Optical Detection of Paraoxon Using Single-Walled Carbon Nanotube Films with Attached Organophosphorus Hydrolase-Expressed Escherichia coli
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Carbon Nanotube (CNT) Solution
2.2. Preparation of OPH-Expressing E. coli
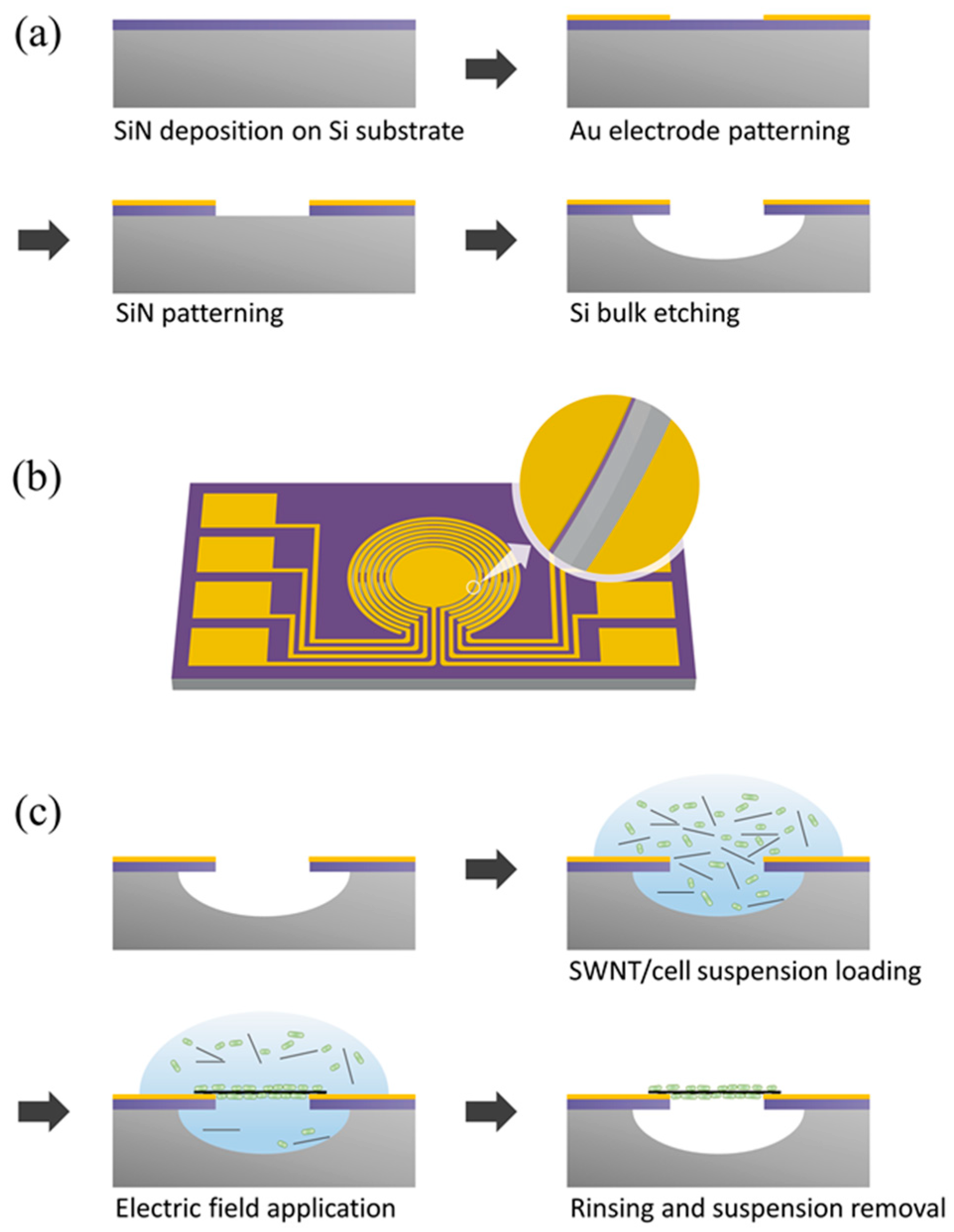
2.3. Fabrication Process for the Cantilever Electrodes
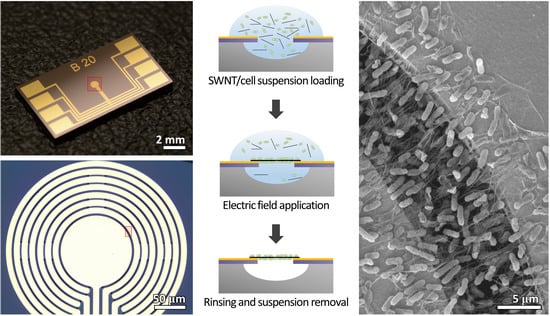
2.4. Fabrication of E. coli-Attached SWNT Film
2.5. Fabrication of Paraoxon-Sensing Platform

2.6. Paraoxon Measurement Setup
3. Results and Discussion
3.1. E. coli-Attached SWNT Film Fabrication
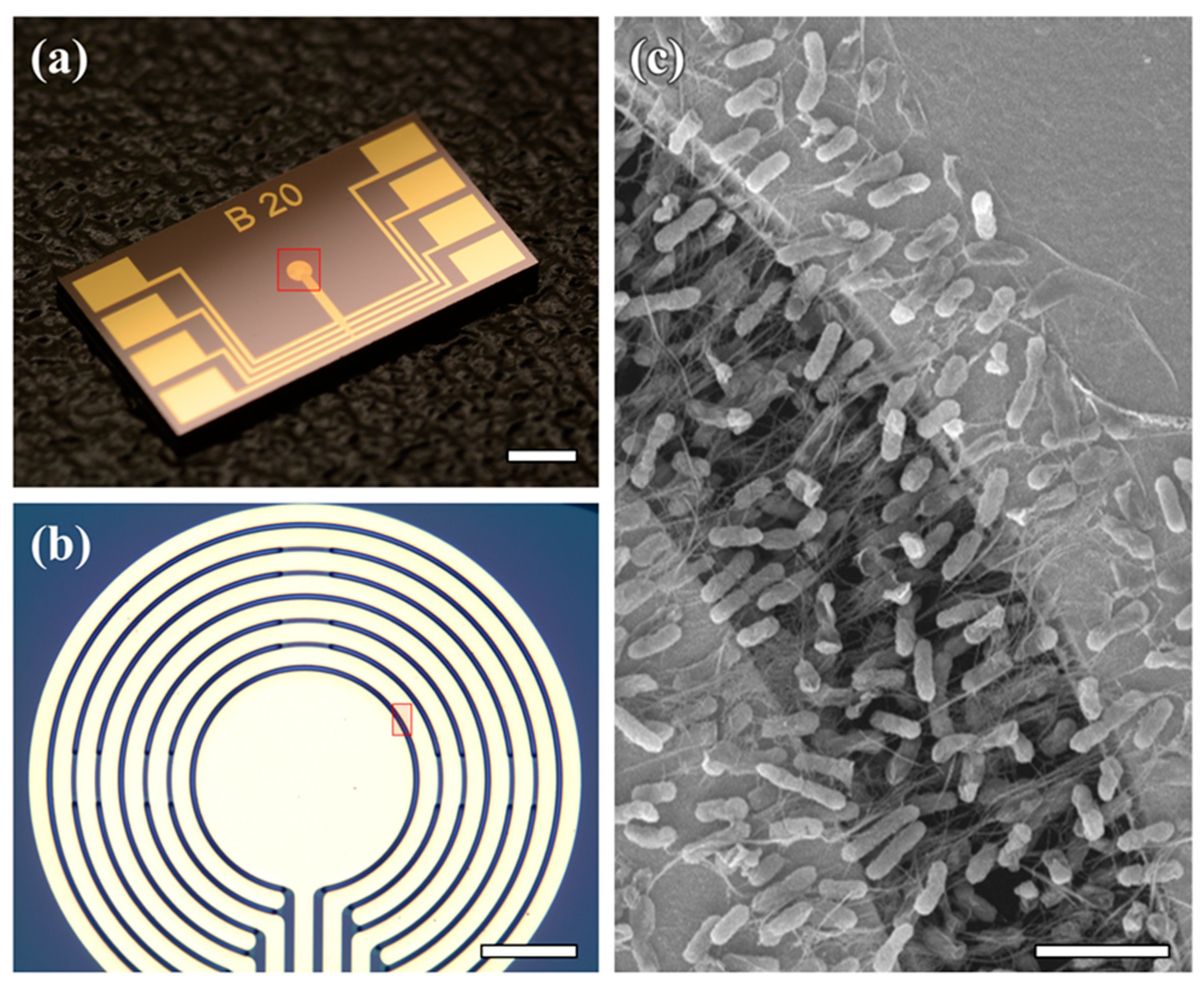
3.2. Structural Advantage of E. coli-Attached SWNT Film
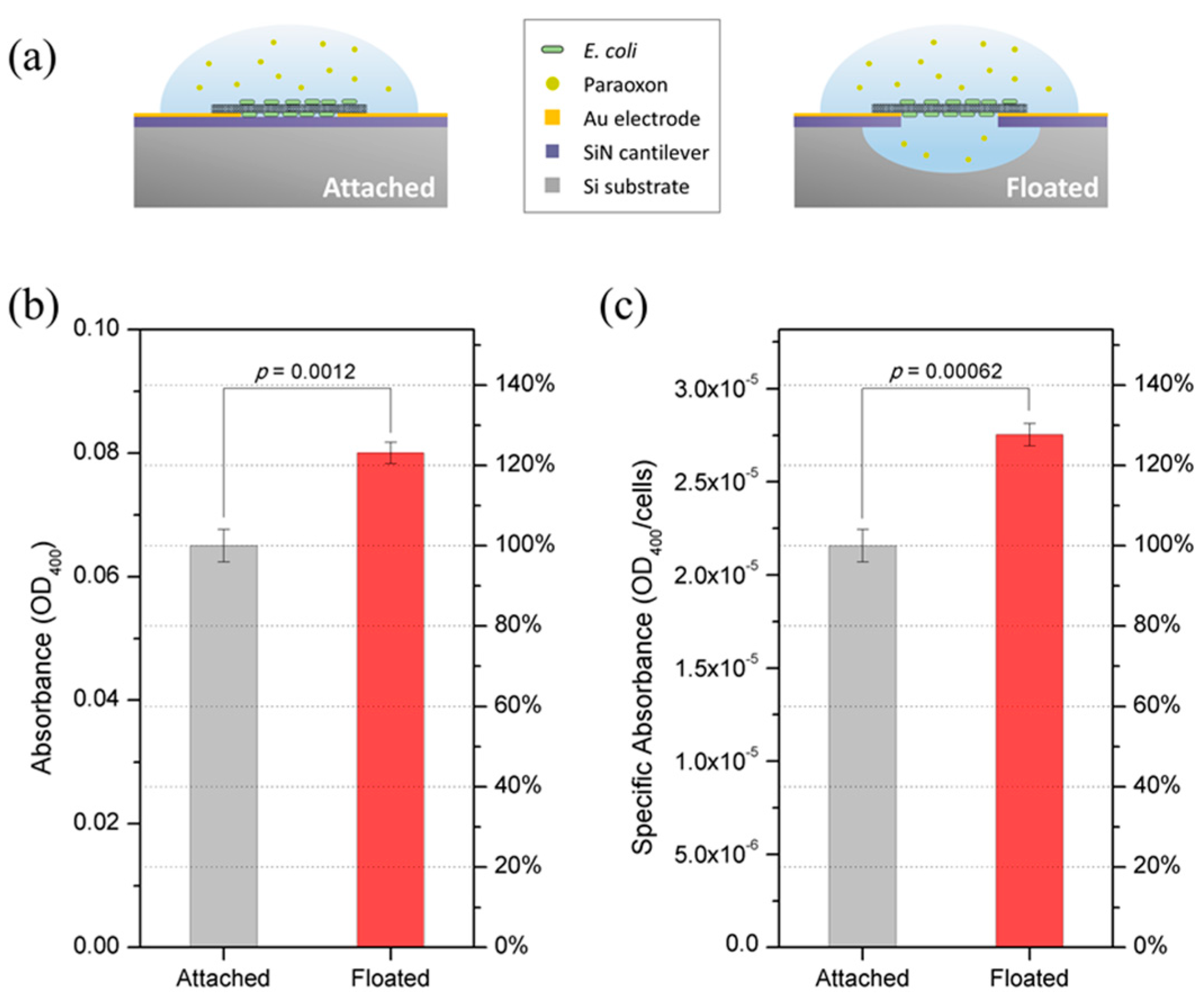
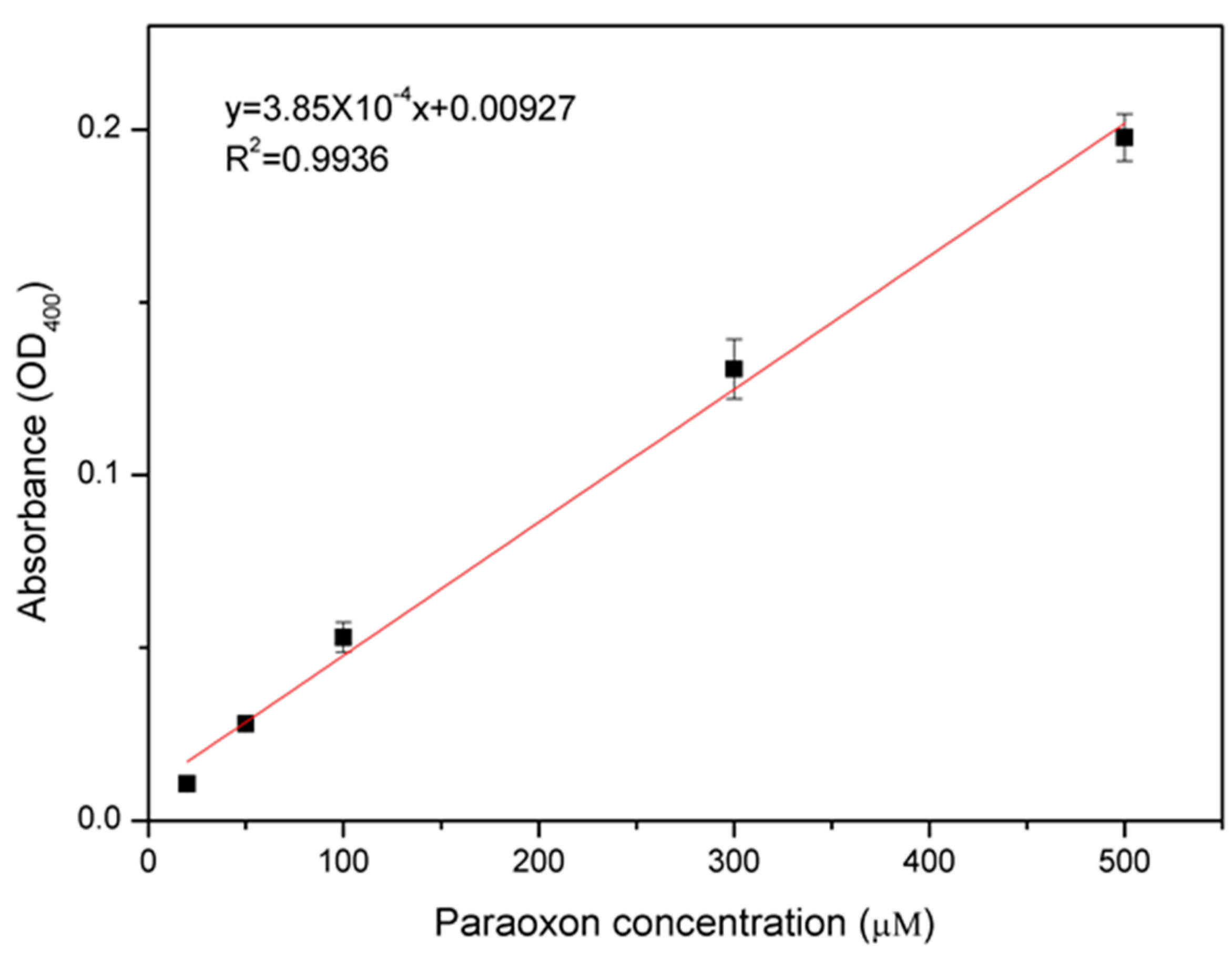
3.3. Paraoxon Measurement and Comparison with Other Whole-Cell OP Sensors
| Immobilization Method | Cell Type | Substrate | Specific Activity (μmol·min−1·mm−2) | Time for Cell Immobilization | Long-Term Storage Stability | Reference |
|---|---|---|---|---|---|---|
| MAP-based adhesion | E. coli BL21 | Paraoxon | 19.06 | >2 h | 28 d (80%) | [21] |
| Glutaraldehyde-based chemical crosslinking | Sphingomonas sp. JK1 | 4(methyl parathion) | 43.18 | >1 h | 18 d (80%) | [25] |
| Glutaraldehyde-based chemical crosslinking | Sphingomonas sp. JK1 | 4(methyl parathion) | 43.12 | >1 h | 32 d (90%) | [26] |
| Adsorption by Van der Waals force | E. coli BL21 | Paraoxon | 119.91 | <100 s | 37 d (90%), 67 d (80%) | This study |
| Equipment | Conventional Spectrophotometer | Conventional Spectrophotometer | Microplate Reader | Microvolume Spectrophotometer |
|---|---|---|---|---|
| Sample container | cuvette (disposable) | cuvette (quartz) | 96-well microplate | Directly on the equipment |
| Sample volume (μL) | 70 | 50 | 200 | 0.5 |
| Light path length (mm) | 10 | 10 | 6.5 | 0.5 |
| Required area (mm2) | 1.374 | 0.981 | 6.038 | 0.196 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cremisini, C.; di Sario, S.; Mela, J.; Pilloton, R.; Palleschi, G. Evaluation of the use of free and immobilised acetylcholinesterase for paraoxon detection with an amperometric choline oxidase based biosensor. Anal. Chim. Acta 1995, 311, 273–280. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, H.; John, R. Development of a quantitative relationship between inhibition percentage and both incubation time and inhibitor concentration for inhibition biosensors—Theoretical and practical considerations. Biosens. Bioelectron. 2001, 16, 1119–1126. [Google Scholar] [CrossRef]
- Sherma, J. Pesticides. Anal. Chem. 1993, 65, 40R–54R. [Google Scholar] [CrossRef]
- Miller, J.K.; Lenz, D.E. Development of an immunoassay for diagnosis of exposure to toxic organophosphorus compounds. J. Appl. Toxicol. 2001, 21, S23–S26. [Google Scholar] [CrossRef] [PubMed]
- Musameh, M.M.; Gao, Y.; Hickey, M.; Kyratzis, I.L. Application of Carbon Nanotubes in the Extraction and Electrochemical Detection of Organophosphate Pesticides: A Review. Anal. Lett. 2012, 45, 783–803. [Google Scholar] [CrossRef]
- Ghanem, E.; Raushel, F.M. Detoxification of organophosphate nerve agents by bacterial phosphotriesterase. Toxicol. Appl. Pharmacol. 2005, 207, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.M.-H.; Mulchandani, A.; Chen, W. Functional analysis of organophosphorus hydrolase variants with high degradation activity towards organophosphate pesticides. Protein Eng. Des. Sel. 2006, 19, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Mello, S.V.; Coutures, C.; Leblanc, R.M.; Cheng, T.-C.; Rastogi, V.K.; DeFrank, J.J. Interaction between organophosphorous hydrolase and paraoxon studied by surface chemistry in situ at air–water interface. Talanta 2001, 55, 881–887. [Google Scholar] [CrossRef]
- Aubert, S.D.; Li, Y.; Raushel, F.M. Mechanism for the Hydrolysis of Organophosphates by the Bacterial Phosphotriesterase. Biochemistry (Mosc.) 2004, 43, 5707–5715. [Google Scholar] [CrossRef] [PubMed]
- Dumas, D.P.; Caldwell, S.R.; Wild, J.R.; Raushel, F.M. Purification and properties of the phosphotriesterase from Pseudomonas diminuta. J. Biol. Chem. 1989, 264, 19659–19665. [Google Scholar] [PubMed]
- Wong, K.-Y.; Gao, J. The Reaction Mechanism of Paraoxon Hydrolysis by Phosphotriesterase from Combined QM/MM Simulations†. Biochemistry (Mosc.) 2007, 46, 13352–13369. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Zhan, C.-G.; Ornstein, R.L. Theoretical studies of reaction pathways and energy barriers for alkaline hydrolysis of phosphotriesterase substrates paraoxon and related toxic phosphofluoridate nerve agents. J. Chem. Soc. Perkin Trans 2 2001, 2355–2363. [Google Scholar]
- Singh, A.K.; Flounders, A.W.; Volponi, J.V.; Ashley, C.S.; Wally, K.; Schoeniger, J.S. Development of sensors for direct detection of organophosphates. Part I: Immobilization, characterization and stabilization of acetylcholinesterase and organophosphate hydrolase on silica supports. Biosens. Bioelectron. 1999, 14, 703–713. [Google Scholar] [CrossRef]
- Van Dyk, J.S.; Pletschke, B. Review on the use of enzymes for the detection of organochlorine, organophosphate and carbamate pesticides in the environment. Chemosphere 2011, 82, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.G.; Lim, G.-B.; Cha, H.J. Functional periplasmic secretion of organophosphorous hydrolase using the twin-arginine translocation pathway in Escherichia coli. J. Biotechnol. 2005, 118, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Lee, S.Y. Secretory and extracellular production of recombinant proteins using Escherichia coli. Appl. Microbiol. Biotechnol. 2004, 64, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Mulchandani, A.; Mulchandani, P.; Kaneva, I.; Chen, W. Biosensor for Direct Determination of Organophosphate Nerve Agents Using Recombinant Escherichia coli with Surface-Expressed Organophosphorus Hydrolase. 1. Potentiometric Microbial Electrode. Anal. Chem. 1998, 70, 4140–4145. [Google Scholar] [PubMed]
- Mulchandani, A.; Kaneva, I.; Chen, W. Biosensor for Direct Determination of Organophosphate Nerve Agents Using Recombinant Escherichia coli with Surface-Expressed Organophosphorus Hydrolase. 2. Fiber-Optic Microbial Biosensor. Anal. Chem. 1998, 70, 5042–5046. [Google Scholar] [CrossRef] [PubMed]
- Richins, R.D.; Kaneva, I.; Mulchandani, A.; Chen, W. Biodegradation of organophosphorus pesticides by surface-expressed organophosphorus hydrolase. Nat. Biotech. 1997, 15, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, M.; Mulchandani, A.; Chen, W. Simultaneous degradation of organophosphorus pesticides and p-nitrophenol by a genetically engineered Moraxella sp. with surface-expressed organophosphorus hydrolase. Biotechnol. Bioeng. 2001, 76, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Choi, B.-H.; Seo, J.H.; Lim, G.; Cha, H.J. Mussel adhesive protein-based whole cell array biosensor for detection of organophosphorus compounds. Biosens. Bioelectron. 2013, 41, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.G.; Choi, S.S.; Cha, H.J. Enhanced Biodegradation of Toxic Organophosphate Compounds Using Recombinant Escherichia coli with Sec Pathway-Driven Periplasmic Secretion of Organophosphorus Hydrolase. Biotechnol. Prog. 2006, 22, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.G.; Kim, C.S.; Seo, J.H.; Kim, I.G.; Choi, S.S.; Ha, J.H.; Nam, S.W.; Lim, G.; Cha, H.J. Coexpression of molecular chaperone enhances activity and export of organophosphorus hydrolase in Escherichia coli. Biotechnol. Prog. 2012, 28, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Seo, J.H.; Kang, D.G.; Cha, H.J. Engineered whole-cell biocatalyst-based detoxification and detection of neurotoxic organophosphate compounds. Biotechnol. Adv. 2014, 32, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; D’Souza, S.F. An optical microbial biosensor for detection of methyl parathion using Sphingomonas sp. immobilized on microplate as a reusable biocomponent. Biosens. Bioelectron. 2010, 26, 1292–1296. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; D’Souza, S.F. Immobilization of microbial cells on inner epidermis of onion bulb scale for biosensor application. Biosens. Bioelectron. 2011, 26, 4399–4404. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Jha, S.K.; D’Souza, S.F. Optical microbial biosensor for detection of methyl parathion pesticide using Flavobacterium sp. whole cells adsorbed on glass fiber filters as disposable biocomponent. Biosens. Bioelectron. 2006, 21, 2100–2105. [Google Scholar] [CrossRef] [PubMed]
- Michelini, E.; Roda, A. Staying alive: New perspectives on cell immobilization for biosensing purposes. Anal. Bioanal. Chem. 2012, 402, 1785–1797. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.; Lewis, S. Sax’s Dangerous Properties of Industrial Materials; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Cassidy, M.B.; Lee, H.; Trevors, J.T. Environmental applications of immobilized microbial cells: A review. J. Ind. Microbiol. 1996, 16, 79–101. [Google Scholar] [CrossRef]
- Park, J.K.; Chang, H.N. Microencapsulation of microbial cells. Biotechnol. Adv. 2000, 18, 303–319. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial Biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; An, T.; Choi, W.; Kim, C.S.; Cha, H.J.; Lim, G. Site-specific immobilization of microbes using carbon nanotubes and dielectrophoretic force for microfluidic applications. RSC Adv. 2013, 4, 1347–1351. [Google Scholar] [CrossRef]
- Kanai, Y.; Khalap, V.R.; Collins, P.G.; Grossman, J.C. Atomistic Oxidation Mechanism of a Carbon Nanotube in Nitric Acid. Phys. Rev. Lett. 2010, 104. [Google Scholar] [CrossRef]
- Lee, G.-W.; Kumar, S. Dispersion of Nitric Acid-Treated SWNTs in Organic Solvents and Solvent Mixtures. J. Phys. Chem. B 2005, 109, 17128–17133. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.W.; Popa-Nita, S.; Shapter, J.G. Measurement of functionalised carbon nanotube carboxylic acid groups using a simple chemical process. Carbon 2006, 44, 1137–1141. [Google Scholar] [CrossRef]
- An, T.; Kim, K.S.; Hahn, S.K.; Lim, G. Real-time, step-wise, electrical detection of protein molecules using dielectrophoretically aligned SWNT-film FET aptasensors. Lab. Chip 2010, 10, 2052–2056. [Google Scholar] [CrossRef] [PubMed]
- Cang-Rong, J.T.; Pastorin, G. The influence of carbon nanotubes on enzyme activity and structure: Investigation of different immobilization procedures through enzyme kinetics and circular dichroism studies. Nanotechnology 2009, 20. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, I.; Kim, G.H.; Kim, C.S.; Cha, H.J.; Lim, G. Optical Detection of Paraoxon Using Single-Walled Carbon Nanotube Films with Attached Organophosphorus Hydrolase-Expressed Escherichia coli. Sensors 2015, 15, 12513-12525. https://doi.org/10.3390/s150612513
Kim I, Kim GH, Kim CS, Cha HJ, Lim G. Optical Detection of Paraoxon Using Single-Walled Carbon Nanotube Films with Attached Organophosphorus Hydrolase-Expressed Escherichia coli. Sensors. 2015; 15(6):12513-12525. https://doi.org/10.3390/s150612513
Chicago/Turabian StyleKim, Intae, Geon Hwee Kim, Chang Sup Kim, Hyung Joon Cha, and Geunbae Lim. 2015. "Optical Detection of Paraoxon Using Single-Walled Carbon Nanotube Films with Attached Organophosphorus Hydrolase-Expressed Escherichia coli" Sensors 15, no. 6: 12513-12525. https://doi.org/10.3390/s150612513