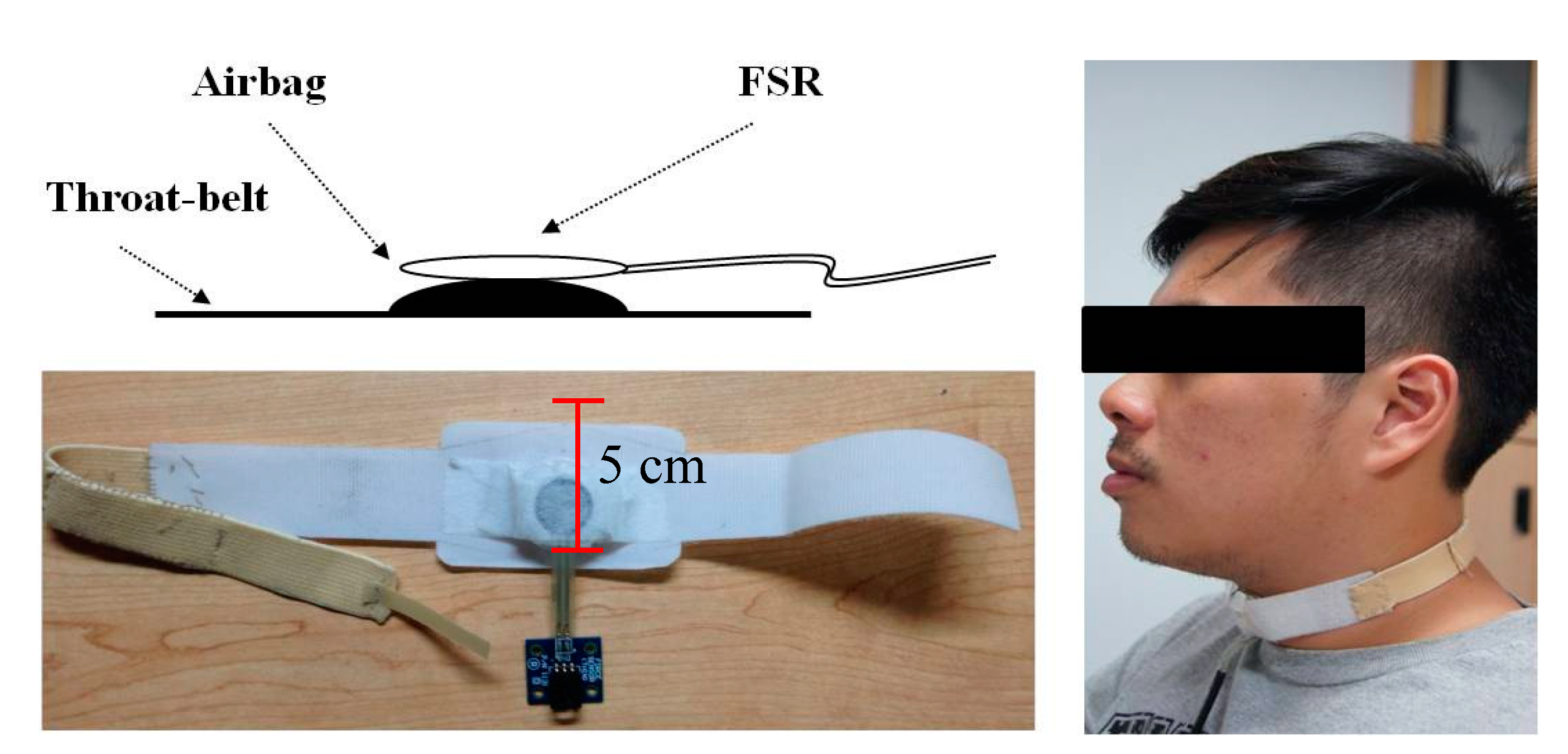
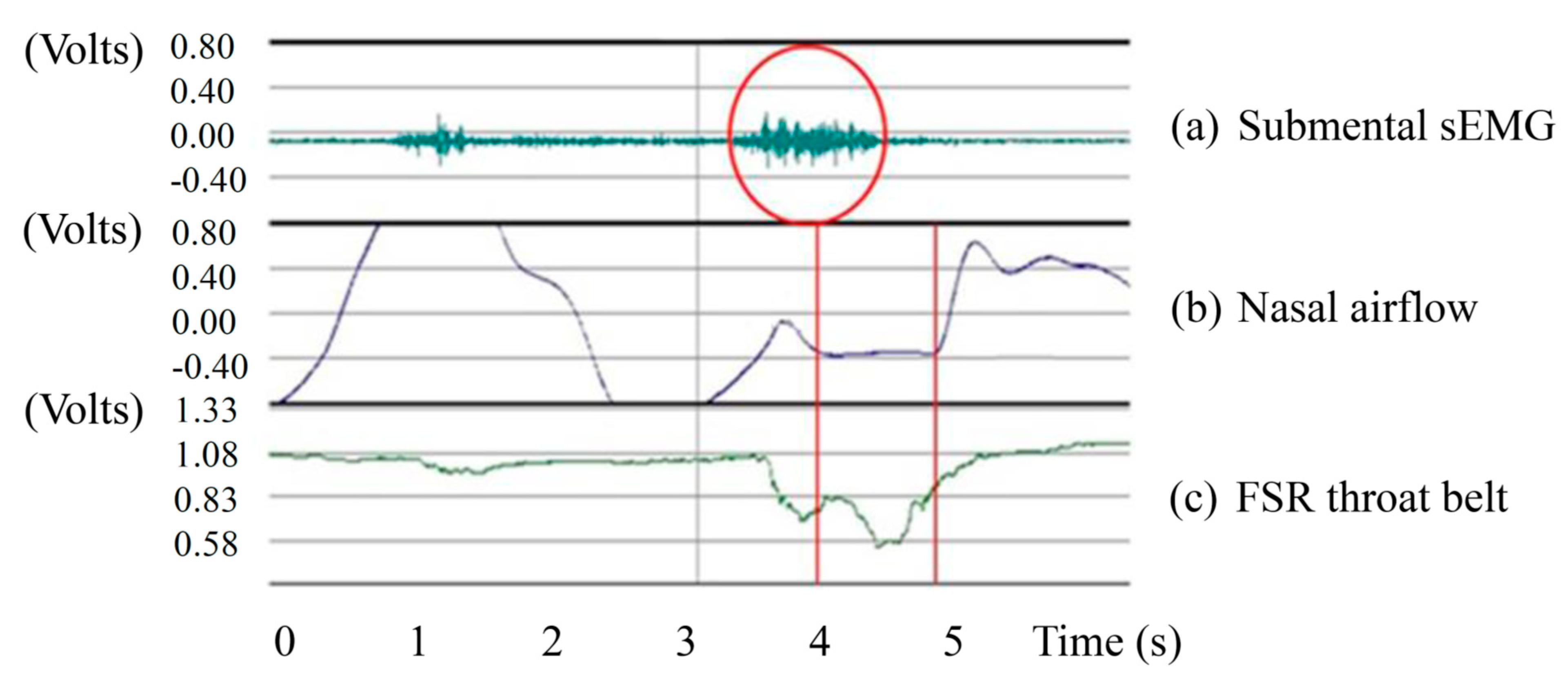
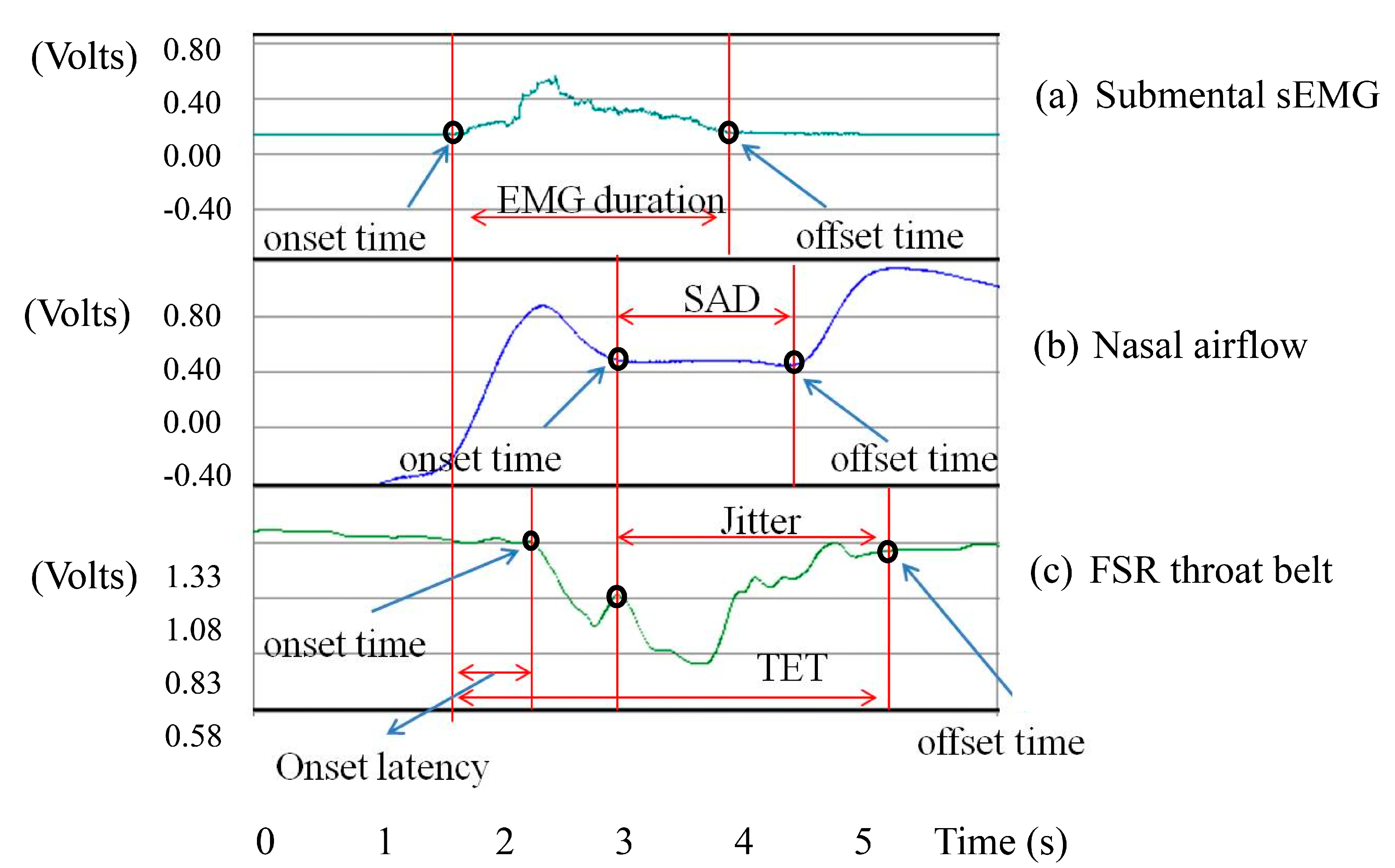
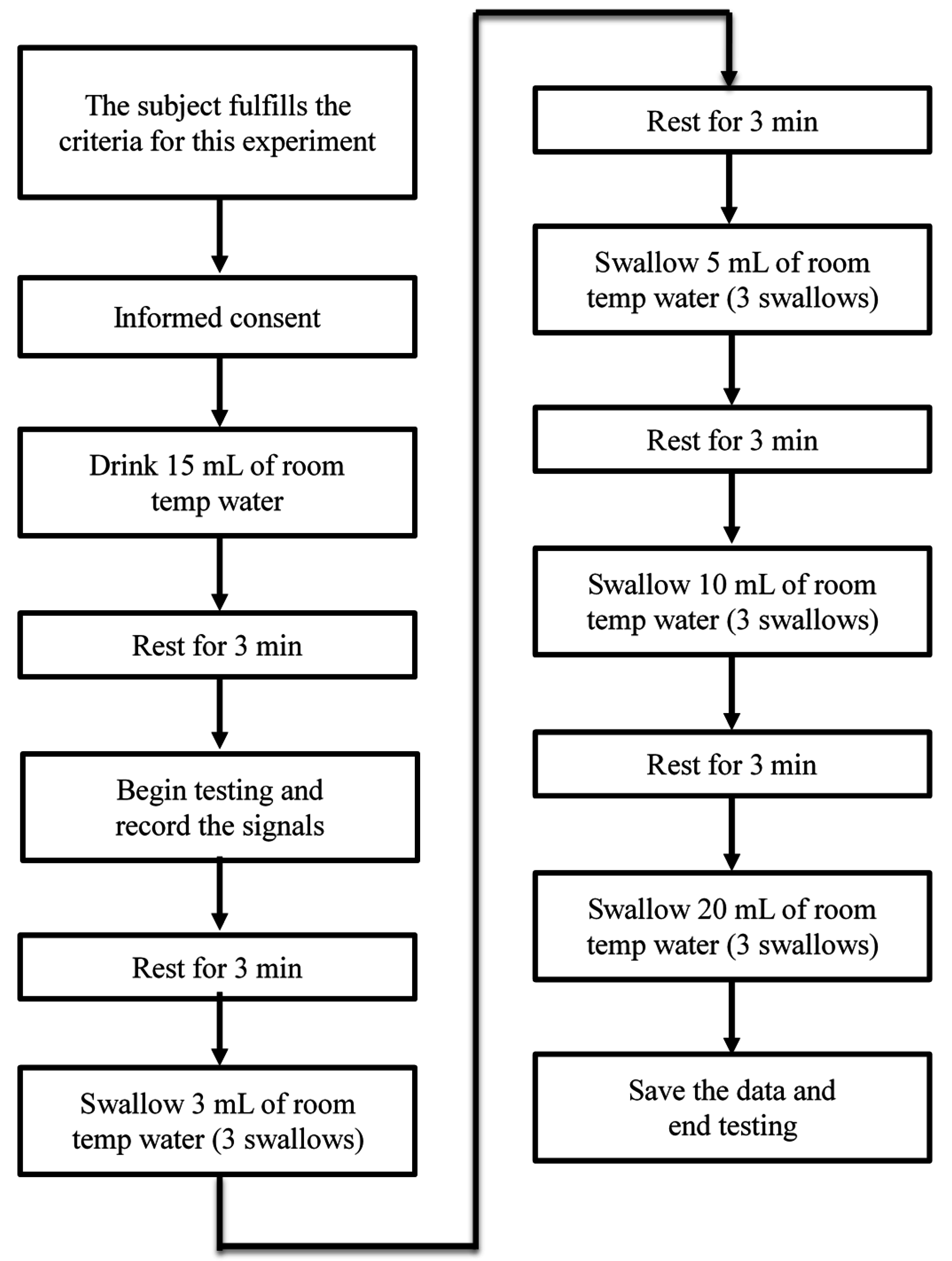
In this section, we will show the results measured by the submental muscle sEMG, the nasal airflow, and the FSR throat belt, as well as the correlation analysis between them. Our goal is to show that the proposed belt can have good sensitivity to the effects of age, the volume of water swallowed, or the gender of the subjects performing the swallowing functions.
4.1.1. The Results of sEMG and Nasal Air Flow
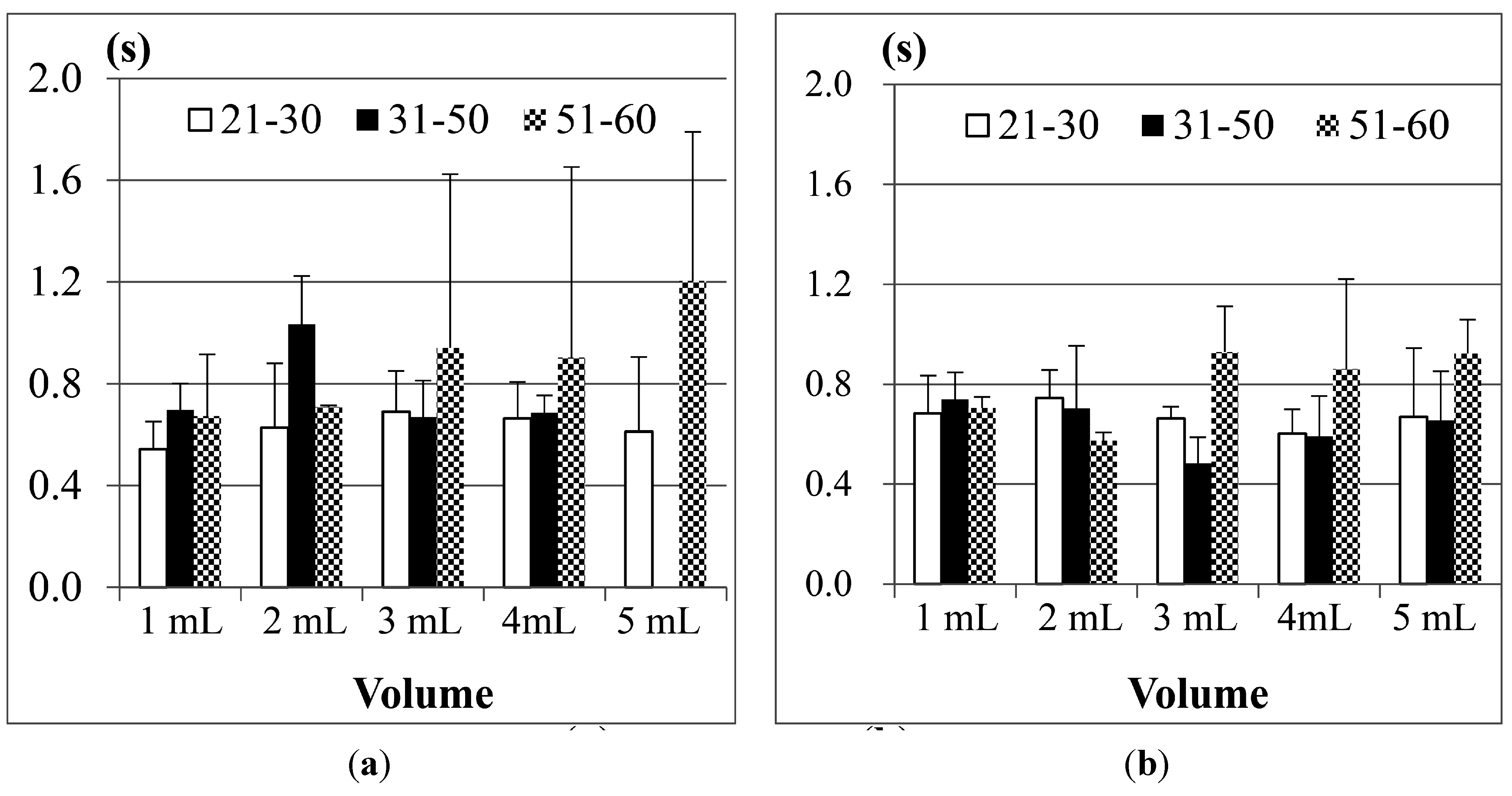
Figure 10 shows the comparison results of sEMG in different age groups, where (a) is for male participants and (b) is for female participants. The first point we found is, although 1 mL water is the smallest water volume, it does not mean that the participants (no matter whether male or female) needed the least time to swallow it. Also, as the age of the participants increases, the sEMG duration will have a slight increase. This fact was also found in other previous studies [
21,
23,
24]. A more acceptable reason is that 1 mL water is too small a sample so most participants need to use more strength and time to swallow it. The other point is, for 3, 5 or even 10 mL water, there are no obvious differences among different age groups. This result shows that using sEMG alone would not be a good approach to evaluate the swallowing function, because the duration time of sEMG actually cannot represent the whole swallowing process (however, in some previous studies, like [
18,
24], they evaluated the swallowing function by only observing the strength or the duration of sEMG).
Figure 10.
Comparison of the average duration time of the submental sEMG in different age groups for (a) male; and (b) female participants. The X-axis lists the water volumes and the Y-axis shows the mean duration time.
Figure 10.
Comparison of the average duration time of the submental sEMG in different age groups for (a) male; and (b) female participants. The X-axis lists the water volumes and the Y-axis shows the mean duration time.
Note that the results of the 31 to 50 years old male group in the 20 mL trials are not included in
Figure 10a. This is because the participants in this group peformed piecemeal swallowing (also known as “piecemeal deglutition” [
13]), where they divided the water into smaller volumes, and finished the swallowing in several times. In this case, the volume represents a dysphagia limit and the duration time of swallowing fluctuates, which makes measurement and analysis difficult. Thus we treat piecemeal swallowing as a special case and will discuss it later.
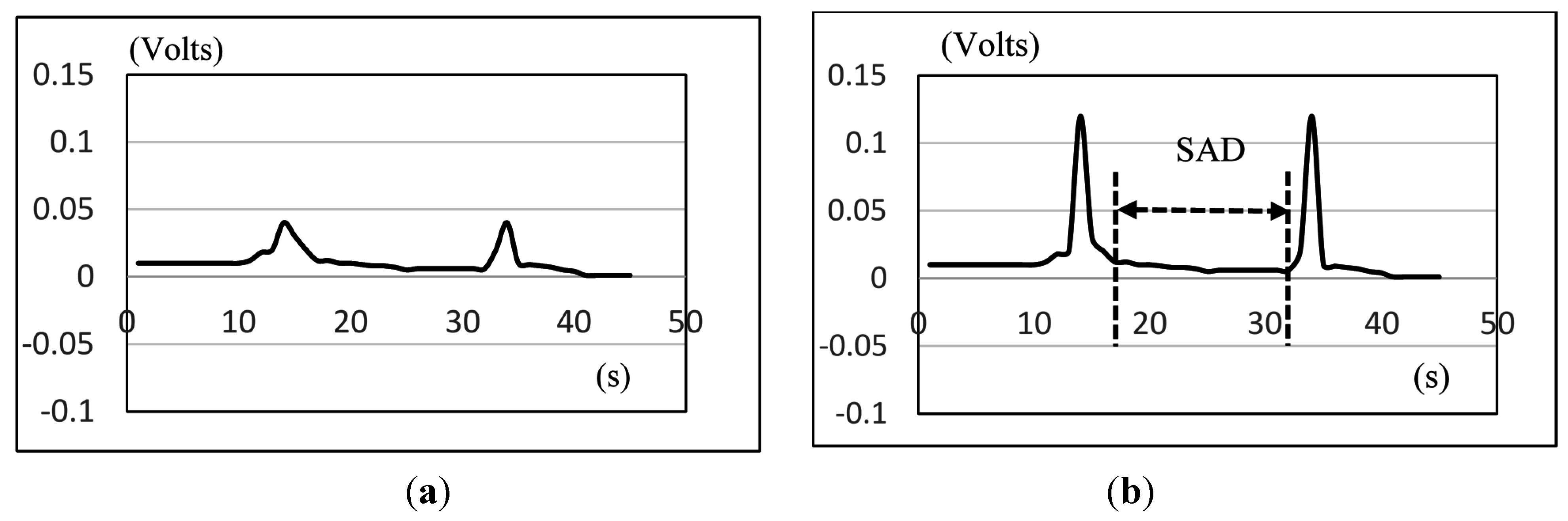
Figure 11 shows the SAD results, calculated from the nasal airflow, where (a) is for male participants; and (b) is for female participants. Here also the 20 mL water results of the male group aged from 31 to 50 years old are not included due to the piecemeal swallowing mentioned above.
Figure 11.
Comparison of the average duration of SAD in different age groups for: (a) male; and (b) female participants. The X-axis lists the water volumes and the Y-axis shows the mean duration time.
Figure 11.
Comparison of the average duration of SAD in different age groups for: (a) male; and (b) female participants. The X-axis lists the water volumes and the Y-axis shows the mean duration time.
As we can see in
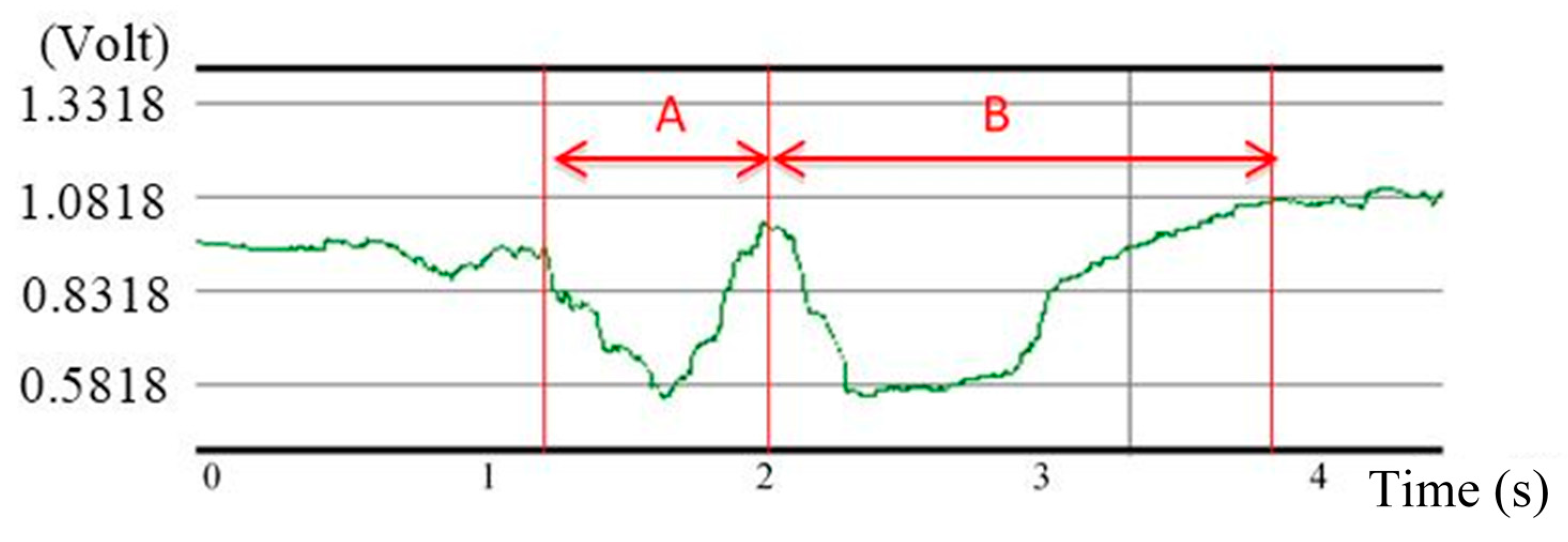
Figure 11, the male and the female participants aged from 51 to 60 years old have obviously longer SAD duration in the 5, 10, and 20 mL tests than the other age participants, but for the participants aged from 21 to 30 and from 31 to 50, there are no significant differences for different volumes of water. We know that when the throat pushes the water or bolus into the stomach via the esophagus, the breathing will undergo a brief apnea period. The duration of this period (
i.e., SAD in
Figure 6) will differ, mostly depending on each one’s swallowing behavior. In our tests, some participants stopped breathing before swallowing for a very short time. Even after swallowing the water, some participants will continue to have this apnea period for a while.
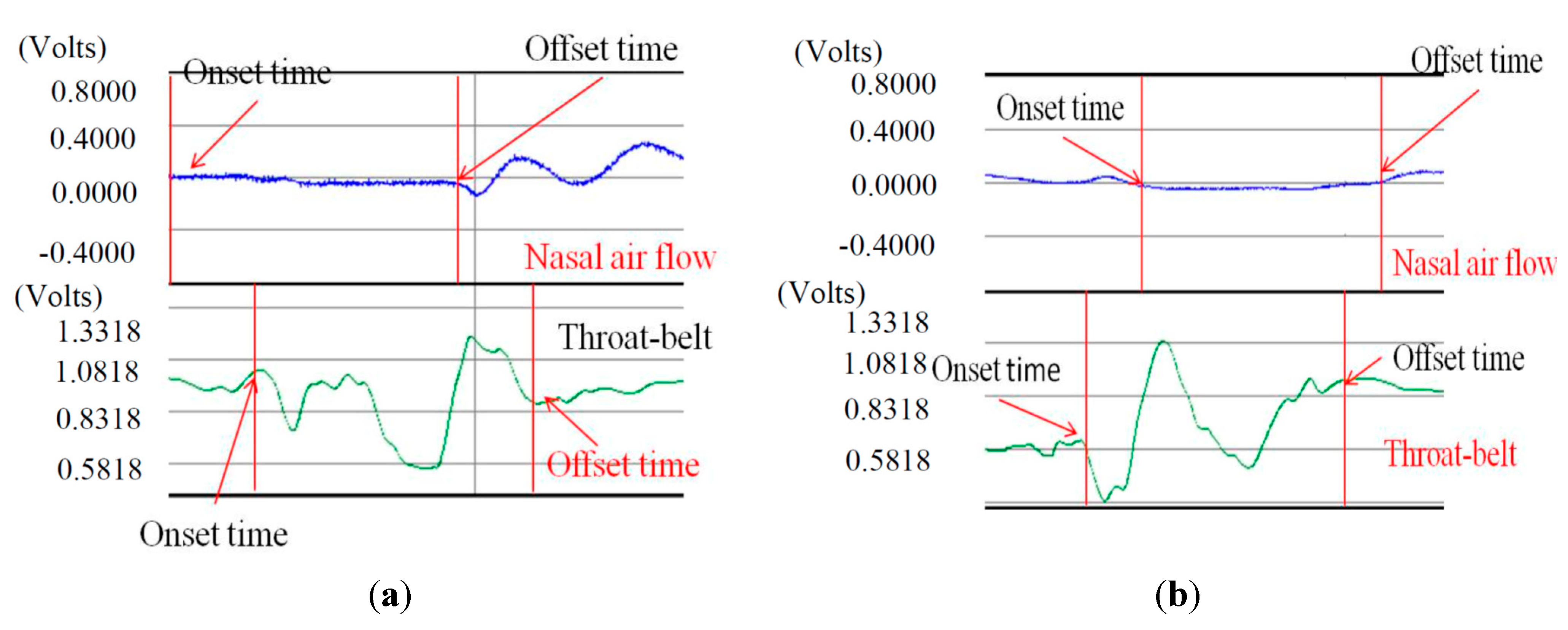
Figure 12 illustrates both of these cases if we compare the nasal airflow signals with the FSR signals from our throat belt.
Figure 12.
Two cases of SAD: (a) the onset time of SAD is earlier than the onset time of FSR; (b) the offset time of SAD is later than the time of FSR.
Figure 12.
Two cases of SAD: (a) the onset time of SAD is earlier than the onset time of FSR; (b) the offset time of SAD is later than the time of FSR.
Figure 12a is the case of which the participant is a 21 year-old male in the 5 mL test. The nasal air flow signals show that the onset time of SAD (
i.e., apnea period) is earlier than the onset time of FSR (
i.e., the time of the thyroid cartilage starts to move). Different from
Figure 12a,
Figure 12b is another case of which the subject is a 40 year-old male in the 10 mL test. In this case, the offset time of SAD happens later than the time the thyroid cartilage stops the motions. This is why in in
Figure 11a there are some cases where the standard deviations are particularly large. In our testing, we find that the swallowing function cannot be distinguished clearly only by sEMG or only by SAD. Thus in the next section, we will add the FSR throat belt to the testing for swallowing function evaluation.
4.1.2. The Results of Jitter and TET by the Throat Belt
Recall that the duration of Jitter and TET (in
Figure 6) can be used to measure the motions of the thyroid cartilage. According to the research of Wang and Chuang
et al. [
21,
23], the duration of Jitter and TET will be affected by the bolus volume. Here we use the FSR throat belt to measure Jitter and TET, and compare the effects of the bolus volumes on different genders and age groups.
(1) Comparison between men and women
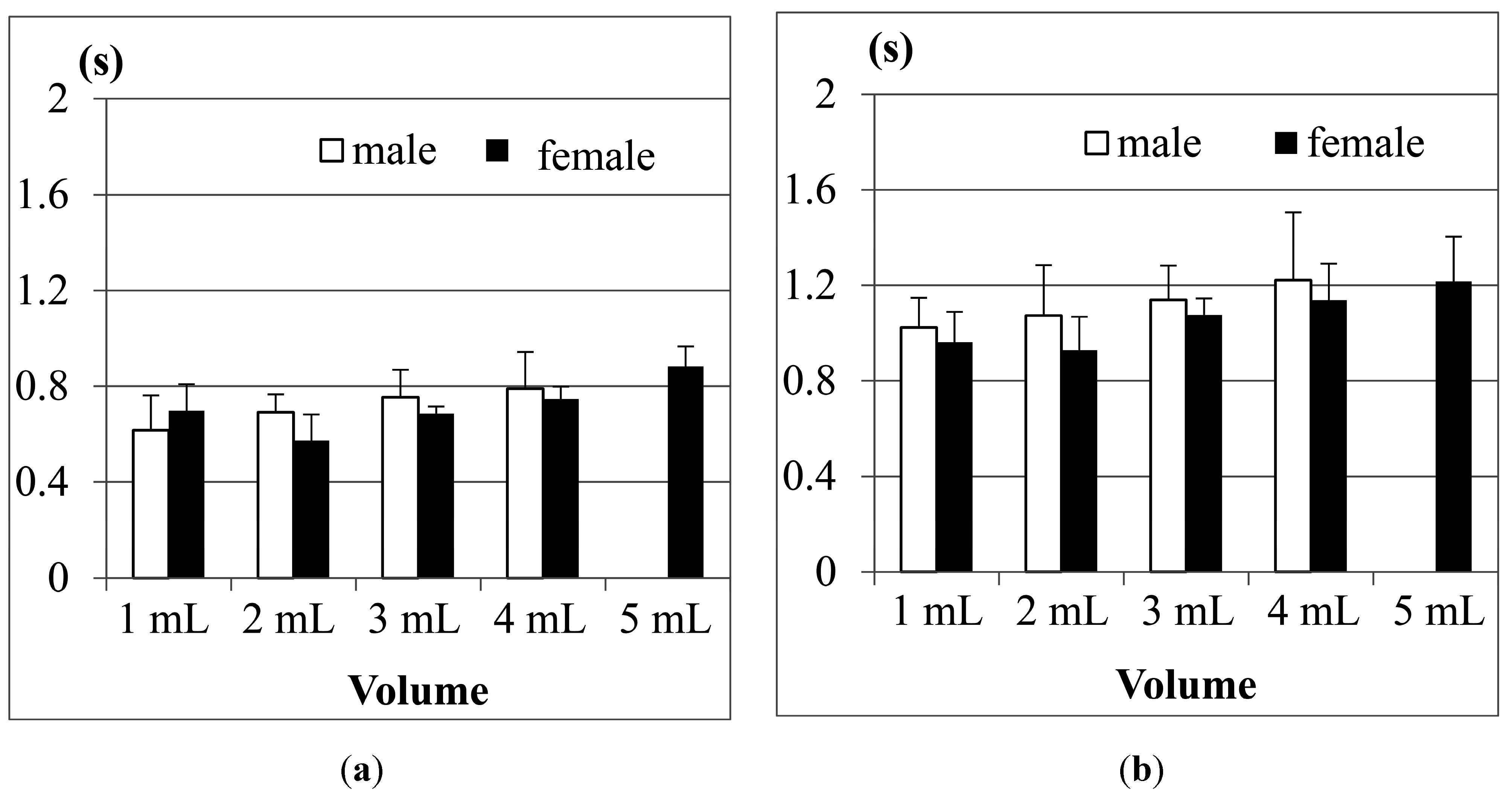
Figure 13a shows the results of Jitter, and
Figure 13b shows the results of TET for the participants aged from 21 to 30 years old in tests with different water volumes. The results show that both male and female participants have longer Jitter and TET durations as the volumes of water increase, except for the case of 1 mL of water. The reason is the same as what we mentioned in
Figure 10.
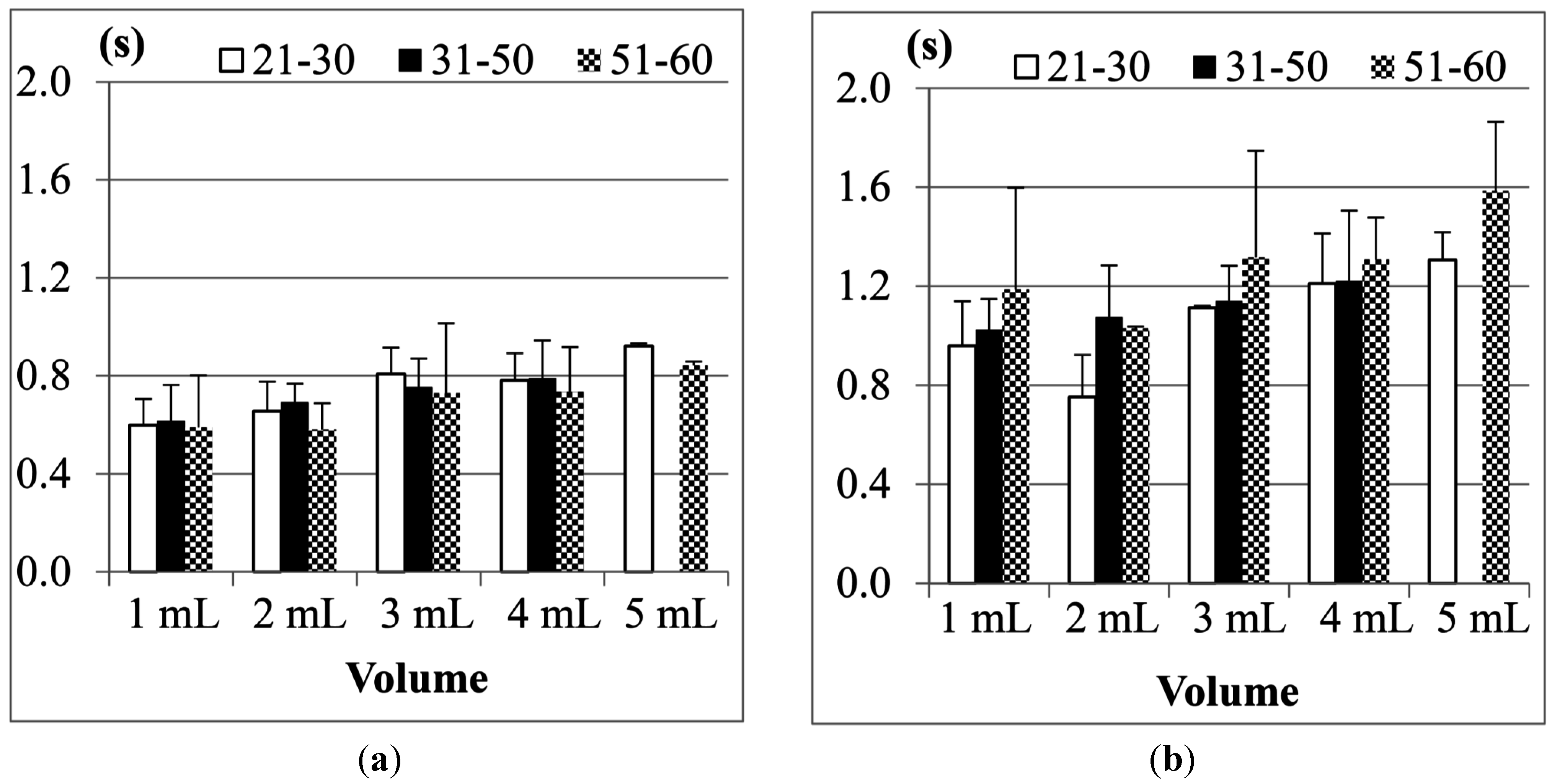
Figure 13.
Average duration of (a) Jitter and (b) TET for the age group 21–30 years old.
Figure 13.
Average duration of (a) Jitter and (b) TET for the age group 21–30 years old.
The results in
Figure 13 also show that both the Jitter and TET of young male participants (aged from 21 to 30), on average, are slightly longer than those of the female participants (aged from 21 to 30) when the volume of water is larger than 5 mL, but for the volumes smaller than 5 mL, the male participants seem to have shorter Jitter and TET. We use pairwise
T-test to evaluate if the male and female participants in this age group have obvious differences (
i.e., research hypothesis).
Table 2 shows the statistical analysis of average Jitter and average TET between the male and female groups aged from 21 to 30 years old for different water volumes. The research hypothesis will be accepted if (
T-value >
F-value) or (
T-value < −
F-value) at the α = 0.05 level. From
Table 2 we can find that in this age group of the participants, the average Jitter or TET between different genders do not have statistically significant differences.
Table 2.
Statistical analysis of average Jitter and average TET between the male and female groups aged from 21 to 30 years old for different water volumes.
Table 2.
Statistical analysis of average Jitter and average TET between the male and female groups aged from 21 to 30 years old for different water volumes.
| (α= 0.05) | 1 mL | 3 mL | 5 mL | 10 mL | 20 mL |
|---|
| Jitter | TET | Jitter | TET | Jitter | TET | Jitter | TET | Jitter | TET |
|---|
| Male’s mean | 0.599 | 0.959 | 0.656 | 0.751 | 0.807 | 1.112 | 0.781 | 1.210 | 0.921 | 1.305 |
| Female’s mean | 0.620 | 1.068 | 0.627 | 1.006 | 0.713 | 1.100 | 0.731 | 1.190 | 0.900 | 1.280 |
| 95% CI * | Upper | 0.876 | 1.285 | 0.740 | 1.365 | 0.804 | 1.162 | 0.816 | 1.332 | 0.989 | 1.368 |
| Lower | 0.573 | 1.017 | 0.593 | 0.913 | 0.678 | 1.046 | 0.695 | 1.076 | 0.861 | 1.205 |
| Degree of freedom (df) | 13 | 13 | 16 | 16 | 12 | 12 | 9 | 9 | 7 | 7 |
| F-value | 2.160 | 2.160 | 2.120 | 2.120 | 2.179 | 2.179 | 2.262 | 2.262 | 2.365 | 2.365 |
| T-value | −0.609 | −1.790 | 1.156 | −1.257 | 1.380 | 0.199 | 1.053 | 0.117 | 0.221 | 0.079 |
| p-value | 0.552 | 0.097 | 0.265 | 0.226 | 0.193 | 0.846 | 0.319 | 0.909 | 0.831 | 0.939 |
| Cohen’s d (Effect size) | −0.321 | −0.943 | 0.545 | −0.577 | 0.770 | 0.111 | 0.638 | 0.071 | 0.148 | 0.053 |
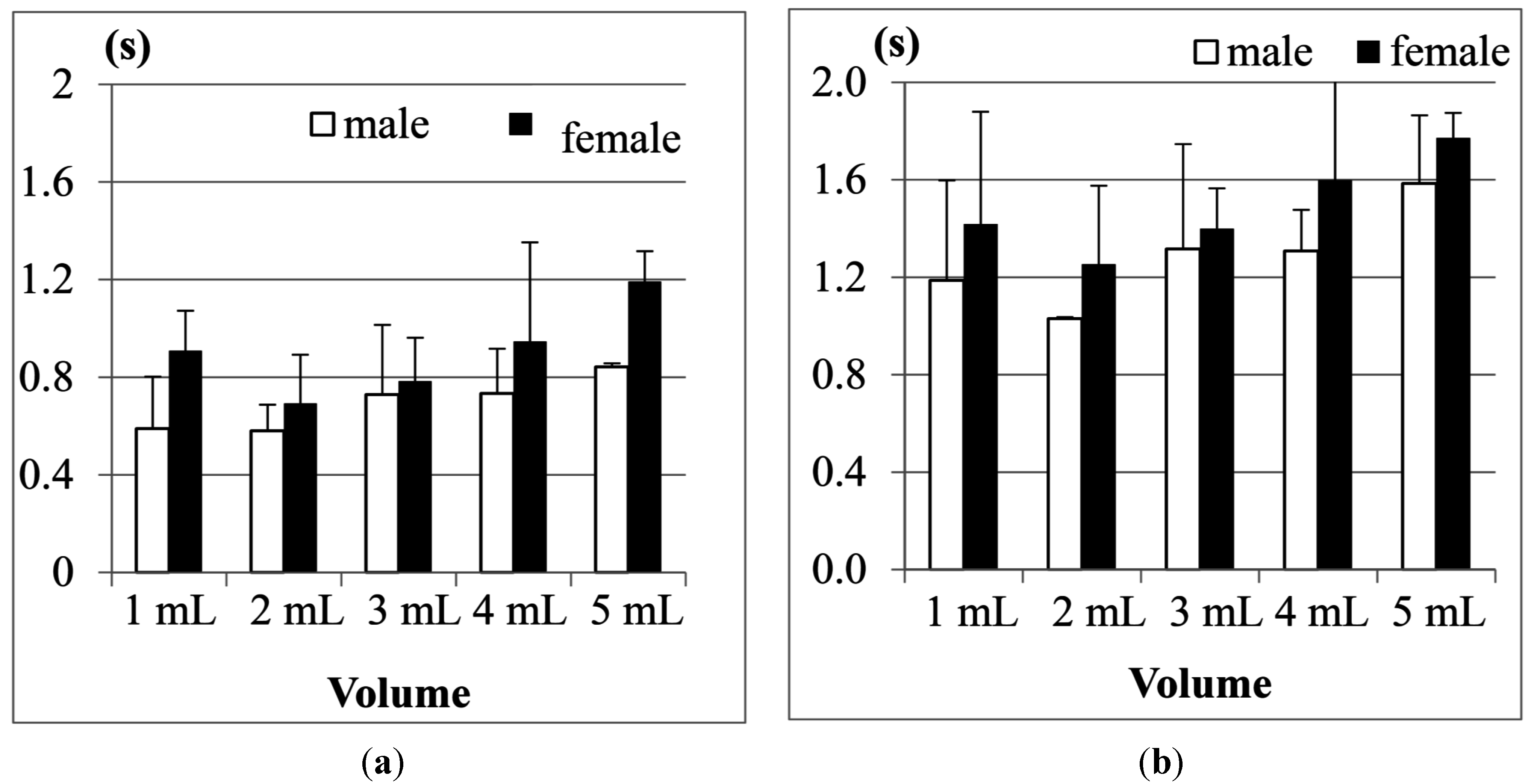
Figure 14 further shows the results of average Jitter and average TET for the 31–50 years old age group. We can find that Jitter and TET become longer as the volume of water increases. Moreover, when we increase the volume of water to 20 mL, the male participants display piecemeal swallowing situations. In this case, they need to swallow the 20 mL water in many times, therefore their Jitter and TET cannot be measured.
Figure 14.
Average duration of (a) Jitter and (b) TET for the age group 31–50 years old.
Figure 14.
Average duration of (a) Jitter and (b) TET for the age group 31–50 years old.
For this age group (31–50), we also find that the male participants in most measurements need longer Jitter and TET durations to finish the swallowing than the female participants. We did pairwise T-tests to compare the male and female groups.
Table 3 shows the statistical analysis of average Jitter and average TET between the male and female groups aged from 31 to 50 years old for different water volumes. We can find that in the 3 mL and 5 mL trials, the average Jitter shows a statistical difference (
i.e.,
T-value >
F-value), while the average TET does not. This result is consistent with the study in [
24], where they found that the male participants performed slightly worse than the female participants in this age group.
Table 3.
Statistical analysis of average Jitter and average TET between the male and female groups aged from 31 to 50 years old for different water volumes.
Table 3.
Statistical analysis of average Jitter and average TET between the male and female groups aged from 31 to 50 years old for different water volumes.
| (α = 0.05) | 1 mL | 3 mL | 5 mL | 10 mL | 20 mL |
|---|
| Jitter | TET | Jitter | TET | Jitter | TET | Jitter | TET | Jitter | TET |
|---|
| Male’s mean | 0.616 | 1.023 | 0.691 | 1.073 | 0.754 | 1.139 | 0.789 | 1.221 | N/A | N/A |
| Female’s mean | 0.698 | 0.962 | 0.573 | 0.928 | 0.686 | 1.076 | 0.746 | 1.137 | 0.883 | 1.215 |
| 95% CI * | Upper | 0.698 | 1.032 | 0.679 | 1.088 | 0.764 | 1.201 | 0.805 | 1.277 | 0.914 | 1.237 |
| Lower | 0.573 | 0.905 | 0.550 | 0.921 | 0.682 | 1.054 | 0.701 | 1.042 | 0.852 | 1.074 |
| Degree of freedom (df) | 18 | 18 | 14 | 14 | 19 | 19 | 17 | 17 | 19 | 19 |
| F-value | 2.101 | 2.101 | 2.145 | 2.145 | 2.093 | 2.093 | 2.110 | 2.110 | 2.093 | 2.093 |
| T-value ** | −1.763 | 0.947 | 5.147 | 1.481 | 2.424 | 1.800 | 0.533 | 0.304 | N/A | N/A |
| p-value | 0.095 | 0.356 | 0.001 | 0.156 | 0.025 | 0.088 | 0.601 | 0.765 | N/A | N/A |
| Cohen’s d (Effect size) | −0.788 | 0.424 | 2.658 | 0.662 | 1.069 | 0.794 | 0.245 | 0.139 | N/A | N/A |
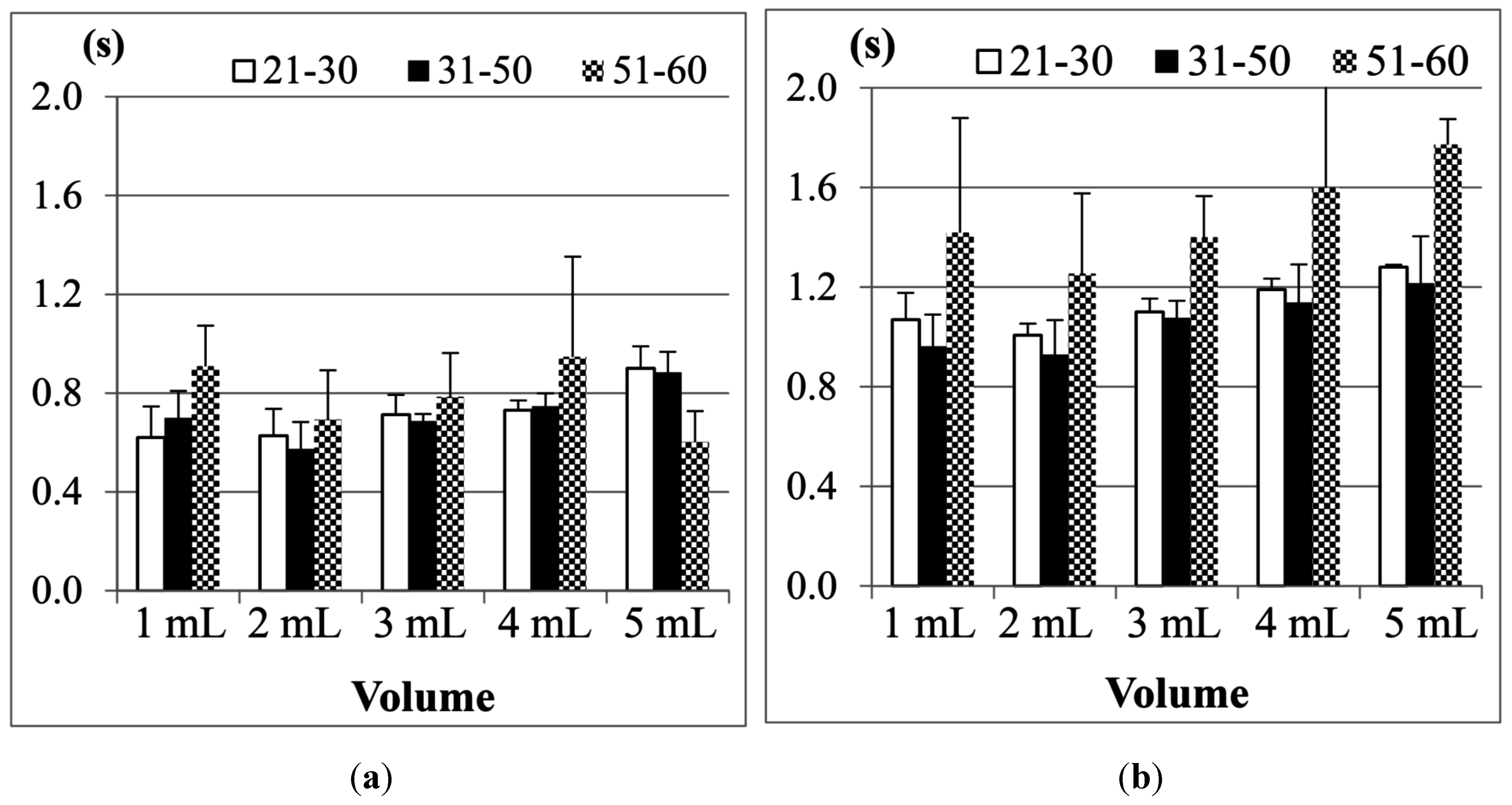
Finally, the results of the 51–60 year old age group are shown in
Figure 15. Compared with the previous two age groups, the participants aged from 51 to 60 obviously needed even longer durationd of Jitter and TET to finish swallowing in all measurements. Particularly, in this age group, we note that the female participants on average need longer Jitter and TET durationd than the male participants, which is quite different from the results in the two previous age groups.
Figure 15.
Average duration of (a) Jitter and (b) TET for the age group 51–60 years old.
Figure 15.
Average duration of (a) Jitter and (b) TET for the age group 51–60 years old.
If we apply a pairwise T-test to compare the male and female participants in this age group (51–60), we find that the durations of Jitter in the 1, 10 and 20 mL measurements show more significant differences (
i.e.,
T-value < −
F-value) than the durations of TET (see
Table 4). From
Figure 13,
Figure 14 and
Figure 15, our testing suggests that the measurement of Jitter can have more distinctive sensitivity than the measurement of TET.
Table 4.
Statistical analysis of average Jitter and average TET between the male and female groups aged from 51 to 60 years old for different water volumes
Table 4.
Statistical analysis of average Jitter and average TET between the male and female groups aged from 51 to 60 years old for different water volumes
| 1 mL | 3 mL | 5 mL | 10 mL | 20 mL |
|---|
| Jitter | TET | Jitter | TET | Jitter | TET | Jitter | TET | Jitter | TET |
|---|
| Male’s mean | 0.589 | 1.187 | 0.580 | 1.029 | 0.728 | 1.316 | 0.733 | 1.307 | 0.842 | 1.584 |
| Female’s mean | 0.908 | 1.418 | 0.693 | 1.253 | 0.784 | 1.399 | 0.946 | 1.598 | 1.192 | 1.771 |
| 95% CI * | Upper | 0.904 | 1.524 | 0.732 | 1.302 | 0.917 | 1.579 | 1.096 | 1.754 | 1.234 | 1.852 |
| Lower | 0.594 | 1.081 | 0.538 | 1.001 | 0.603 | 1.135 | 0.660 | 1.278 | 0.917 | 1.566 |
| Degree of freedom (df) | 10 | 10 | 9 | 9 | 7 | 7 | 7 | 7 | 7 | 7 |
| F-value | 2.228 | 2.228 | 2.262 | 2.262 | 2.365 | 2.365 | 2.365 | 2.365 | 2.365 | 2.365 |
| T-value ** | −3.004 | −1.216 | −1.615 | −1.889 | -0.645 | −0.592 | −2.725 | −2.746 | −4.099 | −5.651 |
| p-value | 0.013 | 0.252 | 0.141 | 0.091 | 0.539 | 0.572 | 0.029 | 0.028 | 0.004 | 0.001 |
| Cohen’s d (Effect size) | −1.734 | −0.702 | −0.978 | −1.144 | −0.456 | −0.419 | −1.828 | −1.942 | −2.898 | −3.996 |
(2) Effects of the Age
The authors in the study [
18,
19] mentioned that age will have more impact on the swallowing ability; when the age increases, the duration of Jitter and TET will become longer. According to this point, we rearrange the data and compare the swallowing ability among different age groups.
Figure 16 shows the results of Jitter and TET for the male participants in three age groups (the data of the participants aged from 31 to 50 years old in the 20 mL measurement was not included due to the piecemeal swallowing).
Figure 16.
Average results of the male participants in different age groups for (a) Jitter and (b) TET.
Figure 16.
Average results of the male participants in different age groups for (a) Jitter and (b) TET.
We find that the participants aged from 51 to 60 years old, on average, need the longest TET (total swallowing time) but the shortest Jitter (the duration of the thyroid cartilage pushing the bolus into the esophagus) to finish the swallowing of water, compared with the other two age groups. This shows that the overall swallowing ability of the elder participants is not as good as the younger participants such that their swallowing needs longer time in the pharyngeal stage. On the other hand, the strength of the thyroid cartilage for the elder participants may be not so good, too. That is why the water bolus will fall into the esophagus in a shorter period, which could potentially bring higher risks of choking injuries.
Figure 17 showed the results of the female participants. As we can see, the female participants aged from 51 to 60 years old also have the longest TET but shorter Jitter, even worse than the male participants. It shows that both of their swallowing function of the thyroid cartilage could deteriorate at a certain rate at this age, but the female participants in this age group may have a higher risk of choking injuries than the male group in their daily eating.
Figure 17.
Average results of the female participants in different age groups for (a) Jitter and (b) TET.
Figure 17.
Average results of the female participants in different age groups for (a) Jitter and (b) TET.
We would like to know if the results shown in
Figure 16 and
Figure 17 have statistical meaning. Therefore we use Analysis of Variance (ANOVA) testing to compare the three different age groups (
i.e., 21–30, 31–50, and 51–60).
Table 5 shows the testing results for Jitter, and
Table 6 for TET. In both tables we list the degree of freedom and the sum of squares for each trial. Also,
F-values are calculated and compared with
F-boundaries at the α = 0.05 level. If (
F-value >
F-boundary) or (
F-value < −
F-boundary), the null hypothesis (
i.e., those three age groups did not have obvious difference) can be rejected. Finally the values of “eta squared” are listed in the last row for reference.
From
Table 5, we find that the parameter “Jitter” does not show significant differences between the different age groups in most water volumes except 20 mL because their
F-values were not large enough. From
Table 6, however, the “TET” parameter shows totally different results. In those five different water volumes, we have up to four
F-values (
i.e., 1, 5, 10, and 20 mL) larger than
F-boundaries. It shows that these three age groups really have a statistical difference in the total swallowing time (TET).
Table 5.
ANOVA testing of “Jitter” for three different age groups in different water volumes.
Table 5.
ANOVA testing of “Jitter” for three different age groups in different water volumes.
| ANOVA of Jitter | 1 mL | 3 mL | 5 mL | 10 mL | 20 mL |
|---|
| Between groups df * | 2 | 2 | 2 | 2 | 2 |
| Within groups df * | 44 | 42 | 41 | 36 | 25 |
| F-boundary (α = 0.05) | 3.20 | 3.22 | 3.22 | 3.26 | 3.39 |
| F-value ** | 1.192 | 0.754 | 0.255 | 1.841 | 15.818 |
| p-value | 0.313 | 0.477 | 0.776 | 0.173 | 0.001 |
| eta squared η2 | 0.051 | 0.035 | 0.012 | 0.093 | 0.559 |
Table 6.
ANOVA testing of “TET” for three different age groups in different water volumes.
Table 6.
ANOVA testing of “TET” for three different age groups in different water volumes.
| ANOVA of TET | 1 mL | 3 mL | 5 mL | 10 mL | 20 mL |
|---|
| Between groups df * | 2 | 2 | 2 | 2 | 2 |
| Within groups df * | 44 | 42 | 41 | 36 | 25 |
| F-boundary (α = 0.05) | 3.20 | 3.22 | 3.22 | 3.26 | 3.39 |
| F-value ** | 7.086 | 1.073 | 5.864 | 6.166 | 7.854 |
| p-value | 0.002 | 0.350 | 0.005 | 0.004 | 0.002 |
| eta squared η2 | 0.243 | 0.044 | 0.222 | 0.255 | 0.428 |
Next, we are interested about which age group has an obvious mean difference of TET from the other two age groups. Thus we apply the Scheffé
post hoc test to
Table 6 for comparing any two age groups.
Table 7,
Table 8,
Table 9 and
Table 10 show the
post hoc test results of TET for the water volumes of 1, 5, 10 and 20 mL, respectively. In each table, if the 95% confidence interval includes 0, the mean difference (μ
i − μ
j) cannot be considered a significant result. As we can see, the 51–60 year-old age group has obvious mean differences (
i.e., the bold numbers) from the other two age groups.
Table 7.
Post hoc test of TET for 1 mL water volume.
Table 7.
Post hoc test of TET for 1 mL water volume.
| Post Hoc Test (1 mL) | Age group (i) | Age Group (j) | Mean Difference (μi − μj) | 95% Confidence Interval |
|---|
| Lower | Upper |
|---|
| Scheffé’s * | 51–60 | 21–30 | 0.152 | −0.077 | 0.381 |
| 51–60 | 31–50 | 0.334 | 0.118 | 0.551 |
| 21–30 | 31–50 | 0.183 | −0.020 | 0.385 |
Table 8.
Post hoc test of TET for 5 mL water volume.
Table 8.
Post hoc test of TET for 5 mL water volume.
| Post Hoc Test (5 mL) | Age Group (i) | Age Group (j) | Mean Difference (μi − μj) | 95% Confidence Interval |
|---|
| Lower | Upper |
|---|
| Scheffé’s * | 51–60 | 21–30 | 0.253 | 0.061 | 0.445 |
| 51–60 | 31–50 | 0.229 | 0.051 | 0.408 |
| 21–30 | 31–50 | −0.023 | −0.178 | 0.131 |
Table 9.
Post hoc test of TET for 10 mL water volume.
Table 9.
Post hoc test of TET for 10 mL water volume.
| Post Hoc Test (10 mL) | Age Group (i) | Age Group (j) | Mean difference (μi − μj) | 95% Confidence Interval |
|---|
| Lower | Upper |
|---|
| Scheffé’s * | 51–60 | 21–30 | 0.312 | 0.035 | 0.589 |
| 51–60 | 31–50 | 0.357 | 0.108 | 0.607 |
| 21–30 | 31–50 | 0.045 | −0.189 | 0.278 |
Table 10.
Post hoc test of TET for 20 mL water volume.
Table 10.
Post hoc test of TET for 20 mL water volume.
| Post Hoc Test (20 mL) | Age Group (i) | Age Group (j) | Mean Difference (μi − μj) | 95% Confidence Interval |
|---|
| Lower | Upper |
|---|
| Scheffé’s * | 51–60 | 21–30 | 0.423 | 0.051 | 0.795 |
| 51–60 | 31–50 | 0.554 | 0.182 | 0.926 |
| 21–30 | 31–50 | 0.131 | −0.241 | 0.503 |
Therefore we can conclude that the factor of age actually has more significant impact on the swallowing ability than the gender factor. The results also suggest that after 50 years of age, no matter whether men or women are considered, the swallowing ability could deteriorate very obviously.
4.1.4. Discussion
From the results described above, we conclude that the FSR throat belt can effectively measure and differentiate the swallowing parameters, Jitter and TET, between different age groups for different volumes of water. Compared with other two parameters sEMG and SAD (nasal air flow), we show that the parameters Jitter and TET have better sensitivity for evaluating the swallowing status. By our testing, we also show that a 20 mL volume would be a swallowing limitation. This fact is very important and useful in developing related healthcare applications [
26,
27]. Also, we show that differences indeed exist between male and female participants, and a deterioration of the swallowing ability will occur with increasing age.
Currently instrument-based approaches (e.g., VFSS) have advantages and limitations. Umay
et al. recommended that bedside tests should be used mainly as initial screening tests [
26]. Therefore the instrument-based approaches should be performed in patients who are at risk for swallowing disorders [
26,
27]. They are advantageous because they can detect aspiration [
28,
29], which aids in the clinical management planning of tube or oral feeding. Nonetheless, the disadvantages of radiation exposure and non-portability make it difficult to apply them in cases of acute stage disease, sickness, in large studies, and for repeated measurements within a short period in follow-up studies. In this paper we use a non-invasive assessment method that does not cause any stress to patients or pose a radiation risk. Furthermore, it should be easily carried or worn, and portable. Equally important, this non-invasive swallowing study tool should be capable of being combined with respiration monitoring for swallowing and respiration coordination studies.
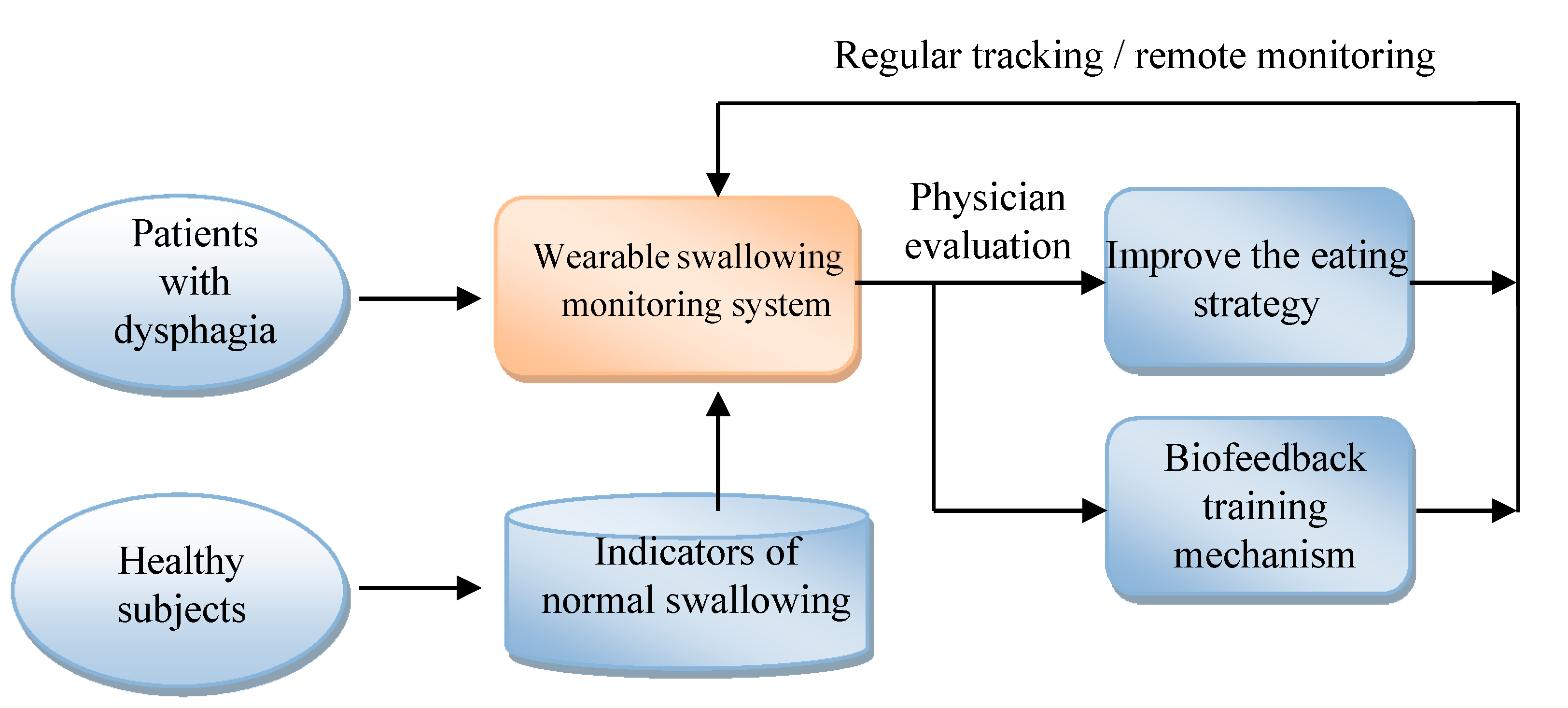
Figure 18 shows the applications that can be developed by the system proposed in this paper. At first, this system can be applied to healthy subjects for building the indicators of normal swallowing patterns. Then we can use this monitoring system to measure the swallowing motions of dysphagia patients. The results can be evaluated by the physicians for eating strategy adjustment or for biofeedback training. All above steps can be repeated for regular tracking and monitoring.
Figure 18.
The applications of the wearable dysphagia monitoring system.
Figure 18.
The applications of the wearable dysphagia monitoring system.
Figure 19.
Design of the holter.
Figure 19.
Design of the holter.
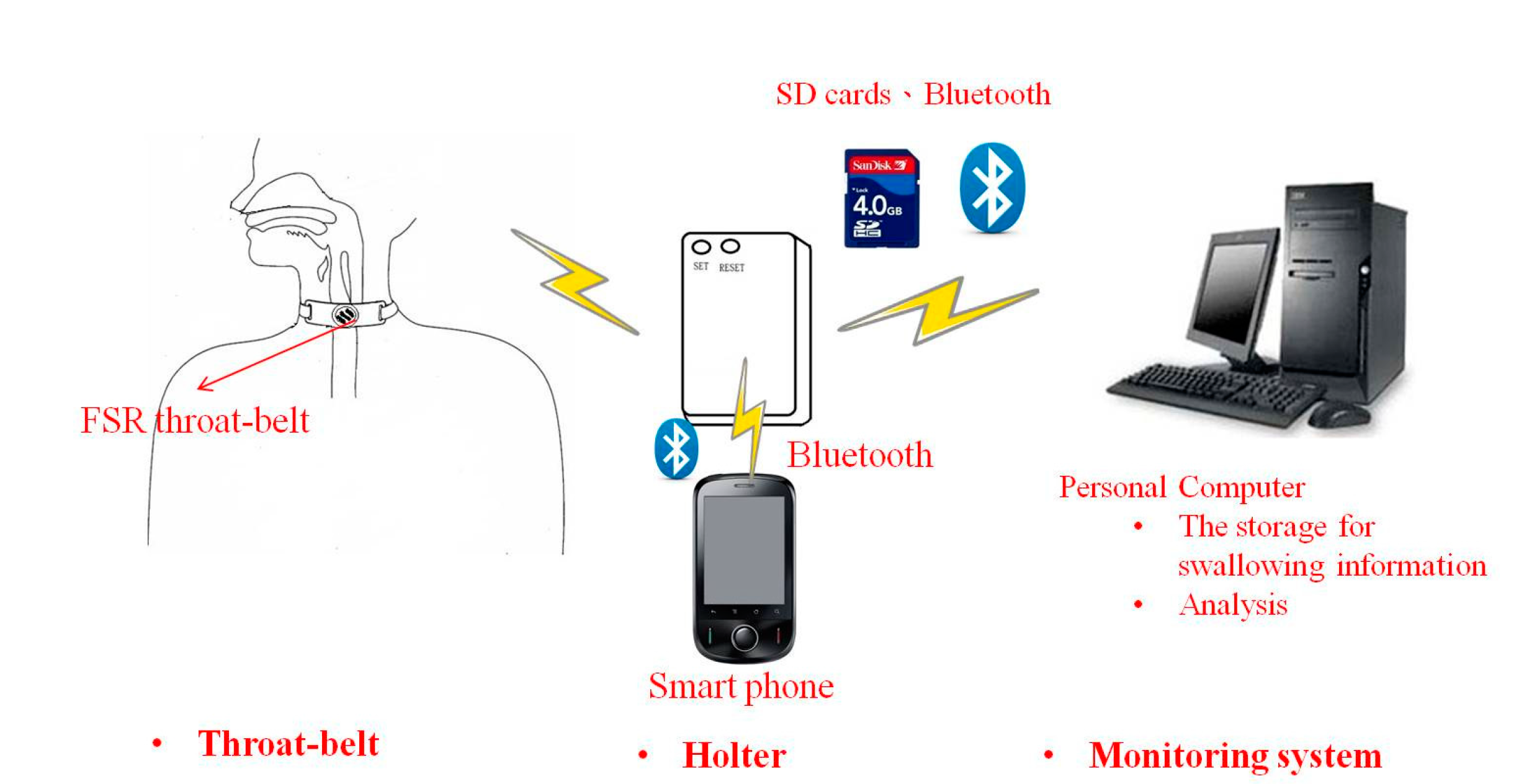
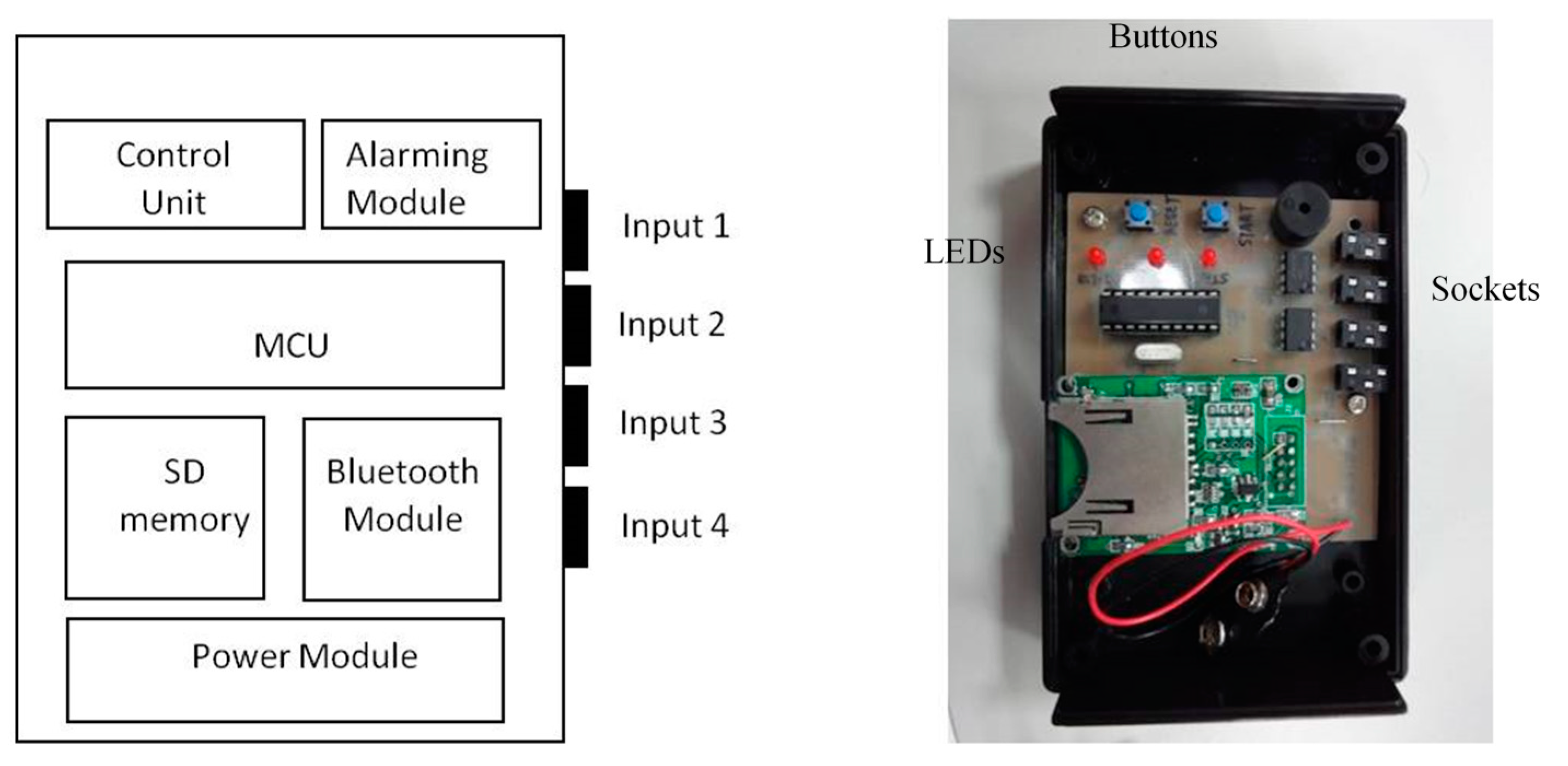
We can also connect the FSR throat belt with a holter to develop portable applications. The holter we implemented is shown in
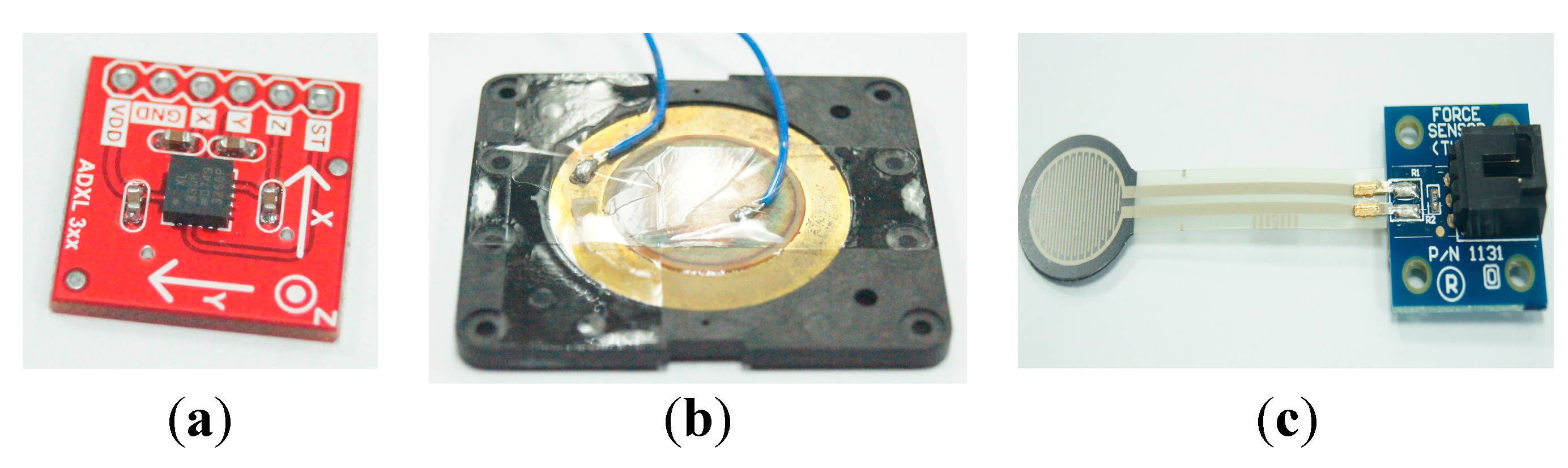
Figure 19. It includes four input signal sockets, two control buttons, three output LEDs and one signal processing circuit. To unify the connection between the holter and different kinds of sensors (
i.e., FSR, sEMG, nasal airflow
etc.), we use 3.5 mm stereo connectors as the input port and refit the sensor output pins with those connectors. In our holter design, we reserved four input ports for future possible connections to other sensors for multivariate data measurements. The signal processing circuit is composed of a microcontroller (MCU), a Bluetooth module, a SD memory module and a power-supply module. The MCU we used is an AT89C2051 [
30], which is a low-power but high performance chip. We use it to do the digital signal preprocessing operations. The data measured by the sensors can be collected on the SD memory card, or be transferred to a mobile phone via the Bluetooth module.