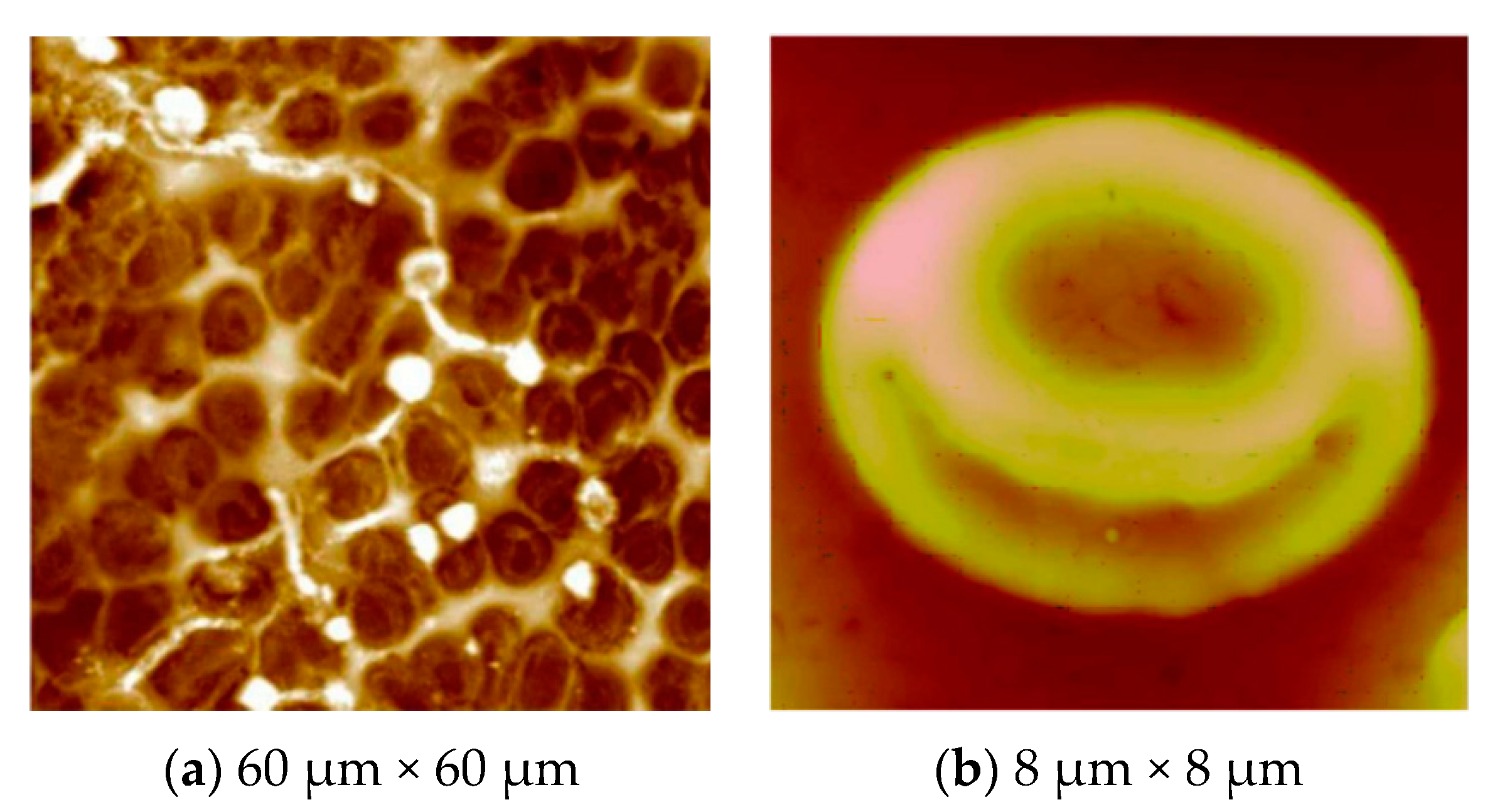
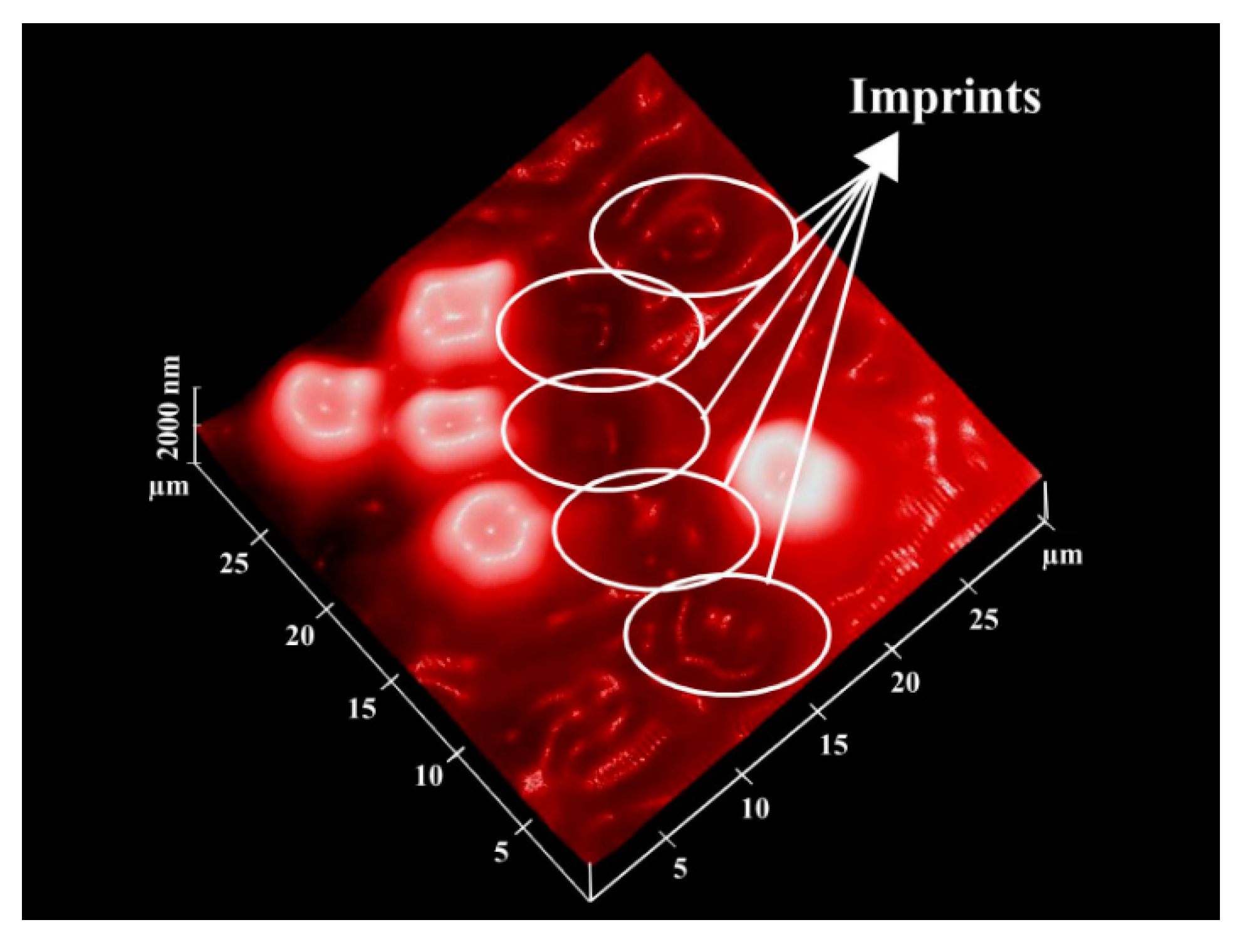
4. ABO Blood Group Typing
4.1. Synthetic Receptors for ABO Blood Group Sensors
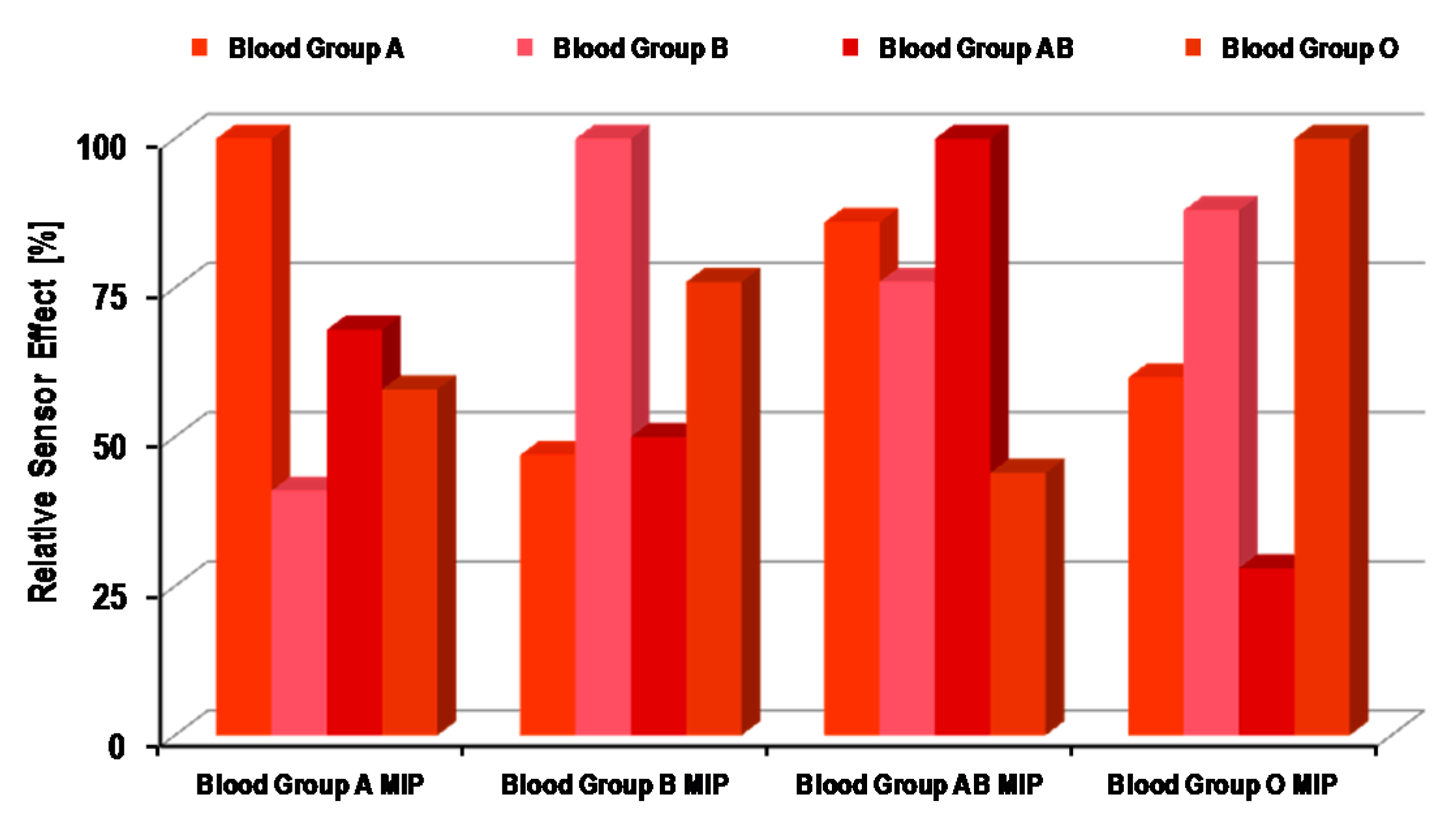

4.2. Natural Receptors for ABO Blood Group Sensors
4.3. Paper-Based Diagnostics for ABO Blood Group Typing
| Blood Group | Number of Samples | Accuracy | Discrepancy | Success Rate (%) |
|---|---|---|---|---|
| A | 12 | 11 | 1 | 92 |
| B | 13 | 11 | 2 | 85 |
| O | 14 | 13 | 1 | 93 |
| AB | 9 | 8 | 1 | 89 |
| Rh | 48 | 46 | 2 | 96 |
4.4. Emerging Strategies in Blood Typing
5. Limitations and Challenges
| Test | Principle | Intended for | Analysis Time | Applicability | Remarks |
|---|---|---|---|---|---|
| Slide | Agglutination | Simple blood group detection | 10 min | Hospitals, clinical laboratories | Least sensitive, but low cost; requires small volume; useful for rapid results |
| Tube | Agglutination | Blood group detection and antibody screening | 10–30 min | Hospitals, clinical laboratories | Intermediate sensitivity and time consuming |
| Microplate | Agglutination | Blood group detection and antibody screening | 10–30 min | Hospitals, clinical laboratories | Fast and highly sensitive |
| Column/gel filtration | Agglutination | Blood group detection and antibody screening | 10–45 min | Hospitals, clinical laboratories | Highly sensitive, but time consuming |
| Molecular blood typing | Nucleic acid amplification methods e.g., PCR-SSP | Blood group difficult to identify by serological methods | >1 h | Used as a supplementary tool with classical methods | Highly sensitive, but a lengthy and tedious procedure |
| Natural antibody sensors (QCM, SPR) | Blood group-specific antibodies, e.g., ( IgM) reaction with antigen | Blood group-associated antigens | 15–30 min | Limited and confined to research laboratories | Sensitive, but expensive; difficult to regenerate |
| Synthetic antibody sensors (QCM) | Geometrical, as well as chemical adherence of RBCs on a synthetic antibody surface | ABO blood-group typing, sub-blood group detection | 10–20 min | Promising, but yet to be established for commercial use | Highly sensitive; easy to regenerate; low cost; useful for several analysis |
| Blood test kits | Lateral assay | Multiparameter identification of blood groups | 5 min | Commercially established and validated | Fast and reliable; suitable for emergency diagnosis |
6. Outlook
Conflicts of Interest
References
- Landsteiner, K. Zur Kenntnis der antifermentativen lytischen and agglutinierenden Wirkung des Blutserums and der lymph. Zentralbl. Bakteriol. Parasit. Infekt. 1900, 27, 357–362. [Google Scholar]
- Landsteiner, K. Ueber Agglutinationserscheinungen normalen menschlichen Blutes. Wien. Klin. Wochenschr. 1901, 14, 1132–1134. [Google Scholar]
- Liu, Z.; Liu, M.; Mercado, T.; Illoh, O.; Davey, R. Extended blood group molecular typing and next-generation sequencing. Transfus. Med. Rev. 2014, 28, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Rowley, M.; Milkins, C. Laboratory aspects of blood transfusion. In Dacie and Lewis Practical Haematology, 10th ed.; Lewis, S.M., Barbara, J.B., Imelda, B., Eds.; Churchill Livingstone: Philadelphia, PA, USA, 2006; pp. 523–554. [Google Scholar]
- Westhoff, C.M.; Reid, M.E. Chapter 6—Abo and related antigens and antibodies. In Blood Banking and Transfusion Medicine, 2nd ed.; Hillyer, C.D., Silberstein, L.E., Ness, P.M., Anderson, K.C., Roback, J.D., Eds.; Churchill Livingstone: Philadelphia, PA, USA, 2007; pp. 69–79. [Google Scholar]
- Malomgre, W.; Neumeister, B. Recent and future trends in blood group typing. Anal. Bioanal. Chem. 2009, 393, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Lee, S.H.; Ahn, C.H.; Lee, J.Y.; Kwon, T.H. Disposable integrated microfluidic biochip for blood typing by plastic microinjection moulding. Lab Chip 2006, 6, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Heucke, U.W.; Cobet, U. Blood group typing by ultrasound backscattering—Quantitative measurements of agglutinates and their shear-dependent behavior. Instrum. Sci. Technol. 2000, 28, 311–321. [Google Scholar] [CrossRef]
- Dolmashkin, A.A.; Dubrovskii, V.A.; Zabenkov, I.V. Blood group typing based on recording the elastic scattering of laser radiation using the method of digital imaging. Quantum Electron. 2012, 42, 409–416. [Google Scholar] [CrossRef]
- Doubrovski, V.A.; Dolmashkin, A.A. Human blood group typing based on digital photographs of RBC agglutination process. Opt. Spectrosc. 2010, 109, 263–267. [Google Scholar] [CrossRef]
- Karpasitou, K.; Drago, F.; Crespiatico, L.; Paccapelo, C.; Truglio, F.; Frison, S.; Scalamogna, M.; Poli, F. Blood group genotyping for Jka/Jkb, Fya/Fyb, S/s, K/k, Kpa/Kpb, Jsa/Jsb, Coa/Cob, and Lua/Lub with microarray beads. Transfusion 2008, 48, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, A.; Lieberzeit, P.A.; Dickert, F.L. Chemical sensors based on molecularly imprinted sol-gel materials. Materials 2010, 3, 2196–2217. [Google Scholar] [CrossRef]
- Wagner, T.; Maris, R.J.; Ackermann, H.-J.; Otto, R.; Beging, S.; Poghossian, A.; Schöning, M.J. Handheld measurement device for field-effect sensor structures: Practical evaluation and limitations. Sens. Actuators B Chem. 2007, 127, 217–223. [Google Scholar] [CrossRef]
- Mujahid, A.; Afzal, A.; Glanzing, G.; Leidl, A.; Lieberzeit, P.A.; Dickert, F.L. Imprinted sol–gel materials for monitoring degradation products in automotive oils by shear transverse wave. Anal. Chim. Acta 2010, 675, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Seidler, K.; Lieberzeit, P.A.; Dickert, F.L. Application of yeast imprinting in biotechnology and process control. Analyst 2009, 134, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Pichon, V.; Chapuis-Hugon, F. Role of molecularly imprinted polymers for selective determination of environmental pollutants—A review. Anal. Chim. Acta 2008, 622, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Piletsky, S.A.; Turner, N.W.; Laitenberger, P. Molecularly imprinted polymers in clinical diagnostics—Future potential and existing problems. Med. Eng. Phys. 2006, 28, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K.; Mosbach, K. Plastic antibodies: Developments and applications. Trends Biotechnol. 1998, 16, 468–475. [Google Scholar] [CrossRef]
- Ye, L.; Mosbach, K. Molecular imprinting: Synthetic materials as substitutes for biological antibodies and receptors. Chem. Mater. 2008, 20, 859–868. [Google Scholar] [CrossRef]
- Schirhagl, R.; Latif, U.; Dickert, F.L. Atrazine detection based on antibody replicas. J. Mater. Chem. 2011, 21, 14594–14598. [Google Scholar] [CrossRef]
- Dai, H.; Xiao, D.; He, H.; Li, H.; Yuan, D.; Zhang, C. Synthesis and analytical applications of molecularly imprinted polymers on the surface of carbon nanotubes: A review. Microchim. Acta 2015, 182, 893–908. [Google Scholar] [CrossRef]
- Sharma, P.S.; Iskierko, Z.; Pietrzyk-Le, A.; D'Souza, F.; Kutner, W. Bioinspired intelligent molecularly imprinted polymers for chemosensing: A mini review. Electrochem. Commun. 2015, 50, 81–87. [Google Scholar] [CrossRef]
- Schirhagl, R.; Podlipna, D.; Lieberzeit, P.A.; Dickert, F.L. Comparing biomimetic and biological receptors for insulin sensing. Chem. Comm. 2010, 46, 3128–3130. [Google Scholar] [CrossRef] [PubMed]
- Piletska, E.; Piletsky, S.; Karim, K.; Terpetschnig, E.; Turner, A. Biotin-specific synthetic receptors prepared using molecular imprinting. Anal. Chim. Acta 2004, 504, 179–183. [Google Scholar] [CrossRef] [Green Version]
- Shinkai, S.; Takeuchi, M. Molecular design of synthetic receptors with dynamic, imprinting, and allosteric functions. Biosens. Bioelectron. 2004, 20, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K.; Mosbach, K. Molecularly imprinted polymers and their use in biomimetic sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Whitcombe, M.J.; Chianella, I.; Larcombe, L.; Piletsky, S.A.; Noble, J.; Porter, R.; Horgan, A. The rational development of molecularly imprinted polymer-based sensors for protein detection. Chem. Soc. Rev. 2011, 40, 1547–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.; Yang, G.; Chen, Y. Rapid and efficient chiral separation of nateglinide and its l-enantiomer on monolithic molecularly imprinted polymers. J. Chromatogr. A 2005, 1090, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.X.; Gao, H.J.; Zhang, L.M.; Chen, X.Q.; Qiao, X.G. The biomimetic immunoassay based on molecularly imprinted polymer: A comprehensive review of recent progress and future prospects. J. Food Sci. 2011, 76, R69–R75. [Google Scholar] [CrossRef] [PubMed]
- Sellergren, B.; Allender, C.J. Molecularly imprinted polymers: A bridge to advanced drug delivery. Adv. Drug Deliver. Rev. 2005, 57, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Lieberzeit, P.; Dickert, F. Rapid bioanalysis with chemical sensors: Novel strategies for devices and artificial recognition membranes. Anal. Bioanal. Chem. 2008, 391, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Wulff, G. Molecular imprinting in cross-linked materials with the aid of molecular templates—A way towards artificial antibodies. Angew. Chem. Int. Ed. 1995, 34, 1812–1832. [Google Scholar] [CrossRef]
- Defreese, J.L.; Katz, A. Synthesis of a confined class of chiral organic catalysts via bulk imprinting of silica. Chem. Mater. 2005, 17, 6503–6506. [Google Scholar] [CrossRef]
- Ge, Y.; Turner, A.P.F. Too large to fit? Recent developments in macromolecular imprinting. Trend. Biotechnol. 2008, 26, 218–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayden, O.; Dickert, F.L. Selective microorganism detection with cell surface imprinted polymers. Adv. Mater. 2001, 13, 1480–1483. [Google Scholar] [CrossRef]
- Nishino, H.; Huang, C.-S.; Shea, K.J. Selective protein capture by epitope imprinting. Angew. Chem. Int. Ed. 2006, 45, 2392–2396. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Lieberzeit, P.A.; Gazda-Miarecka, S.; Halikias, K.; Schirk, C.; Kauling, J.; Dickert, F.L. Imprinting as a versatile platform for sensitive materials—Nanopatterning of the polymer bulk and surfaces. Sens. Actuators B Chem. 2005, 111–112, 259–263. [Google Scholar] [CrossRef]
- Mujahid, A.; Iqbal, N.; Afzal, A. Bioimprinting strategies: From soft lithography to biomimetic sensors and beyond. Biotechnol. Adv. 2013, 31, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Dickert, F.L.; Lieberzeit, P.; Gazda-Miarecka, S.; Halikias, K.; Mann, K.-J. Modifying polymers by self-organisation for the mass-sensitive detection of environmental and biogeneous analytes. Sens. Actuators B Chem. 2004, 100, 112–116. [Google Scholar] [CrossRef]
- Dickert, F.L.; Lieberzeit, P.; Miarecka, S.G.; Mann, K.J.; Hayden, O.; Palfinger, C. Synthetic receptors for chemical sensors—Subnano- and micrometre patterning by imprinting techniques. Biosens. Bioelectron. 2004, 20, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Lieberzeit, P.; Glanznig, G.; Jenik, M.; Gazda-Miarecka, S.; Dickert, F.; Leidl, A. Softlithography in chemical sensing—Analytes from molecules to cells. Sensors 2005, 5, 509–518. [Google Scholar] [CrossRef]
- Alizadeh, T. Comparison of different methodologies for integration of molecularly imprinted polymer and electrochemical transducer in order to develop a paraoxon voltammetric sensor. Thin Solid Films 2010, 518, 6099–6106. [Google Scholar] [CrossRef]
- Jenik, M.; Seifner, A.; Krassnig, S.; Seidler, K.; Lieberzeit, P.A.; Dickert, F.L.; Jungbauer, C. Sensors for bioanalytes by imprinting—Polymers mimicking both biological receptors and the corresponding bioparticles. Biosens. Bioelectron. 2009, 25, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Dickert, F.; Hayden, O. Bioimprinting of polymers and sol-gel phases. Selective detection of yeasts with imprinted polymers. Anal. Chem. 2002, 74, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K.; Noworyta, K.; Kutner, W. Imprinted polymer-based enantioselective acoustic sensor using a quartz crystal microbalance. Anal. Commun. 1999, 36, 391–393. [Google Scholar] [CrossRef]
- Dickert, F.L.; Lieberzeit, P.; Hayden, O. Sensor strategies for microorganism detection—From physical principles to imprinting procedures. Anal. Bioanal. Chem. 2003, 377, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Hayden, O.; Mann, K.-J.; Krassnig, S.; Dickert, F.L. Biomimetic ABO blood-group typing. Angew. Chem. Int. Ed. 2006, 45, 2626–2629. [Google Scholar] [CrossRef] [PubMed]
- Seifner, A.; Lieberzeit, P.; Jungbauer, C.; Dickert, F.L. Synthetic receptors for selectively detecting erythrocyte ABO subgroups. Anal. Chim. Acta 2009, 651, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Brouard, D.; Ratelle, O.; Perreault, J.; Boudreau, D.; St-Louis, M. Pcr-free blood group genotyping using a nanobiosensor. Vox Sang. 2015, 108, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Nanameki, K.; Ushijima, H.; Akase, S.; Matsumoto, T.; Kamata, S. The rapid measurement of ABO blood type by using surface-plasmon resonance sensor. Bunseki Kagaku 1999, 48, 669–672. [Google Scholar] [CrossRef]
- Quinn, J.G.; O'Kennedy, R.; Smyth, M.; Moulds, J.; Frame, T. Detection of blood group antigens utilising immobilised antibodies and surface plasmon resonance. J. Immunol. Methods 1997, 206, 87–96. [Google Scholar] [CrossRef]
- Kimura, S.; Yurugi, K.; Segawa, H.; Kuroda, J.; Sato, K.; Nogawa, M.; Yuasa, T.; Egawa, H.; Tanaka, K.; Maekawa, T. Rapid quantitation of immunoglobulin G antibodies specific for blood group antigens a and b by surface plasmon resonance. Transfusion 2005, 45, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Stussi, G.; Huggel, K.; Lutz, H.U.; Schanz, U.; Rieben, R.; Seebach, J.D. Isotype-specific detection of abo blood group antibodies using a novel flow cytometric method. Brit. J. Haematol. 2005, 130, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Hayashiba, K.; Sakai, M.; Kamio, K.; Hayashiba, Y.; Tazaki, M. Identification of blood by using quartz-crystal microbalance covered with antibody IgG. Bull. Fac. Eng. Kyushu Sangyo Univ. 2001, 38, 169–172. [Google Scholar]
- Tessier, L.; Patat, F.; Schmitt, N.; Lethiecq, M.; Frangin, Y.; Guilloteau, D. Significance of mass and viscous loads discrimination for an at-quartz blood group immunosensor. Sens. Actuators B Chem. 1994, 19, 698–703. [Google Scholar] [CrossRef]
- Krupin, O.; Wang, C.; Berini, P. Selective capture of human red blood cells based on blood group using long-range surface plasmon waveguides. Biosens. Bioelectron. 2014, 53, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Then, W.L.; Li, L.; Shen, W. Paper-based device for rapid typing of secondary human blood groups. Anal. Bioanal. Chem. 2014, 406, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Al-Tamimi, M.; Shen, W.; Zeineddine, R.; Tran, H.; Garnier, G. Validation of paper-based assay for rapid blood typing. Anal. Chem. 2012, 84, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Noiphung, J.; Talalak, K.; Hongwarittorrn, I.; Pupinyo, N.; Thirabowonkitphithan, P.; Laiwattanapaisal, W. A novel paper-based assay for the simultaneous determination of Rh typing and forward and reverse ABO blood groups. Biosens. Bioelectron. 2015, 67, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Then, W.; Li, M.; McLiesh, H.; Shen, W.; Garnier, G. The detection of blood group phenotypes using paper diagnostics. Vox Sang. 2015, 108, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Knowles, S.; Regan, F. Blood cell antigens and antibodies: Erythrocytes, platelets, and granulocytes. In Dacie and Lewis Practical Haematology, 10th ed.; Lewis, S.M., Barbara, J.B., Imelda, B., Eds.; Churchill Livingstone: Philadelphia, PA, USA, 2006; pp. 481–522. [Google Scholar]
- Westhoff, C.M. Molecular DNA based blood group typing. In Transfusion Medicine and Hemostasis, 2nd ed.; Beth, H.S., Christopher, D.H., Charles, S.A., Mikhail, R., Eds.; Elsevier: San Diego, CA, USA, 2013; pp. 141–148. [Google Scholar]
- Prager, M. Molecular genetic blood group typing by the use of PCR-SSP technique. Transfusion 2007, 47, 54S–59S. [Google Scholar] [CrossRef] [PubMed]
- Gassner, C.; Meyer, S.; Frey, B.M.; Vollmert, C. Matrix-assisted laser desorption/ionisation, time-of-flight mass spectrometry–based blood group genotyping—The alternative approach. Transfus. Med. Rev. 2013, 27, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, G.; Shariff, T.; Seul, M.; Vissavajjhala, P.; Hue-Roye, K.; Charles-Pierre, D.; Lomas-Francis, C.; Chaudhuri, A.; Reid, M.E. A flexible array format for large-scale, rapid blood group DNA typing. Transfusion 2005, 45, 680–688. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mujahid, A.; Dickert, F.L. Blood Group Typing: From Classical Strategies to the Application of Synthetic Antibodies Generated by Molecular Imprinting. Sensors 2016, 16, 51. https://doi.org/10.3390/s16010051
Mujahid A, Dickert FL. Blood Group Typing: From Classical Strategies to the Application of Synthetic Antibodies Generated by Molecular Imprinting. Sensors. 2016; 16(1):51. https://doi.org/10.3390/s16010051
Chicago/Turabian StyleMujahid, Adnan, and Franz L. Dickert. 2016. "Blood Group Typing: From Classical Strategies to the Application of Synthetic Antibodies Generated by Molecular Imprinting" Sensors 16, no. 1: 51. https://doi.org/10.3390/s16010051






