Reduced Graphene Oxide Modified the Interdigitated Chain Electrode for an Insulin Sensor
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Apparatus
2.2. Fabrication of rGO-ICE
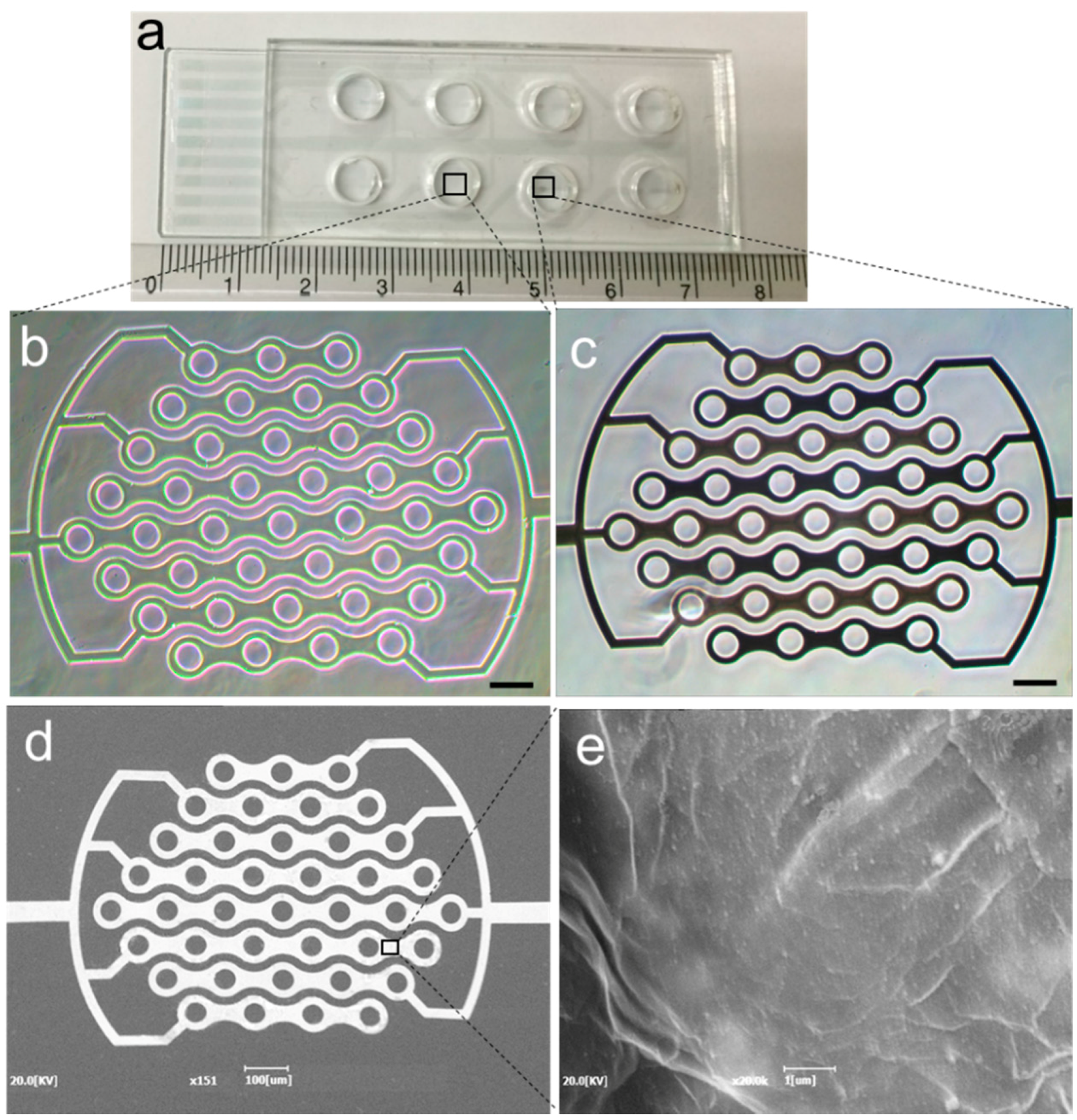
2.3. Development of rGO-ICE Based Insulin Sensor
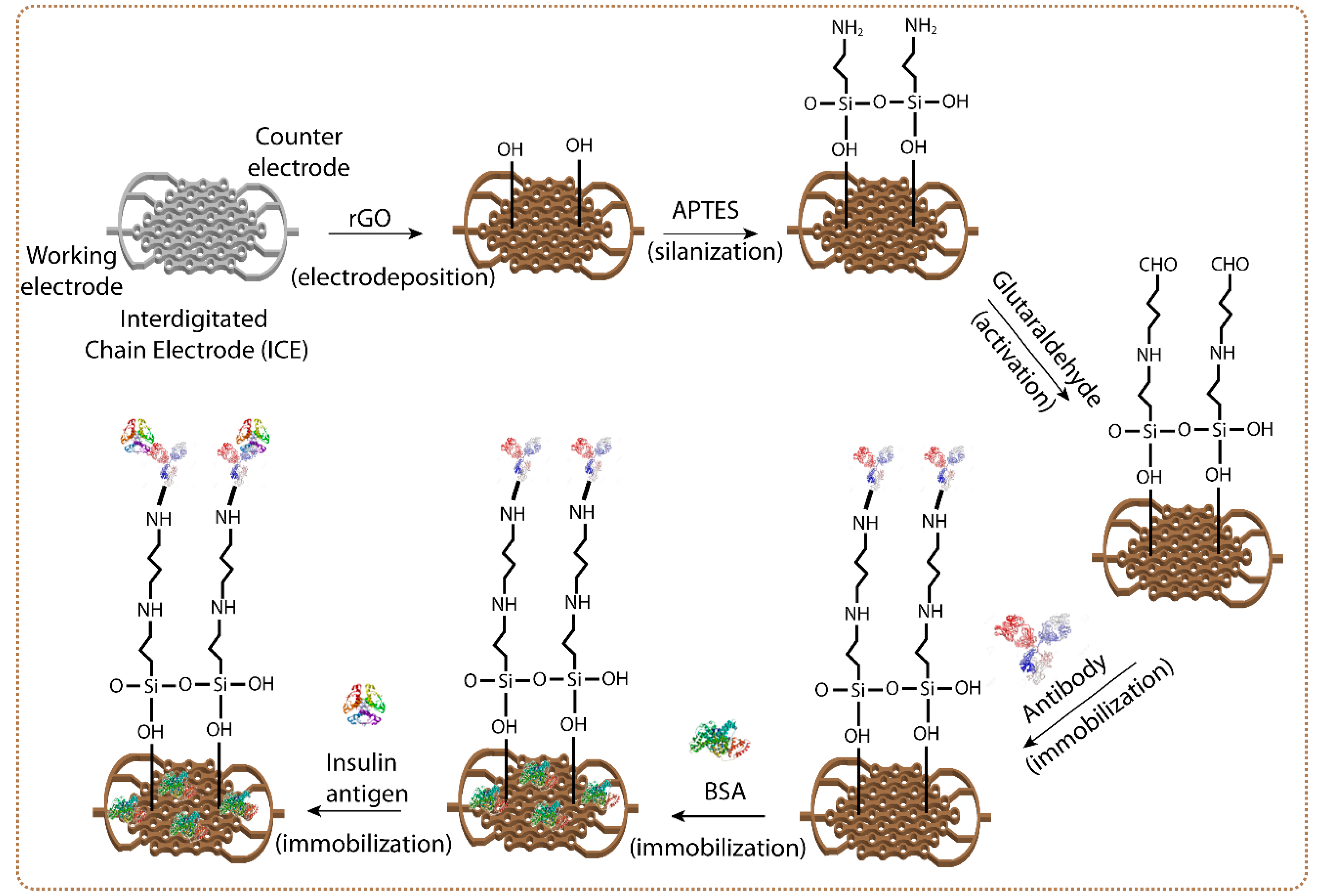
3. Results and Discussion
3.1. Impedance Characteristics of rGO Deposited ICE
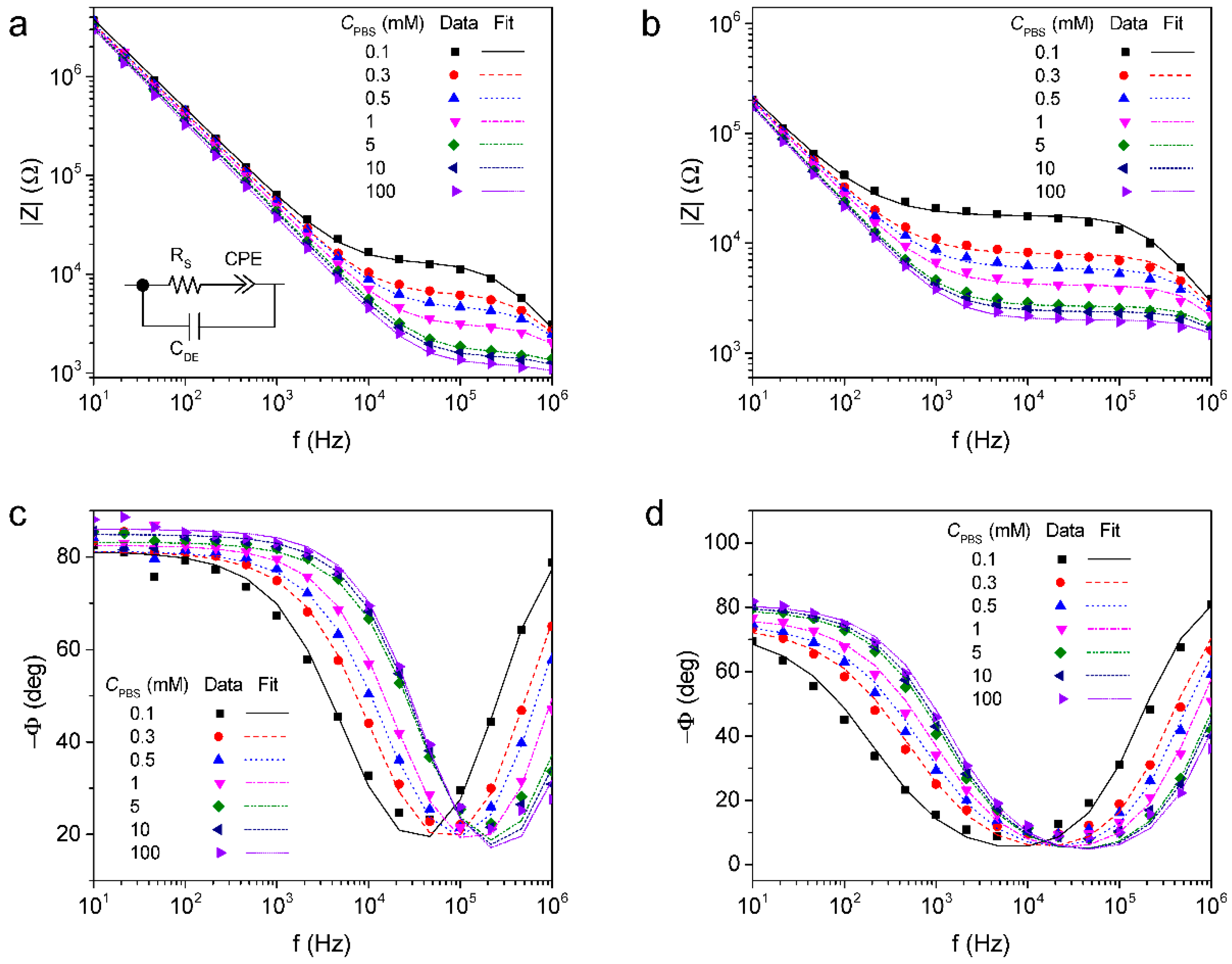
| Electrode | CPBS (mM) | RS [Ω] | CPE | CDE [×10−12 F] | |
|---|---|---|---|---|---|
| T [×10−9 Ω−1 sP] | P | ||||
| Bare-ICE | 0.1 | 13104 ± 439.5 | 6.256 ± 0.265 | 0.901 ± 0.006 | 53.92 ± 2.205 |
| 0.3 | 6329 ± 247.85 | 6.96 ± 0.347 | 0.900 ± 0.007 | 55.8 ± 3.46 | |
| 0.5 | 4616 ± 177.07 | 6.908 ± 0.331 | 0.901 ± 0.006 | 56.79 ± 3.879 | |
| 1 | 3038 ± 107.43 | 6.789 ± 0.298 | 0.915 ± 0.005 | 59.31 ± 4.577 | |
| 5 | 1671 ± 43.82 | 7.070 ± 0.216 | 0.923 ± 0.004 | 67.48 ± 5.0678 | |
| 10 | 1481 ± 40.995 | 6.249 ± 0.20 | 0.942 ± 0.004 | 68.78 ± 5.973 | |
| 100 | 1235 ± 47.21 | 6.267 ± 0.275 | 0.954 ± 0.005 | 74.13 ± 9.766 | |
| rGO-ICE | 0.1 | 17571 ± 382.02 | 168.0 ± 7.575 | 0.812 ± 0.009 | 53.91 ± 1.833 |
| 0.3 | 7896 ± 169.28 | 164.5 ± 6.857 | 0.824 ± 0.008 | 56.33 ± 2.316 | |
| 0.5 | 5832 ± 126.34 | 158.9 ± 6.566 | 0.833 ± 0.007 | 58.19 ± 2.660 | |
| 1 | 4106 ± 85.78 | 151.7 ± 5.974 | 0.852 ± 0.007 | 61.0 ± 3.088 | |
| 5 | 2656 ± 41.0 | 144.1 ± 4.23 | 0.882 ± 0.005 | 64.6 ± 3.02 | |
| 10 | 2384 ± 35.4 | 140.5 ± 3.969 | 0.890 ± 0.004 | 66.75 ± 3.138 | |
| 100 | 2003 ± 33.38 | 140.4 ± 4.389 | 0.898 ± 0.005 | 69.5 ± 3.99 | |
3.2. EIS Analysis of BSA/Ab-Ins/GA/APTES/rGO-ICE for Insulin Detection
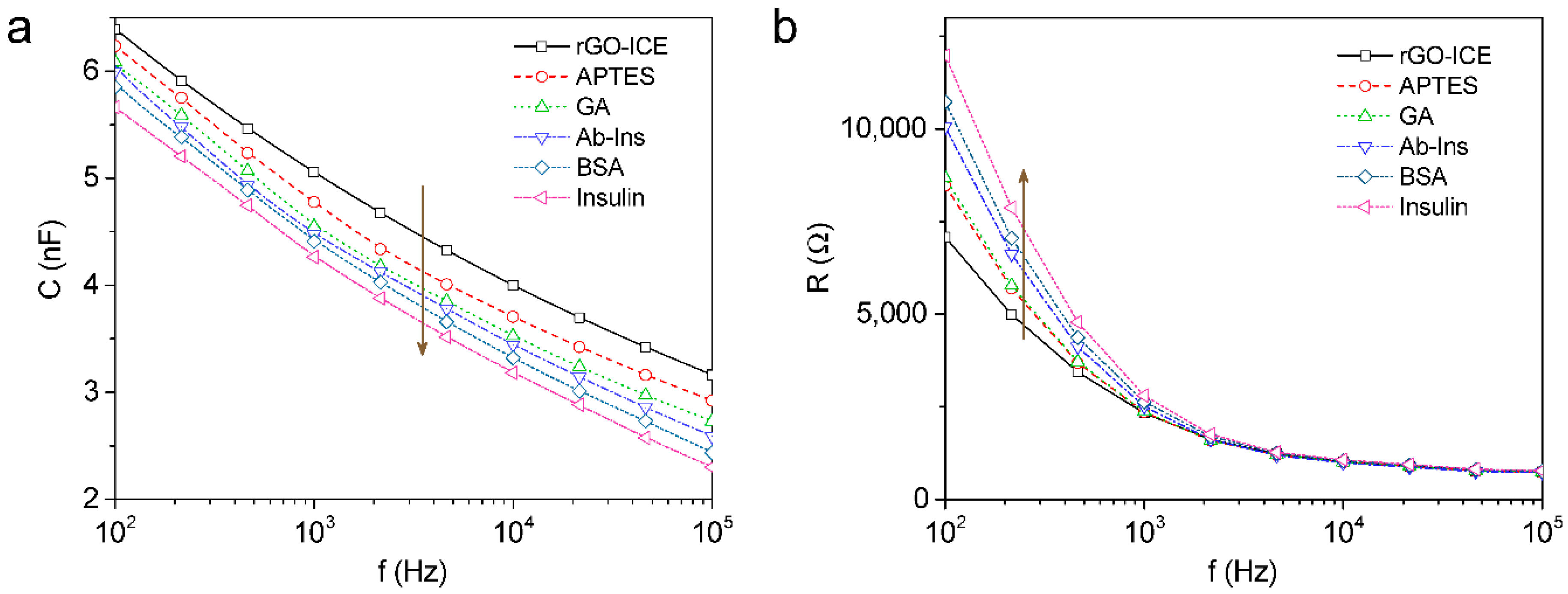
3.3. Capacitive Detection of Insulin
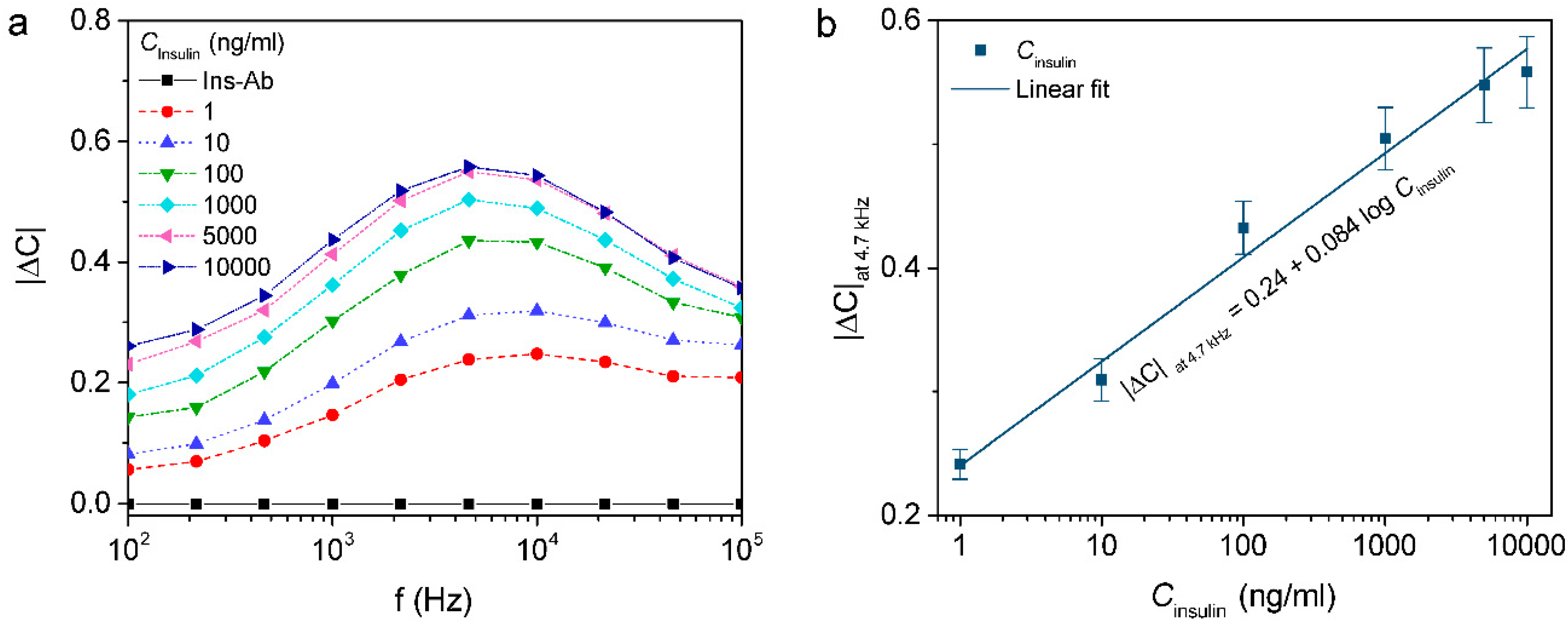
3.4. Capacitive Detection of Insulin in Human Serum
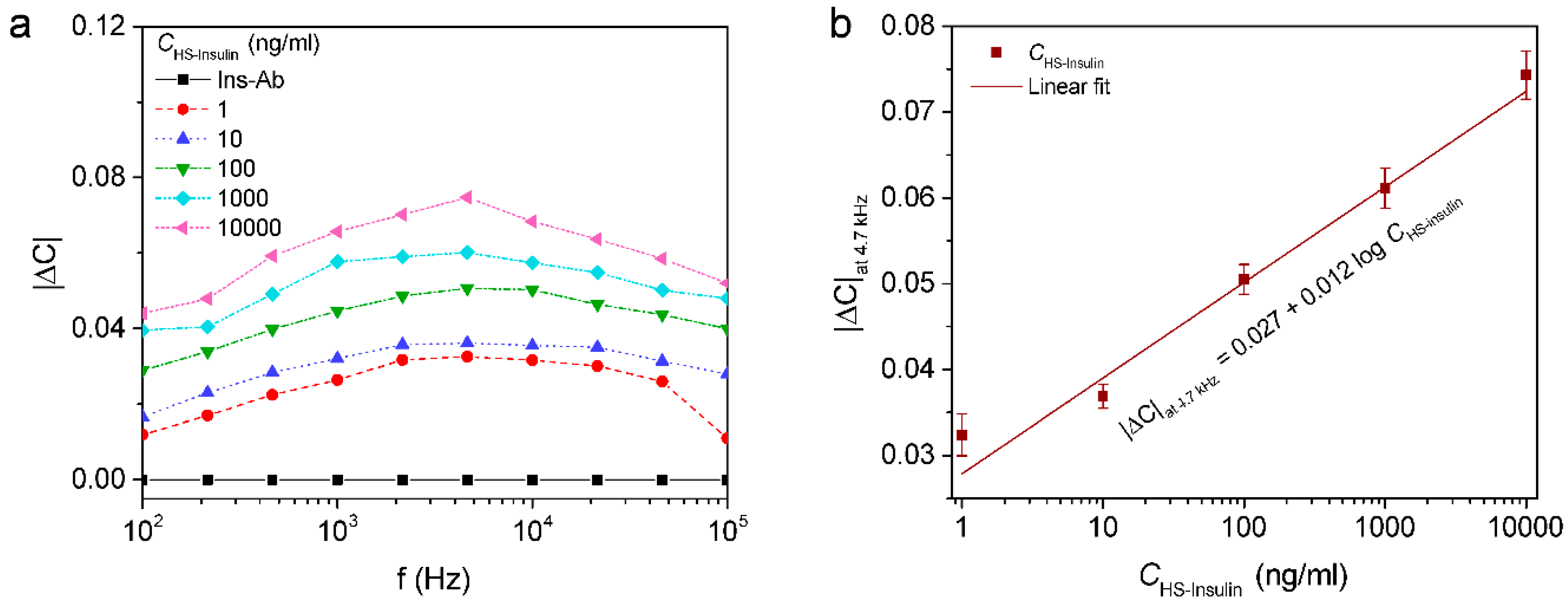
| Electrode | Method | Linear Range (nM) | Detection Limit (nM) | Ref. |
|---|---|---|---|---|
| SiO2NPs-Nafion/GCE a | DPV | 10–50 | 2.8 | [31] |
| NiNPs/ITO | CA | 1–125 | 0.01 | [32] |
| EDA b-CNFs c-NiO | CA | 20–1020 | 12.1 | [33] |
| SPE d/MWCNT e/NiONPs f | CA | 20–260 | 6.1 | [34] |
| CNT-NiCoO2/Nafion | CA | 17.2–5430 | 37.93 | [35] |
| GCE/CNT | CV | 3.45–68.97 | 1.34 | [36] |
| Guanine/NiOxNPs/GCE | CA | 100–1000 | 0.022 | [37] |
| Ni(OH)2–GN g/GCE | CA | 800–6400 | 200 | [38] |
| rGO/ITO | EIS | 0.17–172.4 | 0.086 | This work |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, H.I.; Beauchamp, J.L. Mapping disulfide bonds in insulin with the route 66 method: Selective cleavage of S-C bonds using alkali and alkaline earth metal enolate complexes. J. Am. Soc. Mass. Spectr. 2009, 20, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Ayajiki, K.; Kashiwagi, A.; Masada, M.; Okamura, T. Malfunction of vascular control in lifestyle-related diseases: Mechanisms underlying endothelial dysfunction in the insulin-resistant state. J. Pharmacol. Sci. 2004, 96, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Yeh, C.J.; Lin, C.L.; Yeh, S.Y.; Kao, C.H. Statins can delay insulin use and reduce diabetes-related diseases in asian patients with type 2 diabetes. Medicine 2015, 94. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitu; (WHO/NCD/NCS/99.2); World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Gerasimov, J.Y.; Schaefer, C.S.; Yang, W.W.; Grout, R.L.; Lai, R.Y. Development of an electrochemical insulin sensor based on the insulin-linked polymorphic region. Biosens. Bioelectron. 2013, 42, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Dib, S.A.; Freire, M.B.S.; Miranda, W.L.; Russo, E.M.K. Detection of insulin-antibodies by radioassay and elisa—Interrelation and correlation with metabolic control in type-I diabetes. Braz. J. Med. Biol. Res. 1994, 27, 1167–1180. [Google Scholar] [PubMed]
- Zandstra, G.J.; Wijnberg, I.D. Evaluation of several screening tests for determination of the igG concentration of foals with the turbidimetric immunoassay as reference method. Equine Vet. J. 2015, 47, 2–3. [Google Scholar] [CrossRef]
- Chen, Z.H.; Caulfield, M.P.; McPhaul, M.J.; Reitz, R.E.; Taylor, S.W.; Clarke, N.J. Quantitative insulin analysis using liquid chromatography-tandem mass spectrometry in a high-throughput clinical laboratory. Clin. Chem. 2013, 59, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Poghossian, A.; Yoshinobu, T.; Simonis, A.; Ecken, H.; Luth, H.; Schoning, M.J. Penicillin detection by means of field-effect based sensors: Enfet, capacitive eis sensor or laps? Sens. Actuators B Chem. 2001, 78, 237–242. [Google Scholar] [CrossRef]
- Schoning, M.J.; Thust, M.; Muller-Veggian, M.; Kordos, P.; Luth, H. A novel silicon-based sensor array with capacitive eis structures. Sens. Actuators B Chem. 1998, 47, 225–230. [Google Scholar] [CrossRef]
- Daniels, J.S.; Pourmand, N. Label-free impedance biosensors: Opportunities and challenges. Electroanalysis 2007, 19, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Bustin, D.; Mesaros, S.; Tomcik, P.; Rievaj, M.; Tvarozek, V. Application of redox cycling enhanced current at an interdigitated array electrode for iron-trace determination in ultrapure spectral carbon. Anal. Chim. Acta 1995, 305, 121–125. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, T.H.; Li, T.; Heo, K.; Hong, S.; Ko, J.; Kim, Y.; Shin, Y.B.; Kim, M.G. Electrical immunosensor based on a submicron-gap interdigitated electrode and gold enhancement. Biosens. Bioelectron. 2011, 26, 4690–4696. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.I.; Lim, Y.; Shin, H. The effect of channel height and electrode aspect ratio on redox cycling at carbon interdigitated array nanoelectrodes confined in a microchannel. Analyst 2013, 138, 6404–6411. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.W.; Chang, Y.W.; Lee, G.Y.; Cho, S.; Kang, M.J.; Pyun, J.C. A capacitive biosensor based on an interdigitated electrode with nanoislands. Anal. Chim. Acta 2014, 844, 27–34. [Google Scholar] [CrossRef] [PubMed]
- MacKay, S.; Hermansen, P.; Wishart, D.; Chen, J. Simulations of interdigitated electrode interactions with gold nanoparticles for impedance-based biosensing applications. Sensors 2015, 15, 22192–22208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Wang, J.X.; Wang, Z.; Wang, S.C. Improvement of amperometric sensor used for determination of nitrate with polypyrrole nanowires modified electrode. Sensors 2005, 5, 580–593. [Google Scholar] [CrossRef]
- Peik-See, T.; Pandikumar, A.; Nay-Ming, H.; Hong-Ngee, L.; Sulaiman, Y. Simultaneous electrochemical detection of dopamine and ascorbic acid using an iron oxide/reduced graphene oxide modified glassy carbon electrode. Sensors 2014, 14, 15227–15243. [Google Scholar] [CrossRef] [PubMed]
- Ohno, R.; Ohnuki, H.; Wang, H.H.; Yokoyama, T.; Endo, H.; Tsuya, D.; Izumi, M. Electrochemical impedance spectroscopy biosensor with interdigitated electrode for detection of human immunoglobulin A. Biosens. Bioelectron. 2013, 40, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Benvidi, A.; Dehghani-Firouzabadi, A.; Mazloum-Ardakani, M.; Mirjalili, B.B.F.; Zare, R. Electrochemical deposition of gold nanoparticles on reduced graphene oxide modified glassy carbon electrode for simultaneous determination of levodopa, uric acid and folic acid. J. Electroanal. Chem. 2015, 736, 22–29. [Google Scholar] [CrossRef]
- Yagati, A.K.; Min, J.; Cho, S. Electrosynthesis of ergo-np nanocomposite films for bioelectrocatalysis of horseradish peroxidase towards H2O2. J. Electrochem. Soc. 2014, 161, G133–G140. [Google Scholar] [CrossRef]
- Layek, R.K.; Nandi, A.K. A review on synthesis and properties of polymer functionalized graphene. Polymer 2013, 54, 5087–5103. [Google Scholar] [CrossRef]
- Fakhari, A.R.; Sahragard, A.; Ahmar, H. Development of an electrochemical sensor based on reduced graphene oxide modified screen-printed carbon electrode for the determination of buprenorphine. Electroanalysis 2014, 26, 2474–2483. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Zhang, M.M.; Chen, X.; Li, Y.J.; Wang, J. Electrochemical co-reduction synthesis of aupt bimetallic nanoparticles-graphene nanocomposites for selective detection of dopamine in the presence of ascorbic acid and uric acid. Sensors 2015, 15, 16614–16631. [Google Scholar] [CrossRef] [PubMed]
- Berggren, C.; Bjarnason, B.; Johansson, G. Capacitive biosensors. Electroanalysis 2001, 13, 173–180. [Google Scholar] [CrossRef]
- Teixeira, S.; Burwell, G.; Castaing, A.; Gonzalez, D.; Conlan, R.S.; Guy, O.J. Epitaxial graphene immunosensor for human chorionic gonadotropin. Sens. Actuators B Chem. 2014, 190, 723–729. [Google Scholar] [CrossRef]
- Su, H.L.; Li, Z.F.; Huo, Q.S.; Guan, J.Q.; Kan, Q.B. Immobilization of transition metal (Fe2+, Co2+, Vo2+ or Cu2+) schiff base complexes onto graphene oxide as efficient and recyclable catalysts for epoxidation of styrene. RSC Adv. 2014, 4, 9990–9996. [Google Scholar] [CrossRef]
- Yuan, Y.; Yin, M.; Qian, J.C.; Liu, C.S. Site-directed immobilization of antibodies onto blood contacting grafts for enhanced endothelial cell adhesion and proliferation. Soft Matter. 2011, 7, 7207–7216. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Constant-phase-element behavior caused by resistivity distributions in films I. Theory. J. Electrochem. Soc. 2010, 157, C452–C457. [Google Scholar] [CrossRef]
- Gobi, K.V.; Iwasaka, H.; Miura, N. Self-assembled PEG monolayer based SPR immunosensor for label-free detection of insulin. Biosens. Bioelectron. 2007, 22, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Amini, N.; Gholivand, M.B.; Shamsipur, M. Electrocatalytic determination of traces of insulin using a novel silica nanoparticles-nafion modified glassy carbon electrode. J. Electroanal. Chem. 2014, 714, 70–75. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, M.; Yuan, M.; Liu, W.; Hu, J. Nickel nanoparticle-modified electrode for ultra-sensitive electrochemical detection of insulin. Biosens. Bioelectron. 2016, 77, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chu, X.K.; Yuan, S.M.; Zhao, G.C. Ethylenediamine-assisted preparation of carbon nanofiber supported nickel oxide electrocatalysts for sensitive and durable detection of insulin. RSC Adv. 2015, 5, 41317–41323. [Google Scholar] [CrossRef]
- Rafiee, B.; Fakhari, A.R. Electrocatalytic oxidation and determination of insulin at nickel oxide nanoparticles-multiwalled carbon nanotube modified screen printed electrode. Biosens. Bioelectron. 2013, 46, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Arvinte, A.; Westermann, A.C.; Sesay, A.M.; Virtanen, V. Electrocatalytic oxidation and determination of insulin at CNT-nickel-cobalt oxide modified electrode. Sens. Actuators B Chem. 2010, 150, 756–763. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.H. A carbon nanotubes assisted strategy for insulin detection and insulin proteolysis assay. Anal. Chim. Acta 2009, 650, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Noorbakhash, A.; Sharifi, E.; Semnani, A. Highly sensitive sensor for picomolar detection of insulin at physiological ph, using gc electrode modified with guanine and electrodeposited nickel oxide nanoparticles. Biosens. Bioelectron. 2008, 24, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Q.; Hu, L.L.; Li, L.B.; Wang, K.Q. Facile synthesis of nickel hydroxide-graphene nanocomposites for insulin detection with enhanced electro-oxidation properties. RSC Adv. 2014, 4, 46208–46213. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yagati, A.K.; Park, J.; Cho, S. Reduced Graphene Oxide Modified the Interdigitated Chain Electrode for an Insulin Sensor. Sensors 2016, 16, 109. https://doi.org/10.3390/s16010109
Yagati AK, Park J, Cho S. Reduced Graphene Oxide Modified the Interdigitated Chain Electrode for an Insulin Sensor. Sensors. 2016; 16(1):109. https://doi.org/10.3390/s16010109
Chicago/Turabian StyleYagati, Ajay Kumar, Jinsoo Park, and Sungbo Cho. 2016. "Reduced Graphene Oxide Modified the Interdigitated Chain Electrode for an Insulin Sensor" Sensors 16, no. 1: 109. https://doi.org/10.3390/s16010109






