A Machine Learning Framework for Gait Classification Using Inertial Sensors: Application to Elderly, Post-Stroke and Huntington’s Disease Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Acquisition Protocol
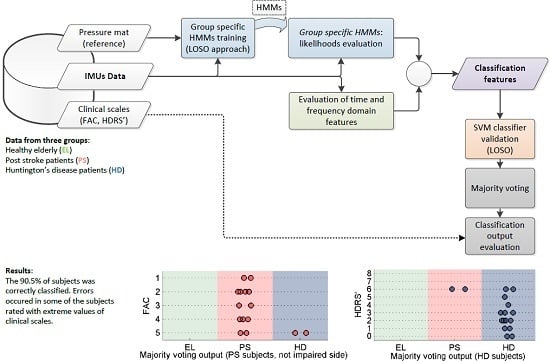
2.3. Gait Classification Strategy
2.3.1. Classification Using Group-Specific HMM Likelihood

2.3.2. SVM Classifier
| HMM-Based Features | H1. Log-likelihood, EL model (limited to a 2-s window) | |
| H2. Log-likelihood, PS model | ||
| H3. Log-likelihood, HD model | ||
| H4. Difference between log-likelihoods given EL and PS models (for all available data) | ||
| H5. Difference between log-likelihoods given EL and HD models | ||
| H6. Difference between log-likelihoods given PS and HD models | ||
| Time Domain Features | T1. Mean value | Evaluated for each channel (84 features) |
| T2. Standard deviation | ||
| T3. Variance | ||
| T4. Maximum | ||
| T5. Minimum | ||
| T6. Range | ||
| Frequency Domain Features | F1. Power at first dominant frequency (P1) | |
| F2. Power at second dominant frequency | ||
| F3. First dominant frequency | ||
| F4. Second dominant frequency | ||
| F5. Total power (PT) | ||
| F6. P1/PT | ||
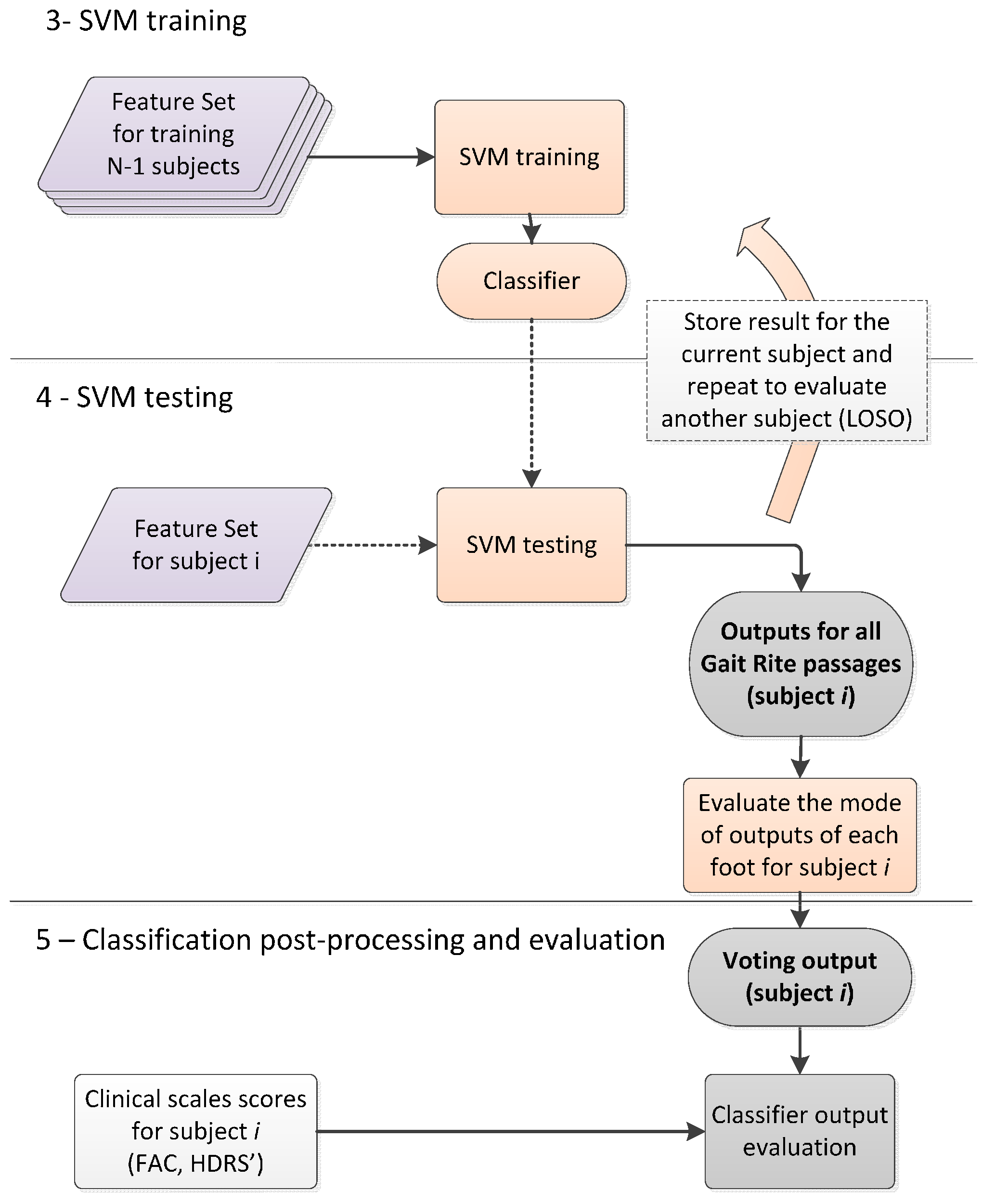
2.3.3. Classification Post-Processing with Majority Voting
2.4. Data Analysis
3. Results
| Group | Number of Passages | Number of Strides Per Passage | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | Total | Min | Max | Mean | SD | Total | |
| EL | 6 | 15 | 12.4 | 3.1 | 124 | 2 | 5 | 3.5 | 0.7 | 862 |
| PS | 2 | 11 | 6.3 | 3.1 | 95 | 3 | 24 | 6.0 | 3.6 | 1144 |
| HD | 4 | 16 | 10.1 | 3.4 | 171 | 2 | 21 | 4.3 | 2.6 | 1467 |
| Overall | 2 | 16 | 9.3 | 4.0 | 390 | 2 | 24 | 4.5 | 2.7 | 3473 |
Gait Classification
| Classification Output | ||||
|---|---|---|---|---|
| EL | PS | HD | ||
| 1. HMM-based information (maximum log-likelihood) | ||||
| Actual Label | EL | 176 (71%) | 0 (0%) | 72 (29%) |
| PS–not imp. side | 0 (0%) | 16 (16.8%) | 79 (83.2%) | |
| PS–imp. side | 0 (0%) | 51 (53.7%) | 44 (46.3%) | |
| HD | 60 (17.5%) | 5 (1.5%) | 277 (81%) | |
| Overall accuracy 66.7% of mat passages | ||||
| 2A. SVM classifier (HMM-based features only) | ||||
| Actual Label | EL | 168 (67.7%) | 50 (20.2%) | 30 (12.1%) |
| PS–not imp. side | 11 (11.6%) | 69 (72.6%) | 15 (15.8%) | |
| PS–imp. side | 0 (0%) | 93 (97.9%) | 2 (2.1%) | |
| HD | 55 (16.1%) | 59 (17.3%) | 228 (66.7%) | |
| Overall accuracy 71.5% of mat passages | ||||
| 2B. SVM classifier (time and frequency domain features only) | ||||
| Actual Label | EL | 182 (73.4%) | 0 (0%) | 66 (26.6%) |
| PS–not imp. side | 3 (3.2%) | 57 (60%) | 35 (36.8%) | |
| PS–imp. side | 1 (1.1%) | 78 (82.1%) | 16 (16.8%) | |
| HD | 63 (18.4%) | 37 (10.8%) | 242 (70.8%) | |
| Overall accuracy 71.7% of mat passages | ||||
| 2C. SVM classifier (all available features) | ||||
| Actual label | EL | 159 (64.1%) | 48 (19.4%) | 41 (16.5%) |
| PS–not imp. side | 0 (0%) | 71 (74.7%) | 24 (25.3%) | |
| PS–imp. side | 0 (0%) | 88 (92.6%) | 7 (7.4%) | |
| HD | 39 (11.4%) | 49 (14.3%) | 254 (74.3%) | |
| Overall accuracy 73.3% of mat passages | ||||
| Classification Output | ||||
|---|---|---|---|---|
| EL | PS | HD | ||
| 1. HMM-based information (maximum log-likelihood) | ||||
| Actual Label | EL | 7 (70%) | 0 (0%) | 3 (30%) |
| PS–not imp. side | 0 (0%) | 5 (33.3%) | 10 (66.7%) | |
| PS–imp. side | 0 (0%) | 10 (66.7%) | 5 (33.3%) | |
| HD | 1 (5.9%) | 0 (0%) | 16 (94.1%) | |
| Overall accuracy 76.2% of subjects | ||||
| 2A. SVM classifier (HMM-based features only) | ||||
| Actual Label | EL | 9 (90%) | 0 (0%) | 1 (10%) |
| PS–not imp. side | 1 (6.7%) | 13 (86.7%) | 1 (6.7%) | |
| PS–imp. side | 0 (0%) | 15 (100%) | 0 (0%) | |
| HD | 1 (5.9%) | 4 (23.5%) | 12 (70.6%) | |
| Overall accuracy 85.7% of subjects | ||||
| 2B. SVM classifier (time and frequency domain features only) | ||||
| Actual Label | EL | 8 (80%) | 0 (0%) | 2 (20%) |
| PS–not imp. side | 0 (0%) | 13 (86.7%) | 2 (13.3%) | |
| PS–imp. side | 0 (0%) | 14 (93.3%) | 1 (6.7%) | |
| HD | 3 (17.6%) | 0 (0%) | 14 (82.4%) | |
| Overall accuracy 83.3% of subjects | ||||
| 2C. SVM classifier (all available features) | ||||
| Actual label | EL | 9 (90%) | 1 (10%) | 0 (0%) |
| PS–not imp. side | 0 (0%) | 13 (86.7%) | 2 (13.3%) | |
| PS–imp. side | 0 (0%) | 15 (100%) | 0 (0%) | |
| HD | 0 (0%) | 2 (11.8%) | 15 (88.2%) | |
| Overall accuracy 90.5% of subjects | ||||
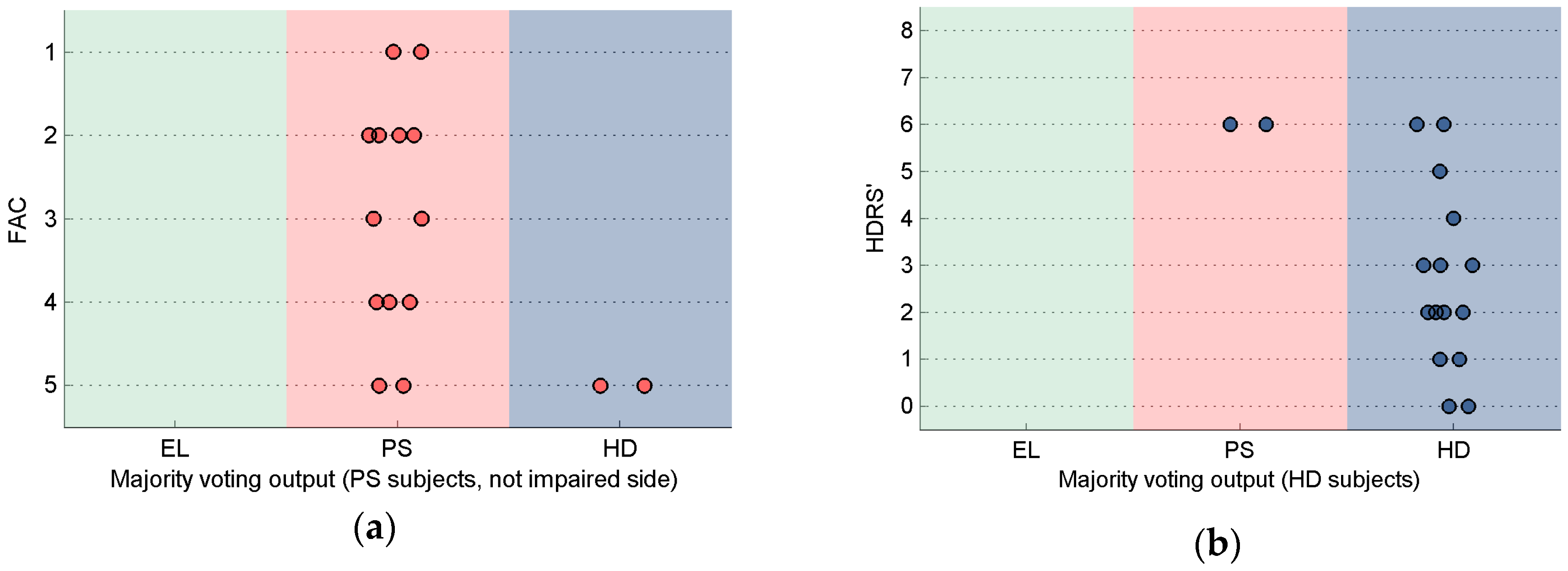
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bonato, P. Wearable sensors and systems. IEEE Eng. Med. Biol. Mag. 2010, 29, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Aminian, K.; Trevisan, C.; Najafi, B.; Dejnabadi, H.; Frigo, C.; Pavan, E.; Telonio, A.; Cerati, F.; Marinoni, E.; Robert, P. Evaluation of an ambulatory system for gait analysis in hip osteoarthritis and after total hip replacement. Gait Post. 2004, 20, 102–107. [Google Scholar] [CrossRef]
- Long, J.D.; Paulsen, J.S.; Marder, K.; Zhang, Y.; Kim, J.I.; Mills, J.A. Tracking motor impairments in the progression of Huntington’s disease. Mov. Disord. 2014, 29, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Giladi, N.; Horak, F.B.; Hausdorff, J.M. Classification of gait disturbances: Distinguishing between continuous and episodic changes. Mov. Disord. 2013, 28, 1469–1473. [Google Scholar] [CrossRef] [PubMed]
- Rueterbories, J.; Spaich, E.G.; Larsen, B.; Andersen, O.K. Methods for gait event detection and analysis in ambulatory systems. Med. Eng. Phys. 2010, 32, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Mâsse, L.C.; Tilert, T.; McDowell, M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Trojaniello, D.; Cereatti, A.; Pelosin, E.; Avanzino, L.; Mirelman, A.; Hausdorff, J.M.; Della, C.U. Estimation of step-by-step spatio-temporal parameters of normal and impaired gait using shank-mounted magneto-inertial sensors: Application to elderly, hemiparetic, parkinsonian and choreic gait. J. Neuroeng. Rehabil. 2014, 11, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Mannini, A.; Sabatini, A.M. Gait phase detection and discrimination between walking-jogging activities using hidden Markov models applied to foot motion data from a gyroscope. Gait Post. 2012, 36, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Pfau, T.; Ferrari, M.; Parsons, K.; Wilson, A. A hidden Markov model-based stride segmentation technique applied to equine inertial sensor trunk movement data. J. Biomech. 2008, 41, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Taborri, J.; Rossi, S.; Palermo, E.; Patanè, F.; Cappa, P. A Novel HMM distributed classifier for the detection of gait phases by means of a wearable inertial sensor network. Sensors 2014, 14, 16212–16234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guenterberg, E.; Yang, A.Y.; Ghasemzadeh, H.; Jafari, R.; Bajcsy, R.; Sastry, S.S. A Method for Extracting Temporal Parameters Based on Hidden Markov Models in Body Sensor Networks with Inertial Sensors. IEEE Tran. Inf. Technol. Biomed. 2009, 13, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, B.; Xu, Y. Human Abnormal Gait Modeling via Hidden Markov Model. In Proceedings of the International Conference on Information Acquisition (ICIA’07), Seogwipo-si, Korea, 8–11 July 2007; pp. 517–522.
- Bae, J.; Tomizuka, M. Gait phase analysis based on a Hidden Markov Model. Mechatronics 2011, 21, 961–970. [Google Scholar] [CrossRef]
- Abaid, N.; Cappa, P.; Palermo, E.; Petrarca, M.; Porfiri, M. Gait detection in children with and without hemiplegia using single-axis wearable gyroscopes. PLoS ONE 2013, 8, 731–752. [Google Scholar] [CrossRef] [PubMed]
- Mannini, A.; Trojaniello, D.; Della Croce, U.; Sabatini, A.M. Hidden Markov Model-Based Strategy for Gait Segmentation using Inertial Sensors: Application to Elderly, Hemiparetic Patients and Huntington’s Disease Patients. In Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Milan, Italy, 25–29 August 2015; pp. 5179–5182.
- Mannini, A.; Sabatini, A.M. Walking speed estimation using foot-mounted inertial sensors: Comparing machine learning and strap-down integration methods. Med. Eng. Phys. 2014, 36, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; Jallon, P. Hidden Markov Models Applied onto Gait Classification. In Proceedings of the 18th European Signal Processing Conference (EUSIPCO-2010), Aalborg, Denmark, 23–27 August 2010; pp. 929–933.
- Schlömer, T.; Poppinga, B.; Henze, N.; Boll, S. Gesture Recognition with a Wii Controller. In Proceedings of the 2nd International Conference on Tangible and Embedded Interaction, Bonn, Germany, 18–20 February 2008; pp. 11–14.
- Nickel, C.; Busch, C.; Rangarajan, S.; Mobius, M. Using Hidden Markov Models for Accelerometer-Based Biometric Gait Recognition. In Proceedings of the IEEE 7th International Colloquium on Signal Processing and its Applications (CSPA), Penang, Malaysia, 4–6 March 2011; pp. 58–63.
- Lakany, H. Extracting a diagnostic gait signature. Patt. Recogn. 2008, 41, 1627–1637. [Google Scholar] [CrossRef]
- Begg, R.K.; Palaniswami, M.; Owen, B. Support vector machines for automated gait classification. IEEE Trans. Biomed. Eng. 2005, 52, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Pogorelc, B.; Bosnić, Z.; Gams, M. Automatic recognition of gait-related health problems in the elderly using machine learning. Multimedia Tools Appl. 2012, 58, 333–354. [Google Scholar] [CrossRef]
- Mannini, A.; Sabatini, A.M. Machine Learning Methods for Classifying Human Physical Activity from On-Body Accelerometers. Sensors 2010, 10, 1154–1175. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Patten, C.; Kothari, D.H.; Zajac, F.E. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Post. 2005, 22, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Koller, W.C.; Trimble, J. The gait abnormality of Huntington’s disease. Neurology 1985, 35, 1450–1450. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, J.-T.; Novak, A.C.; Brouwer, B.; Li, Q. Estimation of spatio-temporal parameters for post-stroke hemiparetic gait using inertial sensors. Gait Post. 2013, 37, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.K.; Quinn, L.; Marder, K.S. Reliability of spatiotemporal gait outcome measures in Huntington’s disease. Mov. Disord. 2005, 20, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Dalton, A.; Khalil, H.; Busse, M.; Rosser, A.; van Deursen, R.; ÓLaighin, G. Analysis of gait and balance through a single triaxial accelerometer in presymptomatic and symptomatic Huntington’s disease. Gait Post. 2013, 37, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.K.; Gill, K.M.; Magliozzi, M.R.; Nathan, J.; Piehl-Baker, L. Clinical gait assessment in the neurologically impaired reliability and meaningfulness. Phys. Ther. 1984, 64, 35–40. [Google Scholar] [PubMed]
- Kremer, H.; Group, H.S. Unified Huntington’s disease rating scale: Reliability and consistency. Mov. Disord. 1996, 11, 136–142. [Google Scholar]
- Palermo, E.; Rossi, S.; Marini, F.; Patanè, F.; Cappa, P. Experimental evaluation of accuracy and repeatability of a novel body-to-sensor calibration procedure for inertial sensor-based gait analysis. Measurement 2014, 52, 145–155. [Google Scholar] [CrossRef]
- Picerno, P.; Cereatti, A.; Cappozzo, A. A spot check for assessing static orientation consistency of inertial and magnetic sensing units. Gait Post. 2011, 33, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Picerno, P.; Cereatti, A.; Cappozzo, A. Joint kinematics estimate using wearable inertial and magnetic sensing modules. Gait Post. 2008, 28, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Favre, J.; Aissaoui, R.; Jolles, B.; de Guise, J.; Aminian, K. Functional calibration procedure for 3D knee joint angle description using inertial sensors. J. Biomech. 2009, 42, 2330–2335. [Google Scholar] [CrossRef] [PubMed]
- Esterman, M.; Tamber-Rosenau, B.J.; Chiu, Y.-C.; Yantis, S. Avoiding non-independence in fMRI data analysis: Leave one subject out. NeuroImage 2010, 50, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Mannini, A.; Genovese, V.; Sabatini, A.M. Online Decoding of Hidden Markov Models for Gait Event Detection Using Foot-Mounted Gyroscopes. IEEE J. Biomed. Health Inf. 2014, 18, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Mannini, A.; Sabatini, A.M. A smartphone-centered wearable sensor network for fall risk assessment in the elderly. In Proceedings of the 10th EAI International Conference on Body Area Networks, Sydney, Australia, 28–30 September 2015; pp. 471–483.
- Rabiner, L.R. A tutorial on HMM and selected applications inspeech recognition. IEEE Proc. 1989, 77, 257–286. [Google Scholar] [CrossRef]
- Mannini, A.; Sabatini, A.M. Accelerometry-based classification of human activities using Markov modeling. Comput. Intell. Neurosci. 2011, 2011, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Vapnik, V. The Nature of Statistical Learning Theory; Springer-Verlag: New York, NY, USA, 2000. [Google Scholar]
- Chang, C.-C.; Lin, C.-J. LIBSVM: A library for support vector machines. ACM Trans. Intell. Syst. Technol. 2011, 2, 1–27. [Google Scholar] [CrossRef]
- Mannini, A.; Intille, S.S.; Rosenberger, M.; Sabatini, A.M.; Haskell, W. Activity recognition using a single accelerometer placed at the wrist or ankle. Med. Sci. Sports Exerc. 2013, 45, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Duin, R.P.W.; Mao, J. Statistical pattern recognition: A review. IEEE Trans. Patt. Anal. Mach. Intell. 2000, 22, 4–37. [Google Scholar] [CrossRef]
- Balasubramanian, C.K.; Neptune, R.R.; Kautz, S.A. Variability in spatiotemporal step characteristics and its relationship to walking performance post-stroke. Gait Post. 2009, 29, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Mannini, A.; Sabatini, A.M.; Intille, S.S. Accelerometry-based recognition of the placement sites of a wearable sensor. Pervas. Mobile Comput. 2015, 21, 62–74. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannini, A.; Trojaniello, D.; Cereatti, A.; Sabatini, A.M. A Machine Learning Framework for Gait Classification Using Inertial Sensors: Application to Elderly, Post-Stroke and Huntington’s Disease Patients. Sensors 2016, 16, 134. https://doi.org/10.3390/s16010134
Mannini A, Trojaniello D, Cereatti A, Sabatini AM. A Machine Learning Framework for Gait Classification Using Inertial Sensors: Application to Elderly, Post-Stroke and Huntington’s Disease Patients. Sensors. 2016; 16(1):134. https://doi.org/10.3390/s16010134
Chicago/Turabian StyleMannini, Andrea, Diana Trojaniello, Andrea Cereatti, and Angelo M. Sabatini. 2016. "A Machine Learning Framework for Gait Classification Using Inertial Sensors: Application to Elderly, Post-Stroke and Huntington’s Disease Patients" Sensors 16, no. 1: 134. https://doi.org/10.3390/s16010134