Design and Evaluation of Potentiometric Principles for Bladder Volume Monitoring: A Preliminary Study
Abstract
:1. Introduction
2. Experimental Section
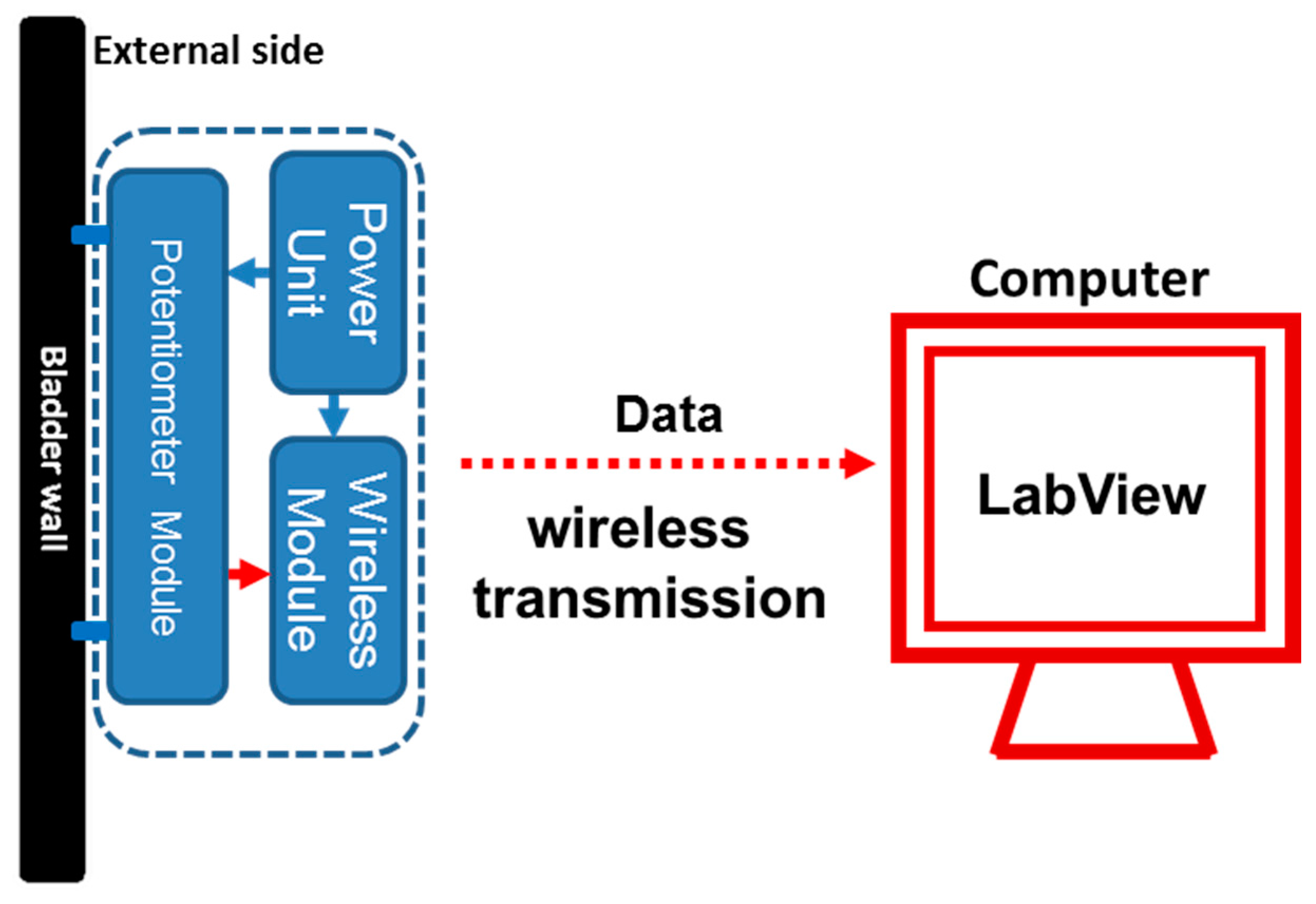
2.1. Basic Structure of the Prototype Potentiometric Platform

2.2. Bladder Geometric Model
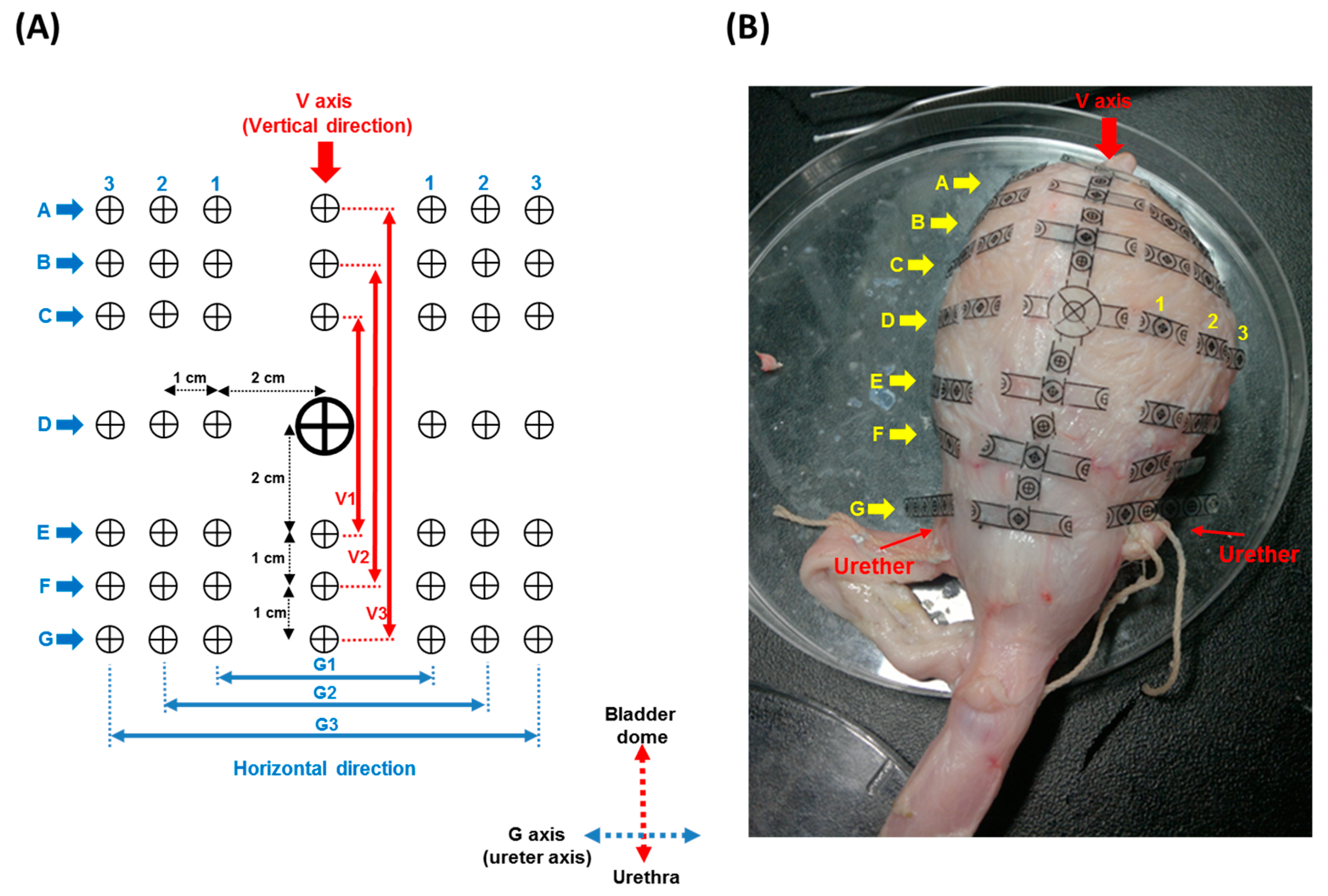
2.3. In Vitro Bladder Testing
3. Results
3.1. Basic Structure of the Prototype Potentiometric Platform
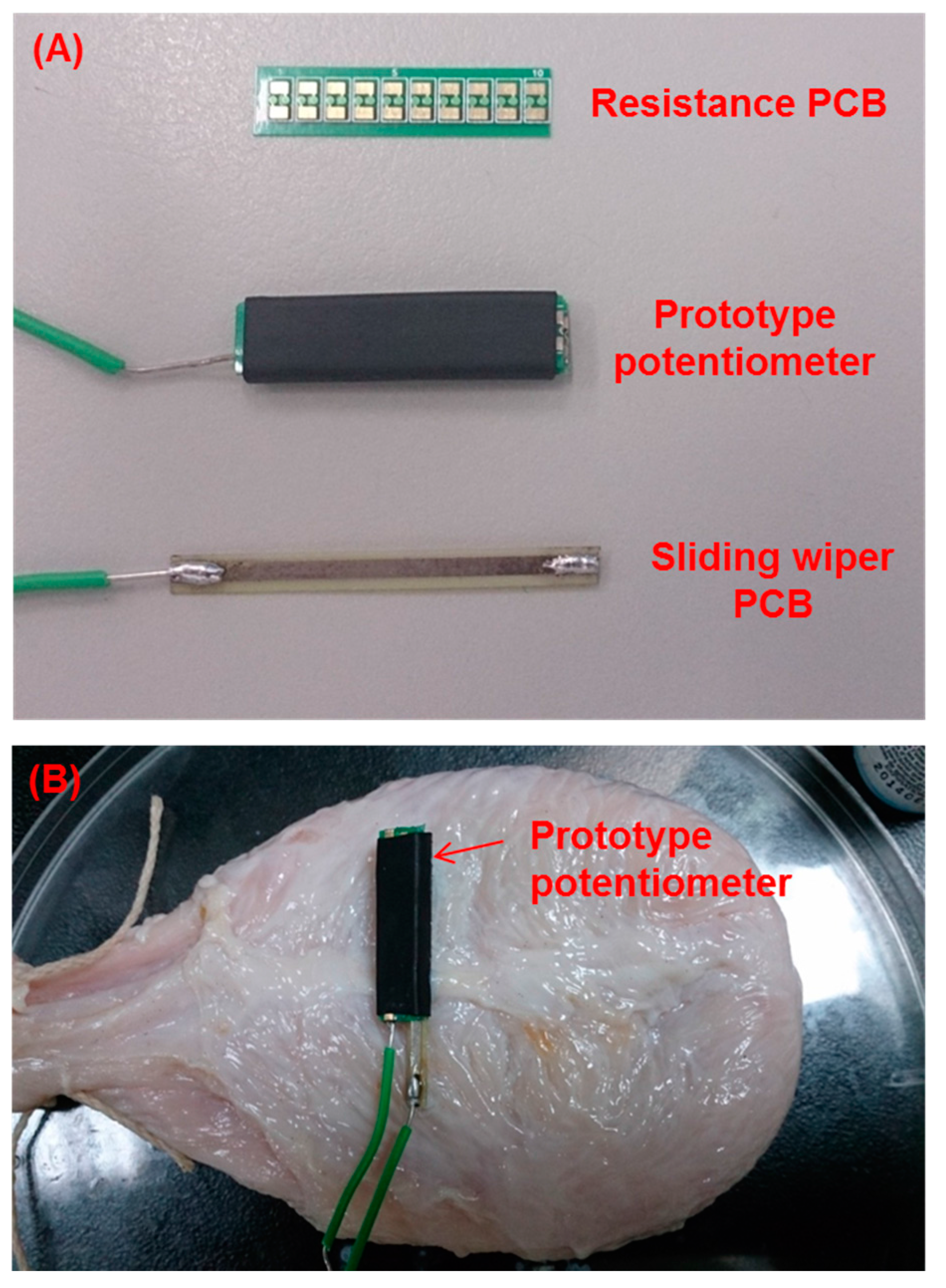
| Components | Length × Width (cm) | Thickness (mm) | Weight (g) |
|---|---|---|---|
| Resistance PCB | 2.4 × 5.6 | 0.28 | 0.3 |
| Sliding wiper PCB | 3.3 × 2.8 | 0.24 | 0.4 |
| Packaged potentiometer | 2.4 × 6.6 | 3.3 | 0.9 |
3.2. Bladder Geometric Model
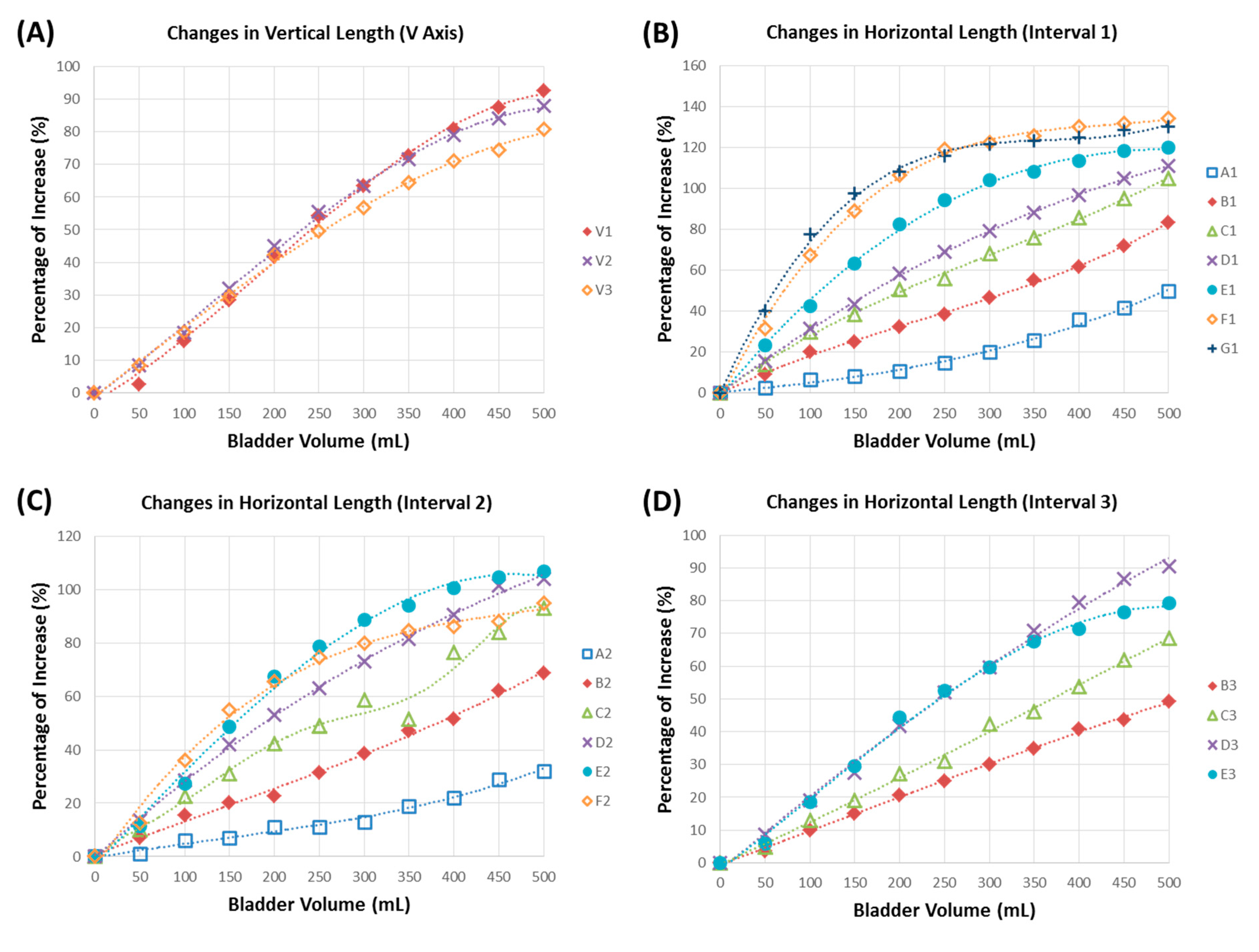
3.3. In Vitro Bladder Testing
| Infusion Volume (mL) | Volume Detection (mL) | Infused Volume/Volume Detected (%) |
|---|---|---|
| 49 ± 6 | 50 | 98.00 |
| 101 ± 18 | 100 | 101.00 |
| 148 ± 20 | 150 | 98.90 |
| 203 ± 20 | 200 | 101.70 |
| 243 ± 24 | 250 | 97.10 |
| 299 ± 18 | 300 | 99.70 |
| 350 ± 31 | 350 | 99.90 |
| 395 ± 21 | 400 | 98.80 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chang, H.Y.; Peng, C.W.; Chen, J.J.J.; Cheng, C.L.; de Groat, W.C. The time-frequency analysis of the pudendo-to-pudendal nerve and pelvic-to-pudendal nerve reflexes in anesthetized intact rats. J. Med. Biol. Eng. 2004, 24, 17–22. [Google Scholar]
- Fowler, C.J.; Griffiths, D.; de Groat, W.C. The neural control of micturition. Nat. Rev. Neurosci. 2008, 9, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.J. Neurological disorders of micturition and their treatment. Brain 1999, 122, 1213–1231. [Google Scholar] [CrossRef] [PubMed]
- Panicker, J.N.; de Seze, M.; Fowler, C.J. Rehabilitation in practice: Neurogenic lower urinary tract dysfunction and its management. Clin. Rehabil. 2010, 24, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Aboseif, S.; Tamaddon, K.; Chalfin, S.; Freedman, S.; Mourad, M.S.; Chang, J.H.; Kaptein, J.S. Sacral neuromodulation in functional urinary retention: An effective way to restore voiding. BJU Int. 2002, 90, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Burks, F.N.; Bui, D.T.; Peters, K.M. Neuromodulation and the neurogenic bladder. Urol. Clin. N. Am. 2010, 37, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Horvath, E.E.; Yoo, P.B.; Amundsen, C.L.; Webster, G.D.; Grill, W.M. Conditional and continuous electrical stimulation increase cystometric capacity in persons with spinal cord injury. Neurourol. Urodyn. 2010, 29, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hou, C.; Zheng, X.; Zhang, W.; Chen, A.; Xu, Z. Design and evaluation of a new bladder volume monitor. Arch. Phys. Med. Rehabil. 2009, 90, 1944–1947. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Skalak, T.C.; Schwenk, T.R.; Mahler, C.M.; Anne, A.; Finnerty, P.W.; Haber, H.L.; Weikle, R.M.; Feldman, M.D. Accuracy of the conductance catheter for measurement of ventricular volumes seen clinically: Effects of electric field homogeneity and parallel conductance. IEEE Trans. Biomed. Eng. 1997, 44, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Schlebusch, T.; Nienke, S.; Leonhardt, S.; Walter, M. Bladder volume estimation from electrical impedance tomography. Physiol. Meas. 2014, 35, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Merks, E.J.W.; Bouakaz, A.; Bom, N.; Lancee, C.T.; van der Steen, A.F.W.; de Jong, N. Design of a multilayer transducer for acoustic bladder volume assessment. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 53, 1730–1738. [Google Scholar] [CrossRef] [PubMed]
- Seif, C.; Herberger, B.; Cherwon, E.; Portillo, F.J.M.; Molitor, A.; Stieglitz, T.; Bohler, G.; Zendler, S.; Junemann, K.P.; Braun, P.M.; et al. Urinary bladder volumetry by means of a single retrosymphysically implantable ultrasound unit. Neurourol. Urodyn. 2004, 23, 680–684. [Google Scholar]
- Mendez, A.; Belghith, A.; Sawan, M. A dsp for sensing the bladder volume through afferent neural pathways. IEEE Trans. Biomed. Circuits Syst. 2014, 8, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Mendez, A.; Sawan, M.; Minagawa, T.; Wyndaele, J.J. Estimation of bladder volume from afferent neural activity. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Lai, C.H.; Kuo, T.S.; Chen, C.C.; Chen, Y.L.; Young, S.T.; Chen, S.C.; Lai, J.S.; Peng, C.W. Dual-channel neuromodulation of pudendal nerve with closed-loop control strategy to improve bladder functions. J. Med. Biol. Eng. 2014, 34, 82–89. [Google Scholar] [CrossRef]
- Provost, B.; Sawan, M. Proposed new bladder volume monitoring device based on impedance measurement. Med. Biol. Eng. Comput. 1997, 35, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, B.J.; Boggs, J.W.; Gustafson, K.J.; Creasey, G.H.; Grill, W.M. Detection of neurogenic detrusor contractions from the activity of the external anal sphincter in cat and human. Neurourol. Urodyn. 2006, 25, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Defreitas, G.; Lemack, G.E. The reproducibility of urodynamic findings in healthy female volunteers: Results of repeated studies in the same setting and after short-term follow-up. Neurourol. Urodyn. 2004, 23, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Rovner, E.S.; Wein, A.J. Evaluation of lower urinary tract symptoms in females. Curr. Opin. Urol. 2003, 13, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Scaldazza, C.V.; Morosetti, C. Effect of different sized transurethral catheters on pressure-flow studies in women with lower urinary tract symptoms. Urol. Int. 2005, 75, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Gill, B.C.; Fletter, P.C.; Zaszczurynski, P.J.; Perlin, A.; Yachia, D.; Damaser, M.S. Fluid volume conductance for determination of bladder volume. In Proceedings of the 3rd IEEE-EMBS International Summer School and Symposium on Medical Devices and Biosensors, Cambridge, MA, USA, 4–6 September 2006; pp. 115–117.
- Rajagopalan, S.; Sawan, M.; Ghafar-Zadeh, E.; Savadogo, O.; Chodavarapu, V.P. A polypyrrole-based strain sensor dedicated to measure bladder volume in patients with urinary dysfunction. Sensors 2008, 8, 5081–5095. [Google Scholar] [CrossRef]
- Lee, D.S.; Kim, S.J.; Sohn, D.W.; Choi, B.; Lee, M.K.; Lee, S.J.; Kim, S.W. Real-time bladder volume monitoring by the application of a new implantable bladder volume sensor for a small animal model. Kaohsiung J. Med. Sci. 2011, 27, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; McClure, T.; Lin, C.K.; Jea, D.; Dabiri, F.; Massey, T.; Sarrafzadeh, M.; Srivastava, M.; Montemagno, C.D.; Schulam, P.; et al. Development of a fully implantable wireless pressure monitoring system. Biomed. Microdevices 2009, 11, 259–264. [Google Scholar]
- Lee, W.S.; Kim, A.; Ziaie, B.; Raghunathan, V.; Powell, C.R. Up-Link: An ultra-low power implantable wireless system for long-term ambulatory urodynamics. In Proceedings of the Biomed. Circuits and Systems Conference (BioCAS), Lausanne, Switzerland, 22–24 October 2014.
- Majerus, S.J.A.; Fletter, P.C.; Damaser, M.S.; Garverick, S.L. Low-Power wireless micromanometer system for acute and chronic bladder-pressure monitoring. IEEE Trans. Biomed. Eng. 2011, 58, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Powell, C.R.; Ziaie, B. An implantable pressure sensing system with electromechanical interrogation scheme. IEEE Trans. Biomed. Eng. 2014, 61, 2209–2217. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; Mirzadeh, H.; Simjoo, M. Hydrophilic interpenetrating polymer networks of poly(dimethyl siloxane) (pdms) as biomaterial for cochlear implants. J. Biomater. Sci. Polym. Ed. 2006, 17, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.E.; Arner, A. Urinary bladder contraction and relaxation: Physiology and pathophysiology. Physiol. Rev. 2004, 84, 935–986. [Google Scholar] [CrossRef] [PubMed]
- Finkbeiner, A.E. In vitro responses of detrusor smooth muscle to stretch and relaxation. Scand. J. Urol. Nephrol. 1999, 201, 5–11. [Google Scholar] [CrossRef]
- De Groat, W.C. Anatomy and physiology of the lower urinary tract. Urol. Clin. N. Am. 1993, 20, 383–401. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-C.; Hsieh, T.-H.; Fan, W.-J.; Lai, C.-H.; Chen, C.-L.; Wei, W.-F.; Peng, C.-W. Design and Evaluation of Potentiometric Principles for Bladder Volume Monitoring: A Preliminary Study. Sensors 2015, 15, 12802-12815. https://doi.org/10.3390/s150612802
Chen S-C, Hsieh T-H, Fan W-J, Lai C-H, Chen C-L, Wei W-F, Peng C-W. Design and Evaluation of Potentiometric Principles for Bladder Volume Monitoring: A Preliminary Study. Sensors. 2015; 15(6):12802-12815. https://doi.org/10.3390/s150612802
Chicago/Turabian StyleChen, Shih-Ching, Tsung-Hsun Hsieh, Wen-Jia Fan, Chien-Hung Lai, Chun-Lung Chen, Wei-Feng Wei, and Chih-Wei Peng. 2015. "Design and Evaluation of Potentiometric Principles for Bladder Volume Monitoring: A Preliminary Study" Sensors 15, no. 6: 12802-12815. https://doi.org/10.3390/s150612802







