Interferometric Reflectance Imaging Sensor (IRIS)—A Platform Technology for Multiplexed Diagnostics and Digital Detection
Abstract
:1. Background and Motivation
2. Interferometric Reflectance Imaging Sensor (IRIS)
2.1. High-Throughput Label-Free Biomass Detection with IRIS

2.2. Digital Detection of Single Particles with SP-IRIS
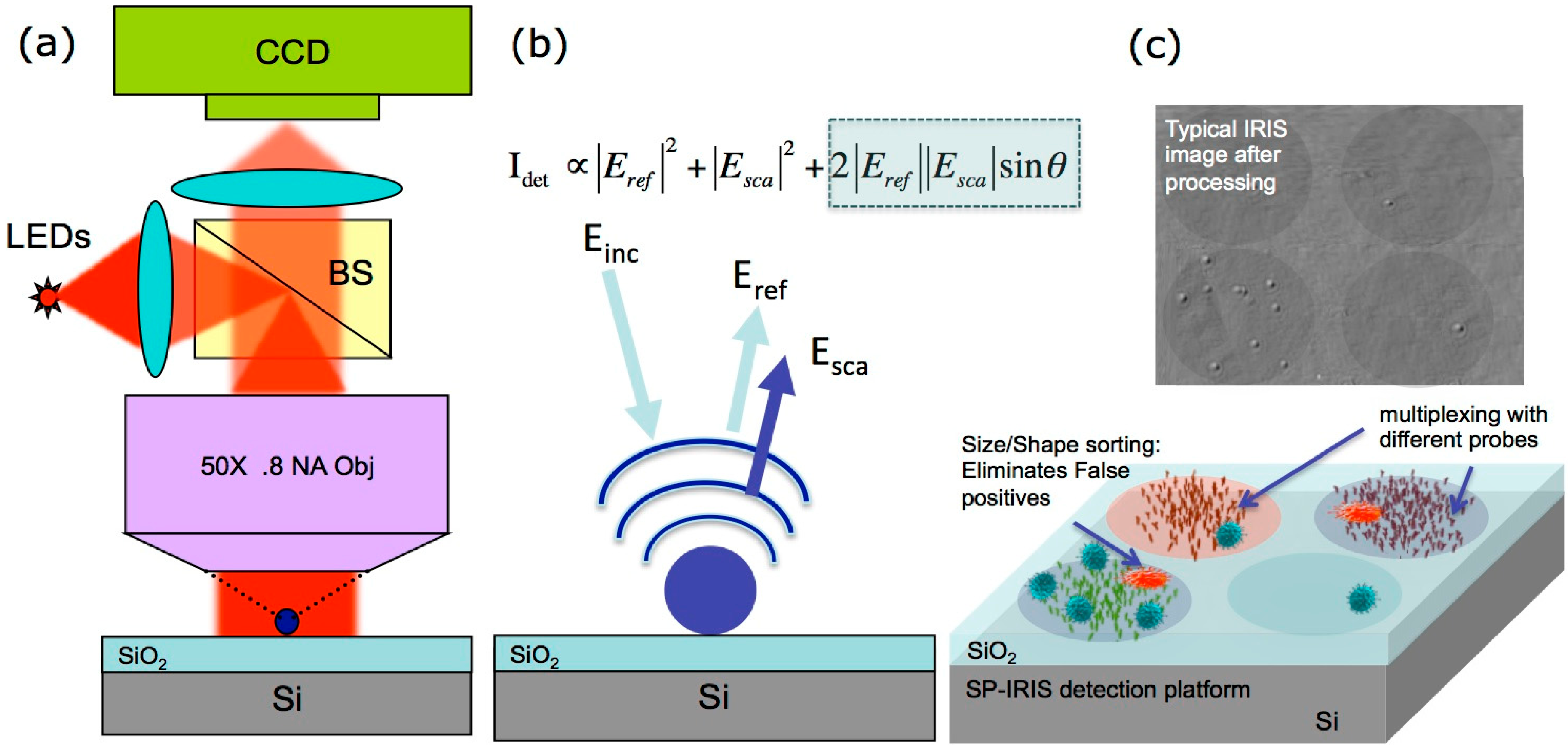
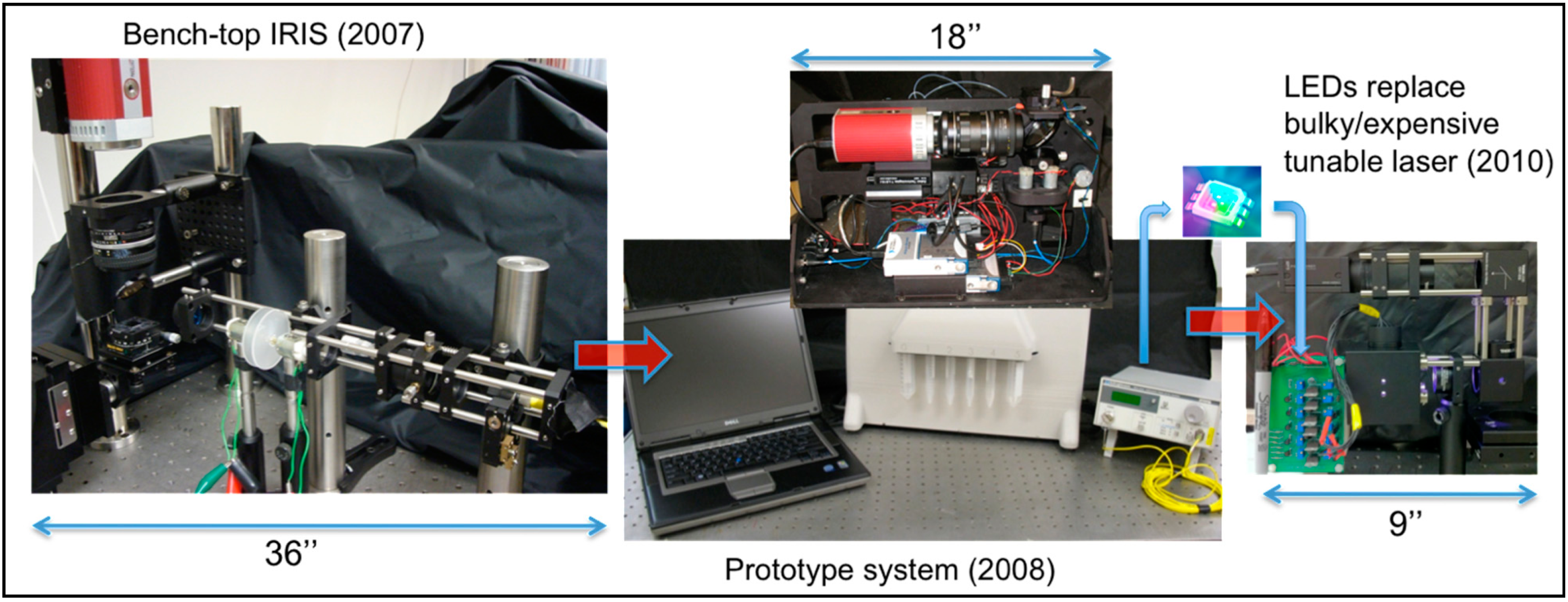
3. Instrumentation

4. Applications and Results
4.1. Label-Free Detection with Protein Microarrays
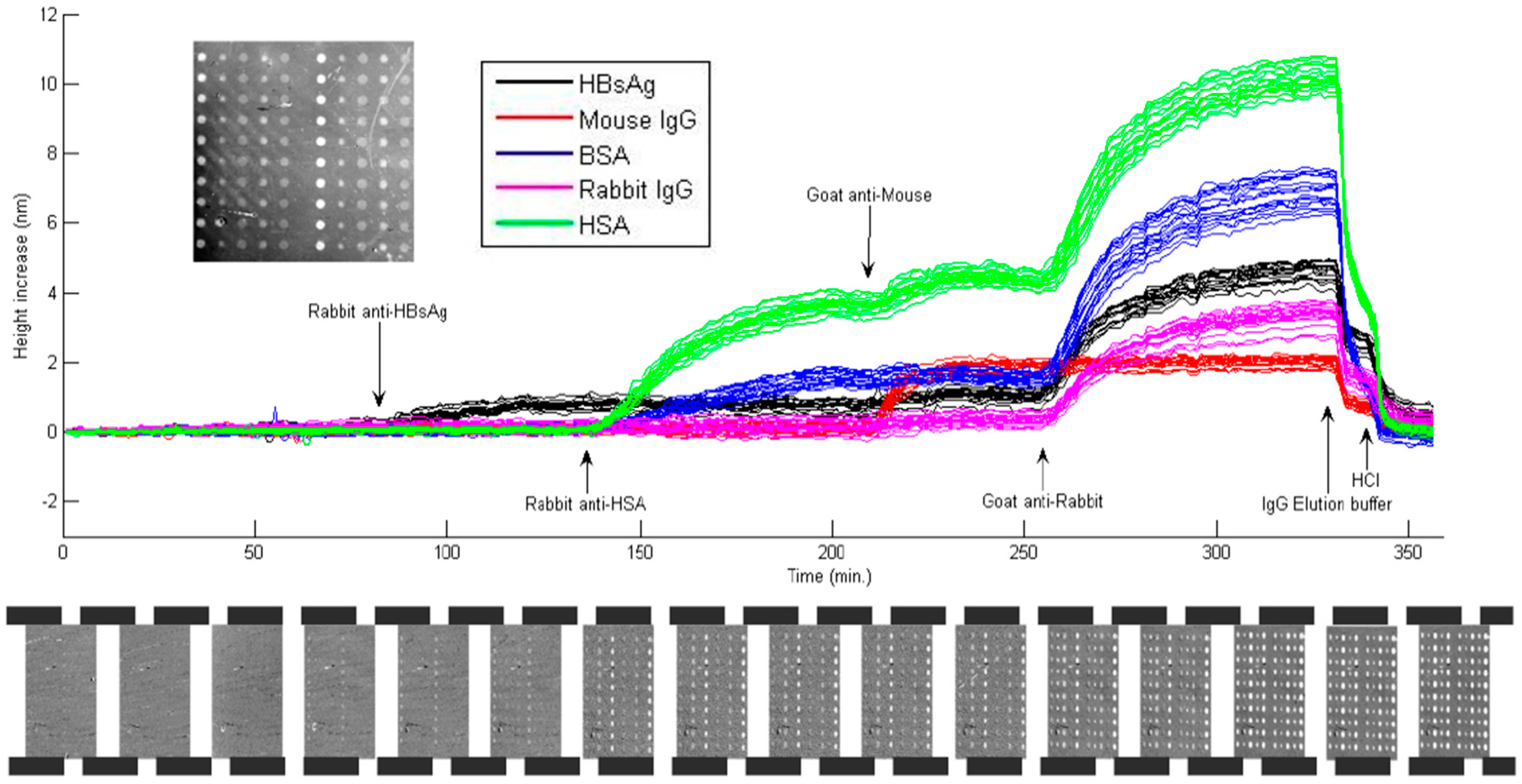
4.2. Label-Free Detection with DNA Microarrays
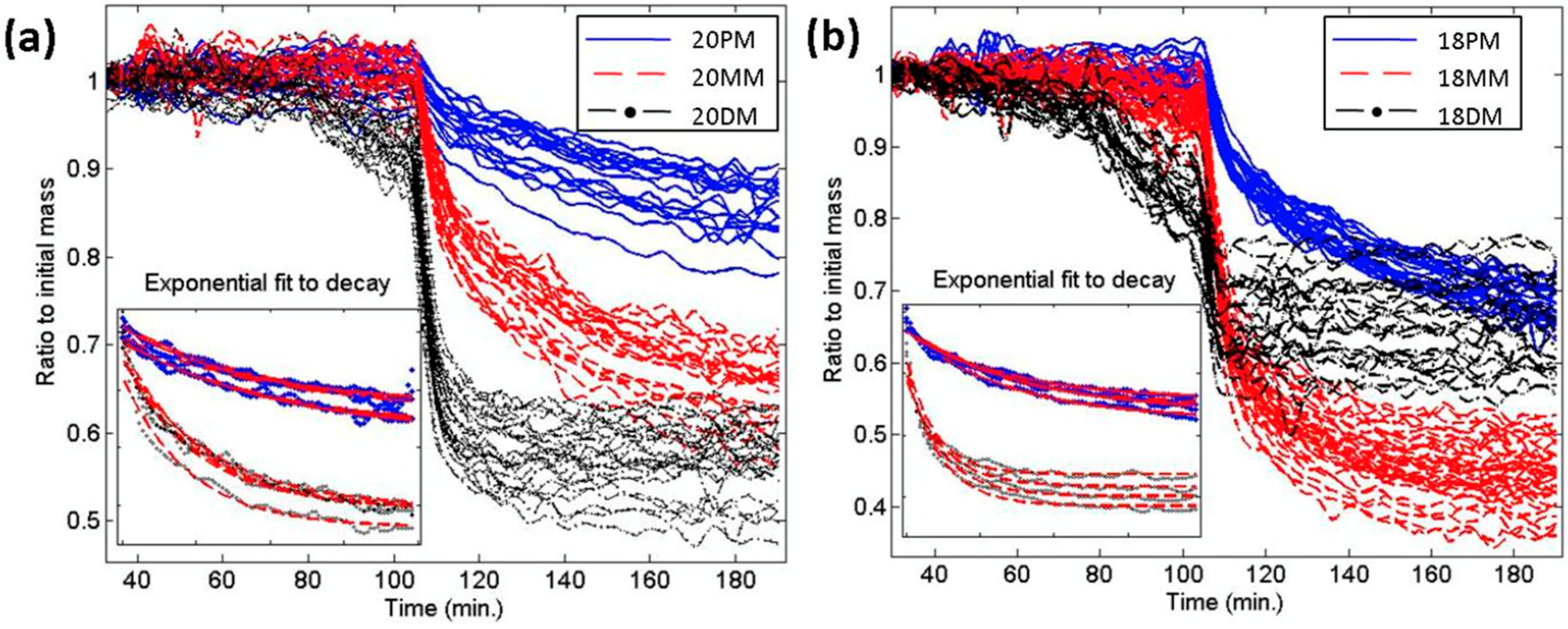
4.3. Protein Detection with DNA Microarrays
4.4. Microarray Quality Control and Calibrated Fluorescence Enhancement (CaFE)
4.5. Single Particle Interferometric Reflectance Imaging Sensor
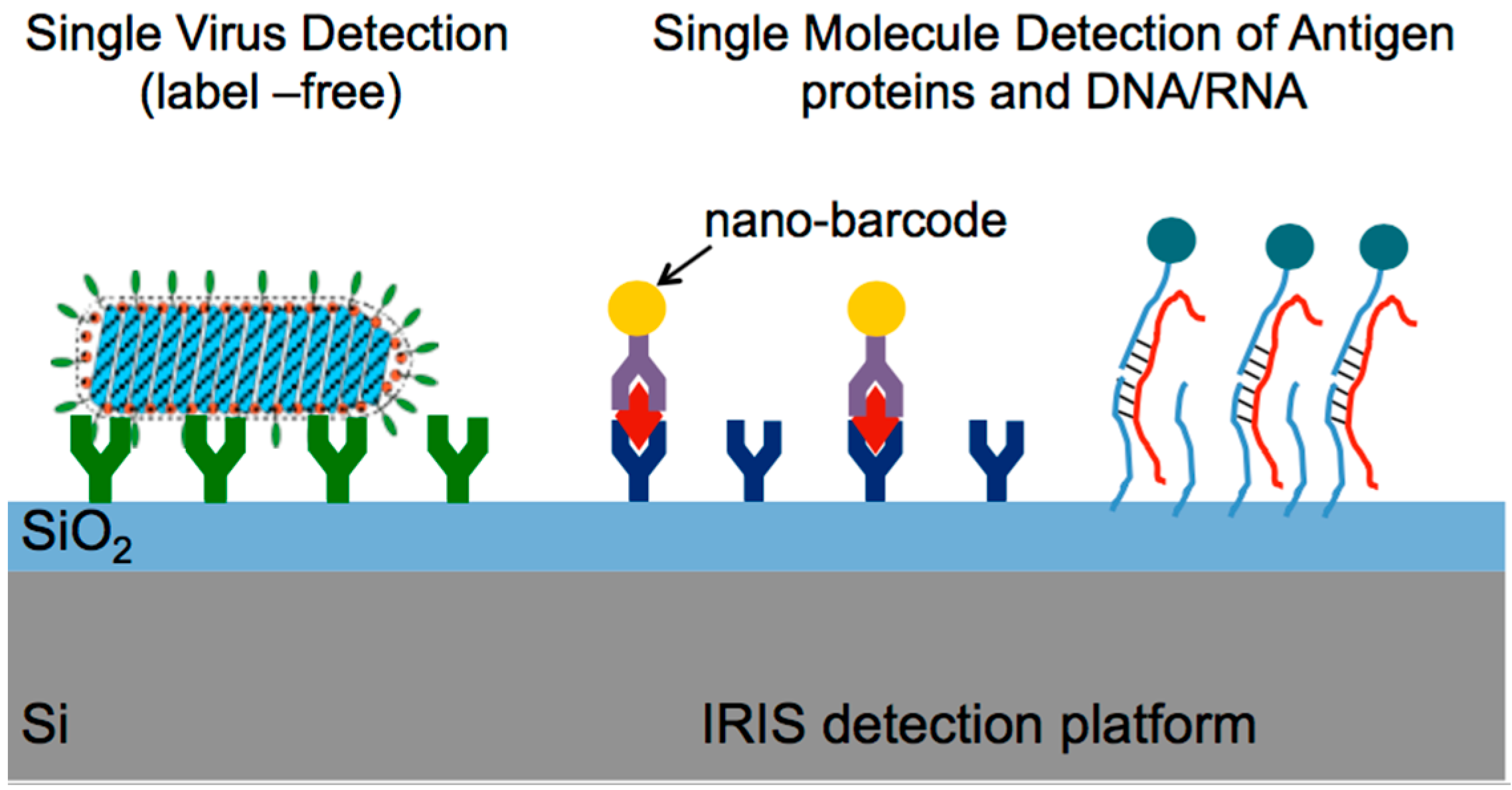
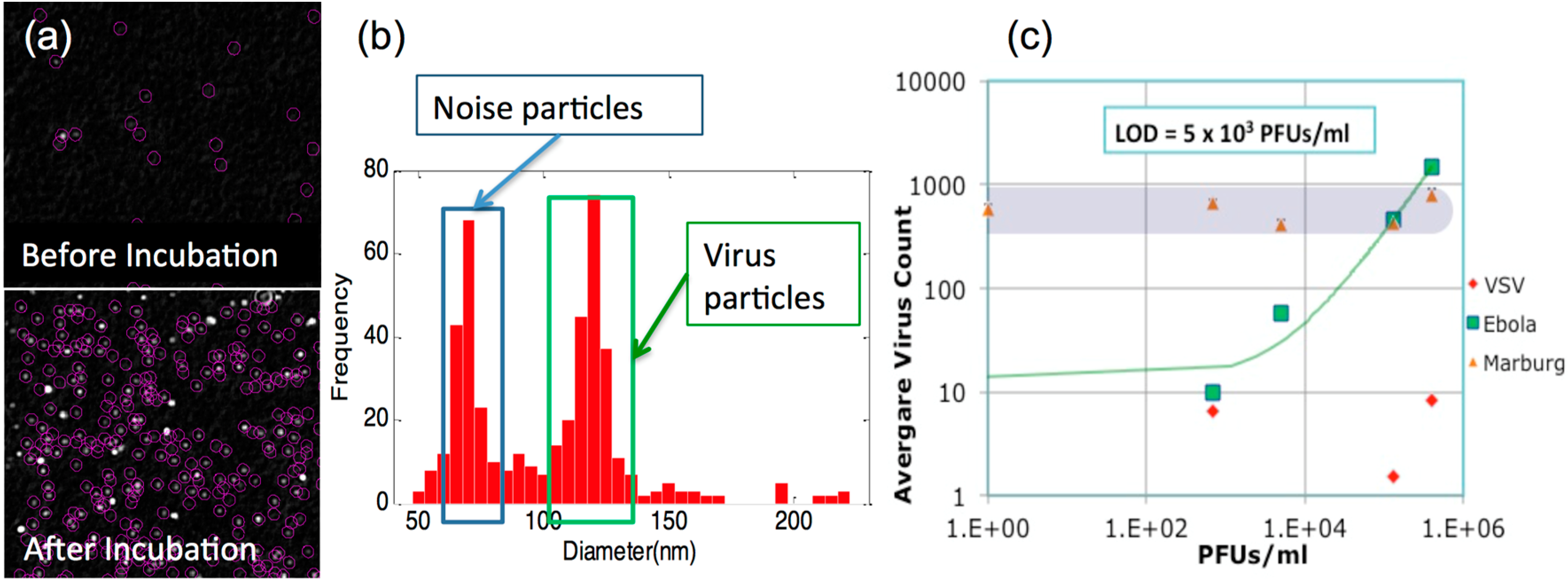
4.5.1. Digital Label-Free Detection of Viruses
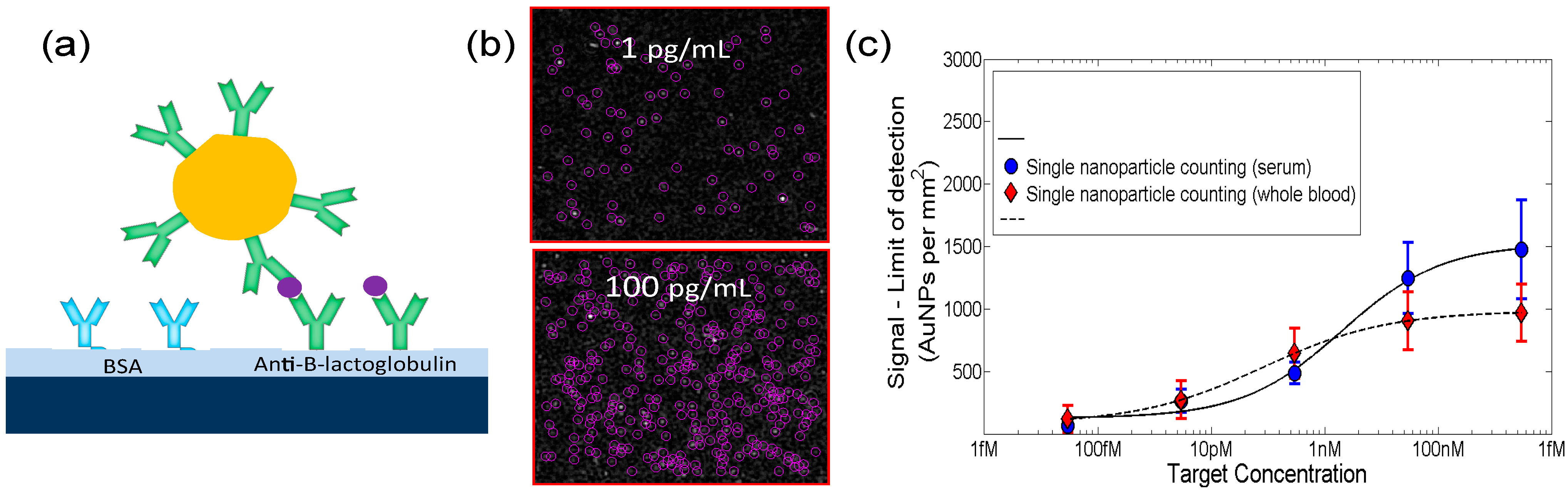
4.5.2. Multiplexed Protein Detection with Au Nanoparticle Labels
5. Alternative Techniques and Performance Comparisons
| Biosensor | Analyte | Limit of Detection | Reference |
|---|---|---|---|
| SP-IRIS | Protein (β-lactoglobulin) | 60 aM | [28] |
| Virus (Ebola-pseudotyped VSV) | 5 × 103 pfu/mL (<10 aM) | [25] | |
| IRIS * | Protein (rabbit-IgG) | 19 ng/mL (~120 pM) | [4] |
| Protein (BSA) | 5.2 pg/mm2 | [14] | |
| Virus (VSV) | 3.5 × 105 pfu/mL | [7] | |
| iSCAT | Protein (IgG1) | 1 ng/mL (~6 pM) | [35] |
| WGM | Protein | 0.03 pg/mm2 | [31] |
| Virus (InfA) | 10 fM | [36] | |
| SPRi | Protein (β2m/cysC) | 1 nM | [37] |
| DNA | 10 nM | [38] |
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Gubala, V.; Harris, L.F.; Ricco, A.J.; Tan, M.X.; Williams, D.E. Point of care diagnostics: Status and future. Anal. Chem. 2012, 84, 487–515. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wu, D.; Li, H.; Xu, C.; Wang, H.; Zhao, Y.; Cai, Y.; Wei, Q.; Du, B. Label-free amperometric immunosensor for the detection of human serum chorionic gonadotropin based on nanoporous gold and grapheme. Anal. Biochem. 2011, 414, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Stoeva, S.I.; Lee, J.S.; Smith, J.E.; Rosen, S.T.; Mirkin, C.A. Multiplexed detection of protein cancer markers with biobarcoded nanoparticle probes. J. Am. Chem. Soc. 2006, 128, 8378–8379. [Google Scholar] [CrossRef] [PubMed]
- Özkumur, E.; Needham, J.W.; Bergstein, D.A.; Gonzalez, R.; Cabodi, M.; Gershoni, J.M.; Goldberg, B.B.; Ünlü, M.S. Label-free and dynamic detection of biomolecular interactions for high-throughput microarray applications. Proc. Natl. Acad. Sci. USA 2008, 105, 7988–7992. [Google Scholar] [CrossRef] [PubMed]
- Özkumur, E.; Yalçın, A.; Cretich, M.; Lopez, C.A.; Bergstein, D.A.; Goldberg, B.B.; Chiari, M.; Ünlü, M.S. Quantification of DNA and protein adsorption by optical phase shift. Biosens. Bioelectron. 2009, 25, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Özkumur, E.; Ahn, S.; Yalçın, A.; Lopez, C.A.; Çevik, E.; Irani, R.J.; DeLisi, C.; Chiari, M.; Ünlü, M.S. Label-free microarray imaging for direct detection of DNA hybridization and single-nucleotide mismatches. Biosens. Bioelectron. 2010, 25, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.A.; Daaboul, G.G.; Vedula, R.S.; Özkumur, E.; Bergstein, D.A.; Geisbert, T.W.; Fawcett, H.E.; Goldberg, B.B.; Connor, J.H.; Ünlü, M.S. Label-free, multiplexed virus detection using spectral reflectance imaging. Biosens. Bioelectron. 2011, 26, 3432–3437. [Google Scholar] [CrossRef] [PubMed]
- Daaboul, G.G.; Yurt, A.; Zhang, X.; Hwang, G.M.; Goldberg, B.B.; Ünlü, M.S. High-throughput detection and sizing of individual low-index nanoparticles and viruses for pathogen identification. Nano Lett. 2010, 10, 4727–4731. [Google Scholar] [CrossRef] [PubMed]
- Yurt, A.; Daaboul, G.G.; Connor, J.H.; Goldberg, B.B.; Ünlü, M.S. Single nanoparticle detectors for biological applications. Nanoscale 2012, 4, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Daaboul, G.G.; Lopez, C.A.; Yurt, A.; Member, S.; Goldberg, B.B. Label-free optical biosensors for virus detection and characterization. Sel. Top. Quantum Electron. IEEE J. 2012, 18, 1422–1433. [Google Scholar] [CrossRef]
- Vedula, R.; Daaboul, G.; Reddington, A.; Özkumur, E.; Bergstein, D.A.; Ünlü, M.S. Self-referencing substrates for optical interferometric biosensors. J. Mod. Opt. 2010, 57, 1564–1569. [Google Scholar] [CrossRef]
- Ünlü, M.S. Digital detection of nanoparticles: Viral diagnostics and multiplexed protein and nucleic acid assays. MRS Proc. 2014. [Google Scholar] [CrossRef]
- Novotny, L.; Hecht, B. Principles of Nano-Optics, 2nd ed.; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Daaboul, G.G.; Vedula, R.S.; Ahn, S.; Lopez, C.A.; Reddington, A.; Ozkumur, E.; Ünlü, M.S. LED-based interferometric reflectance imaging sensor for quantitative dynamic monitoring of biomolecular interactions. Biosens. Bioelectron. 2011, 26, 2221–2227. [Google Scholar] [CrossRef] [PubMed]
- Reddington, A.P. Instrument Design and Optimization of Interferometric Reflectance Imaging Sensors for in Vitro Diagnostics. Ph.D. Thesis, Boston University, Boston, MA, USA, January 2014. [Google Scholar]
- Reddington, A.P.; Monroe, M.R.; Ünlü, M.S. Integrated imaging instrument for self-calibrated fluorescence protein microarrays. Rev. Sci. Instrum. 2013, 84, 103702. [Google Scholar] [CrossRef] [PubMed]
- Monroe, M.R.; Reddington, A.P.; Collins, A.D.; LaBoda, C.; Cretich, M.; Chiari, M.; Little, F.F.; Ünlü, M.S. Multiplexed method to calibrate and quantitate fluorescence signal for allergen-specific IgE. Anal. Chem. 2011, 83, 9485–9491. [Google Scholar] [CrossRef] [PubMed]
- Reddington, A.P.; Member, S.; Trueb, J.T.; Freedman, D.S.; Tuysuzoglu, A.; Member, S.; Daaboul, G.G.; Lopez, C.A.; Karl, W.C.; Member, S.; et al. An Interferometric Reflectance Imaging Sensor for Point of Care Viral Diagnostics. IEEE Trans. Biomed. Eng. 2013, 60, 3276–3283. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Huang, C.-L.; Ozkumur, E.; Zhang, X.; Chinnala, J.; Yalcin, A.; Bandyopadhyay, S.; Russek, S.; Ünlü, M.S.; DeLisi, C.; et al. TATA binding proteins can recognize nontraditional DNA sequences. Biophys. J. 2012, 103, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Lopez, C.A.; Hu, H.; Xia, Y.; Freedman, D.S.; Reddington, A.P.; Daaboul, G.G.; Ünlü, M.S.; Genco, C.A. A high-throughput method to examine protein-nucleotide interactions identifies targets of the bacterial transcriptional regulatory protein Fur. PLoS ONE 2014, 9, e96832. [Google Scholar] [CrossRef] [PubMed]
- Hessner, M.; Singh, V.; Wang, X.; Khan, S.; Tschannen, M.; Zahrt, T. Utilization of a labeled tracking oligonucleotide for visualization and quality control of spotted 70-mer arrays. BMC Genomics 2004, 5, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francesca, B.; Francesco, D.; Vincenza, C.; Silvia, G.; Gabriele, D.C.; Manuela, S.; Luigi, P.; Maurizio, F.; Marcella, C.; Laura, C. High-sensitive microarray substrates specifically designed to improve sensitivity for the identification of fetal paternally inherited sequences in maternal plasma. Clin. Chem. Lab. Med. 2009, 47, 818–823. [Google Scholar]
- Cretich, M.; Monroe, M.R.; Reddington, A.; Zhang, X.; Daaboul, G.G.; Damin, F.; Sola, L.; Unlu, M.S.; Chiari, M. Interferometric silicon biochips for label and label-free DNA and protein microarrays. Proteomics 2012, 12, 2963–2977. [Google Scholar] [CrossRef] [PubMed]
- Sevenler, D.; Ünlü, N.L.; Ünlü, M.S. Nanoparticle biosensing with interferometric reflectance imaging. In Nanobiosensors and Nanobioanalyses; Vestergaard, M.C., Kerman, K., Hsing, I.M., Tamiya, E., Eds.; Springer: Tokyo, Japan, 2015. [Google Scholar]
- Daaboul, G.G.; Lopez, C.A.; Chinnala, J.; Goldberg, B.B.; Connor, J.H.; Ünlü, M.S. Digital sensing and sizing of vesicular stomatitis virus pseudotypes in complex media: A model for ebola and marburg detection. ACS Nano 2014, 8, 6047–6055. [Google Scholar] [CrossRef] [PubMed]
- Luchansky, M.S.; Washburn, A.L.; McClellan, M.S.; Bailey, R.C. Sensitive on-chip detection of a protein biomarker in human serum and plasma over an extended dynamic range using silicon photonic microring resonators and sub-micron beads. Lab Chip 2011, 11, 2042–2044. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, P.E.; Whitman, L.J. Detection limits for nanoscale biosensors. Nano Lett. 2005, 5, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Monroe, M.R.; Daaboul, G.G.; Tuysuzoglu, A.; Lopez, C.A.; Little, F.F.; Ünlü, M.S. Single nanoparticle detection for multiplexed protein diagnostics with attomolar sensitivity in serum and unprocessed whole blood. Anal. Chem. 2013, 85, 3698–3706. [Google Scholar] [CrossRef] [PubMed]
- Steiner, G. Surface plasmon resonance imaging. Anal. Bioanal. Chem. 2004, 379, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Hu, H.; Chen, W.; Lu, M.; Tian, L.; Yu, H.; Long, K.D.; Chow, E.; King, W.P.; Singamaneni, S.; et al. Single nanoparticle detection using photonic crystal enhanced microscopy. Analyst 2014, 139, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, F.; Arnold, S. Whispering-gallery-mode biosensing: Label-free detection down to single molecules. Nat. Meth. 2008, 5, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Lindfors, K.; Kalkbrenner, T.; Stoller, P.; Sandoghdar, V. Detection and spectroscopy of gold nanoparticles using supercontinuum white light confocal microscopy. Phys. Rev. Lett. 2004, 93, 037401. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Arroyo, J.; Kukura, P. Interferometric scattering microscopy (iSCAT): New frontiers in ultrafast and ultrasensitive optical microscopy. Phys. Chem. Chem. Phys. 2012, 14, 15625. [Google Scholar] [CrossRef] [PubMed]
- Ignatovich, F.V.; Novotny, L. Real-time and background-free detection of nanoscale particles. Phys. Rev. Lett. 2006, 96, 013901. [Google Scholar] [CrossRef] [PubMed]
- Piliarik, M.; Sandoghdar, V. Direct optical sensing of single unlabelled proteins and super-resolution imaging of their binding sites. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, F.; Arnold, S.; Keng, D. Single virus detection from the reactive shift of a whispering-gallery mode. Proc. Natl. Acad. Sci. USA 2008, 105, 20701–20704. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Nedelkov, D.; Corn, R.M. Surface plasmon resonance imaging measurements of antibody arrays for the multiplexed detection of low molecular weight protein biomarkers. Anal. Chem. 2006, 78, 6504–6510. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.P.; Grimsrud, T.E.; Liles, M.R.; Goodman, R.M.; Corn, R.M. Surface plasmon resonance imaging measurements of DNA and RNA hybridization adsorption onto DNA microarrays. Anal. Chem. 2001, 73, 1–7. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avci, O.; Ünlü, N.L.; Özkumur, A.Y.; Ünlü, M.S. Interferometric Reflectance Imaging Sensor (IRIS)—A Platform Technology for Multiplexed Diagnostics and Digital Detection. Sensors 2015, 15, 17649-17665. https://doi.org/10.3390/s150717649
Avci O, Ünlü NL, Özkumur AY, Ünlü MS. Interferometric Reflectance Imaging Sensor (IRIS)—A Platform Technology for Multiplexed Diagnostics and Digital Detection. Sensors. 2015; 15(7):17649-17665. https://doi.org/10.3390/s150717649
Chicago/Turabian StyleAvci, Oguzhan, Nese Lortlar Ünlü, Ayça Yalçın Özkumur, and M. Selim Ünlü. 2015. "Interferometric Reflectance Imaging Sensor (IRIS)—A Platform Technology for Multiplexed Diagnostics and Digital Detection" Sensors 15, no. 7: 17649-17665. https://doi.org/10.3390/s150717649







