1. Introduction
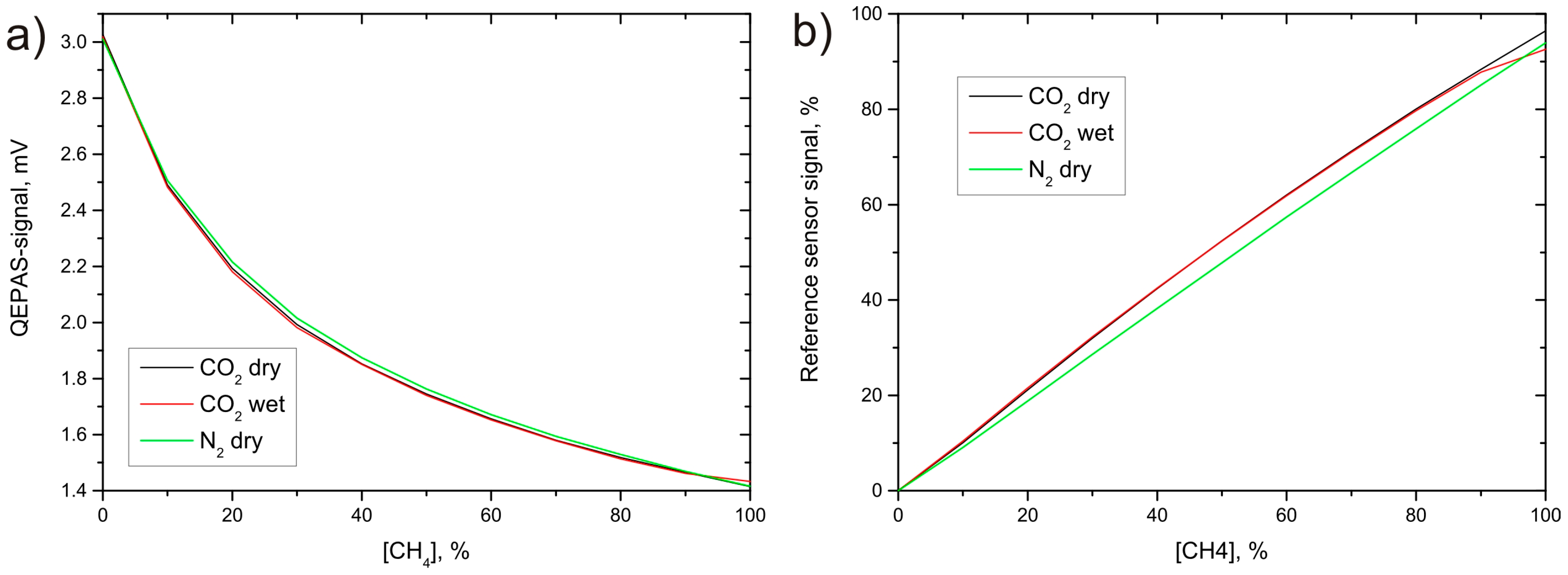
Gas sensing is an important issue for several industrial applications, securing work safety and providing process and quality control. Each application requires certain sensor characteristics such as sensitivity, selectivity, or costs. For biogas sensing, different requirements occur, depending on the utilization of the gas, e.g., direct electric power generation or grid feeding. Biogas is a mixture with about 40%–60% methane (CH
4), 40%–60% carbon dioxide (CO
2), and traces of nitrogen (N
2), oxygen (O
2), hydrogen sulfide (H
2S), hydrogen (H
2) und ammonia (NH
3). Another important component is water vapor, which is contained until saturation. Permanent monitoring is done for CH
4, CO
2, H
2S, and O
2 in most biogas plants. H
2S, and O
2 are commonly measured with electrochemical sensors, while CH
4 and CO
2 are measured optically via absorption spectroscopic methods. The main difficulty in measuring the latter two gases lies in the high concentrations, around 50%, and in minimizing cross sensitivities against other biogas components. Until now, experimental work on CH
4 and CO
2 sensing was focused on small target gas concentrations, reaching low detection limits [
1,
2], whereas measurements of high concentrations as needed for biogas analysis are rare [
3,
4]. A comparison between the new sensor technique and currently used absorption spectroscopic sensors is shown in
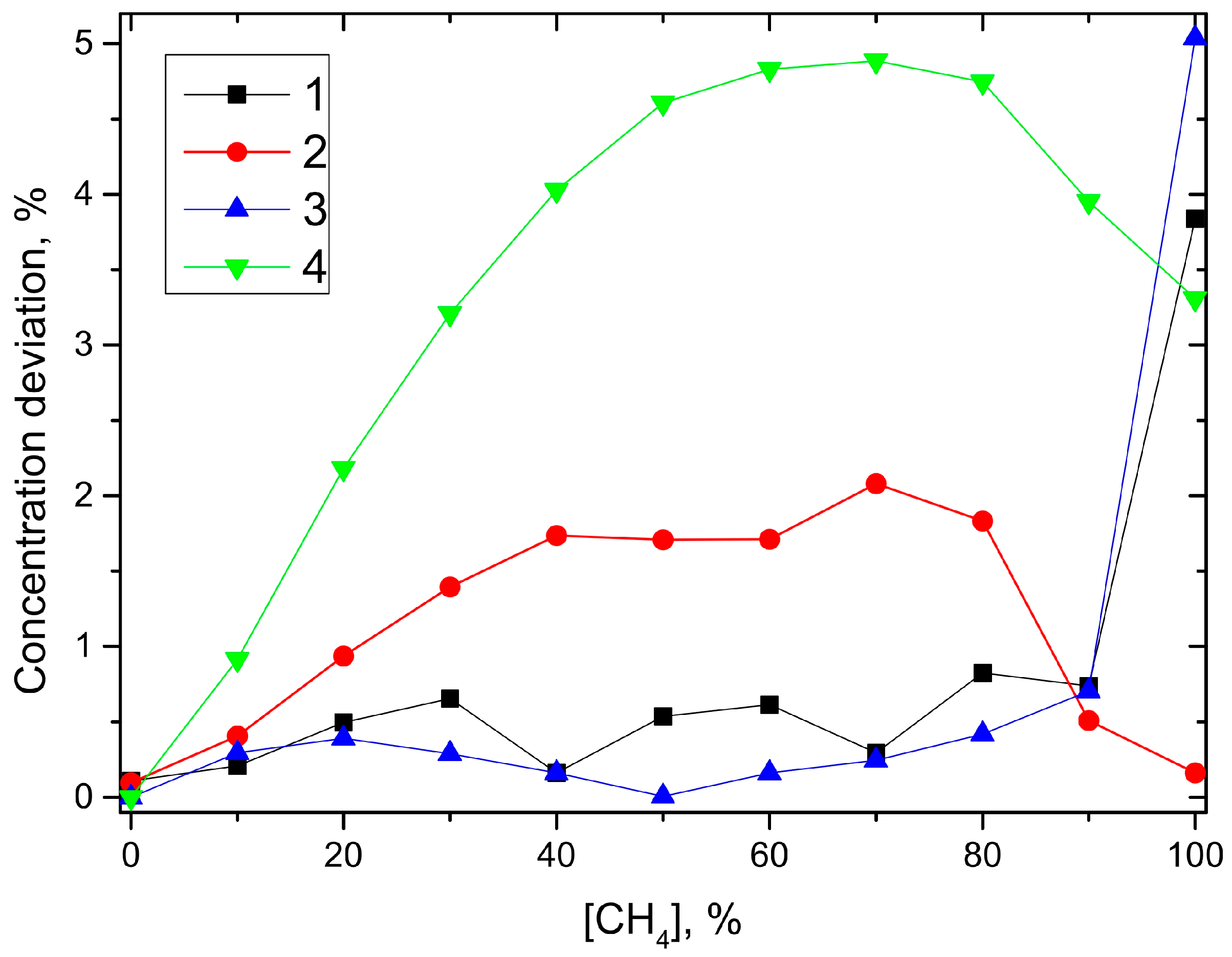
Section 3.2 to underline the performance of the new developed sensor system. Relevant parameters are the sensor precision as defined in [
5] and the cross sensitivity to other species.
The aim of this work is to show the feasibility of a cost efficient LED-based miniaturized photoacoustic sensor for methane and carbon dioxide monitoring in the harsh environment of biogas plants. The technique used to achieve this goal is known as quartz-enhanced photoacoustic spectroscopy (QEPAS) and was first presented by Kosterev
et al. in 2002 [
6]. In this technique, a quartz micro tuning fork (QTF) is used as highly resonant sound detector; typical properties are a resonance frequency of
fr = 32.768 kHz and a quality factor of about
Q = 10,000 at ambient pressure. These sound detectors offer small geometrical dimensions, robust mechanical properties, are low priced (~0.10 €), and insensitive to external sound sources [
6,
7].
An expensive part of many optical sensors is the excitation light source. Especially for QEPAS, most publications show sensors using diode lasers or quantum cascade lasers [
8,
9,
10]. This is due to the good beam quality of laser sources, the high optical output power, and the narrow line width and tunability, which ensure almost cross sensitivity free measurements. However, to achieve a cost efficient sensor, an LED is a more favorable choice, even though it lacks all mentioned optical properties. An important development towards the use of LEDs in QEPAS sensors was done by Liu and coworkers in 2009; they presented the so called off-beam acoustic resonator [
11]. Acoustic micro resonators were used for acoustic signal amplification in QEPAS before, however the off-beam approach comes along with an almost free choice of the resonator diameter. This offers resonators with inner diameters in the mm-range, enabling most of the LED light to pass through the resonator and to generate photoacoustic signal [
12].
As the strongest absorption bands for most molecules lie in the mid infrared (MIR) region, LEDs emitting at wavelengths around λmethane = 3.4 µm and λcarbon = 4.2 µm were chosen to measure methane and carbon dioxide, respectively. Without spectral filtering, the broad spectrum of MIR-LEDs of several hundred nm causes strong cross sensitivities to other biogas components. To overcome this problem, the QEPAS technique is supplemented with an additional absorption path. In this absorption-QEPAS setup, the QEPAS-cell is sealed and filled with the pure target gas and serves as spectral filtering detector, whereas the absorption cell contains the gas mixture under test.
2. Experimental Section
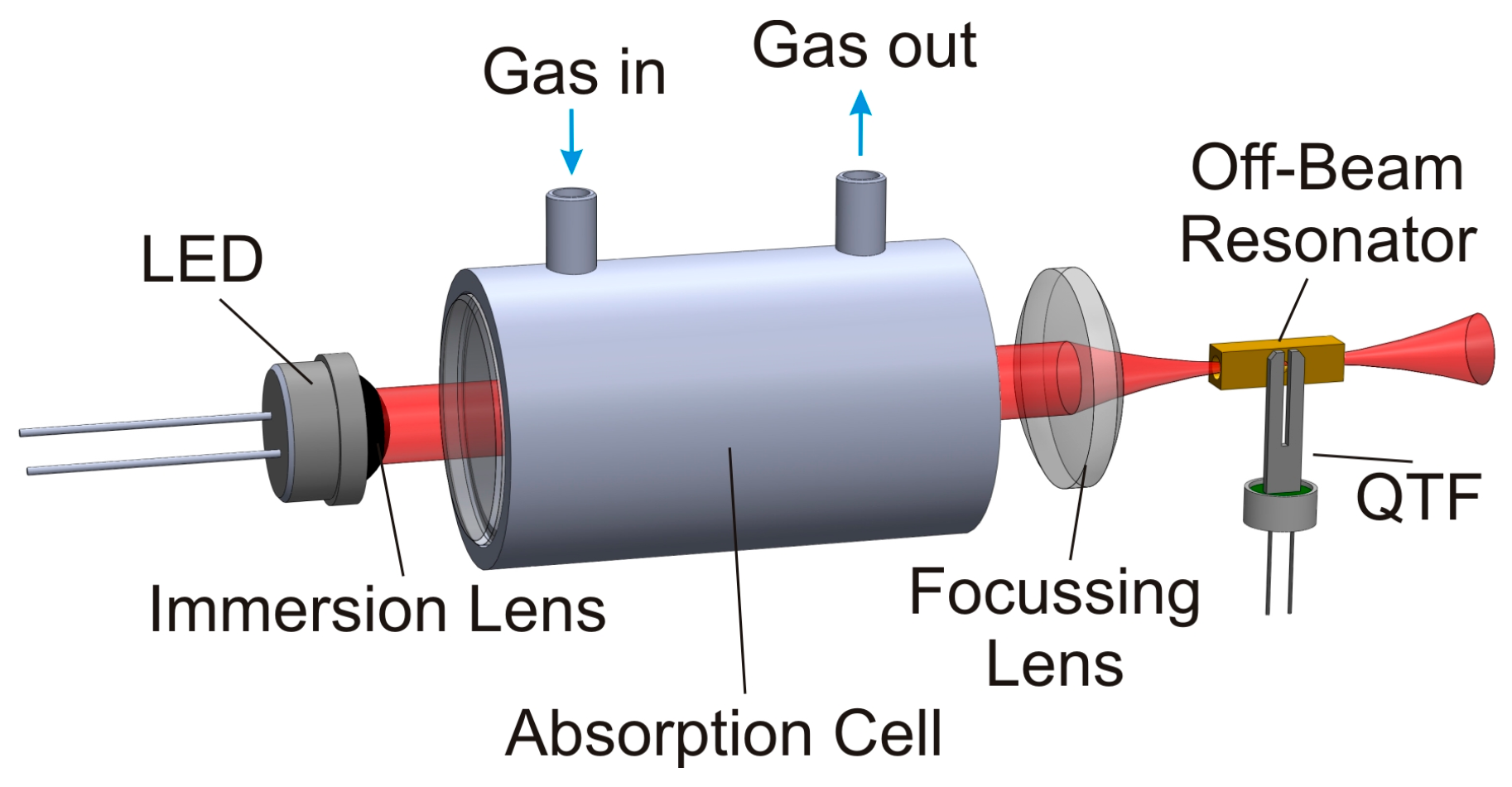
The absorption-QEPAS technique uses a simple optical setup, as shown in
Figure 1. The highly divergent LED light is guided through the absorption path and the acoustic resonator by two lenses. The used light sources were already equipped with a silicon immersion lens for collimation and embedded in a brass heatsink for optimum thermal connectivity. The detection of methane was done with an LED emitting around
λmethane = 3.4 µm with a quasi continuous wave (cw) optical power of
Pmethane = 0.2 mW (IoffeLED Ltd—LED34Sr). The detection of carbon dioxide was realized with an LED emitting around
λcarbon = 4.2 µm with a quasi cw optical power of
Pcarbon = 0.04 mW (IoffeLED Ltd—LED42Sr). These low optical power values can still be used for photoacoustic spectroscopy, as the absorption cross sections in the MIR are strong for both gases under test. However, the absorption band of CO
2 is considerably narrower than the LED emission spectrum, which means that only a small portion of the already low optical output power can contribute to the photoacoustic excitation. Both LEDs are driven by a commercial current driver (Thorlabs Inc.—ITC 133). A thermal stabilization of the LEDs is not required, as the broad spectrum of the LEDs shows only small spectral shift within a reasonable temperature range and the thermal connection to the aluminum housing of the sensor units was designed efficient enough to avoid overheating.
Figure 1.
Sketch of the experimental setup for absorption-QEPAS. The detector cell around the off-beam resonator is not illustrated to allow insight in the resonator setup.
Figure 1.
Sketch of the experimental setup for absorption-QEPAS. The detector cell around the off-beam resonator is not illustrated to allow insight in the resonator setup.
The absorption cells for both sensors are manufactured from stainless steel to avoid damage caused by corrosive biogas components, mainly by H2S. Calcium fluoride was chosen as window material for the absorption path because of its good transmittance in the MIR region (even without coating) and because of its good chemical stability. As the absorption cross sections for both target gases differ strongly, the absorption path length for both sensors had to be tailored, too. Optimum signal behavior in the desired measurement range, around 50% target gas concentration, was achieved for an absorption path length of Lmethane = 4 cm and Lcarbon = 4 mm for the methane and carbon dioxide sensor, respectively. A focusing lens made from black diamondTM chalcogenide glass is used to image the LED light through the QEPAS cell with its acoustic resonator. This focusing lens also serves as input window for the QEPAS cell.
During the absorption-QEPAS measurements presented in the following, the QEPAS cell was filled with the pure target gas and sealed. Prior to that, the acoustic resonator was experimentally optimized for each target gas. The acoustic resonance depends strongly on the speed of sound, which is coupled to the molecular mass of the gas under test [
12,
13]. Therefore, the optimum resonator length for both target gases differs from
lmethane = 7 mm for the methane sensor to
lcarbon = 4 mm for the carbon dioxide sensor. The QTF is glued in close proximity (~150 µm) to the acoustic off-beam resonator. Coupling between both resonators is provided by a small sonic output in the center of the sidewall of the acoustic resonator tube; it has a diameter of
dso = 400 µm. The detailed mechanical setup of the QEPAS cell itself is described in [
12]. A typical resonance curve of the assembled QEPAS cell is shown in
Figure 2. The expected Lorentian shape of the resonance is slightly distorted by a nonlinear background, which can be explained with electrical crosstalk. The inset of
Figure 2 shows an opened QTF (left), ready for mounting in a QEPAS cell and a second device still encapsulated and under vacuum.
Figure 2.
Typical resonance curve of the QTF within the methane QEPAS cell. The inset shows a photograph of a QTF with and without housing.
Figure 2.
Typical resonance curve of the QTF within the methane QEPAS cell. The inset shows a photograph of a QTF with and without housing.
The mechanical motion of the tuning fork, which is created by the photoacoustically-induced sound wave in the acoustic resonator, is measurable via the piezoelectric effect. As the tuning fork material quartz has strong piezoelectric properties, the first symmetrical bending mode of the tuning fork generates an evaluable piezo current. This small current, typically in the range of pA, is amplified with a transimpedance amplifier using a feedback resistor with
R = 10 MΩ. Thereafter, spectral filtering is applied, using a lock-in amplifier (Femto Messtechnik GmbH—LIA-BVD-180-H). A lock-in time constant of
TC = 1 s with a slope efficiency of 12 dB was chosen for all measurements described in the following. These parameters resulted in a measurement time of about 10 s for each data point. The absolute values of the lock-in measurement were recorded by a data acquisition device (National Instruments—NI PCIe-6353). Two analog outputs of this DAQ-device were used with a sampling rate of 1 MS/s to generate the sinusoidal waveform needed for the LED current modulation and the lock-in reference channel. The sinusoidal waveform showed two advantages in comparison to the theoretically more favorable pulsed modulation: first, because of the limited bandwidth of the current controllers, the modulation signal after these modules lost its rectangular shape; and second, the sinusoidal modulation showed higher frequency stability because of the oversampling of this analog signal by about 30 times at 32.7 kHz. Digital ports were used to enable the current controller output and to switch between the measurements of each gas. All processes within the sensor were automatically controlled by an internal PC using tailored National Instruments LabView software. Data recovery was possible using remote access to the unit via Ethernet or wireless LAN.
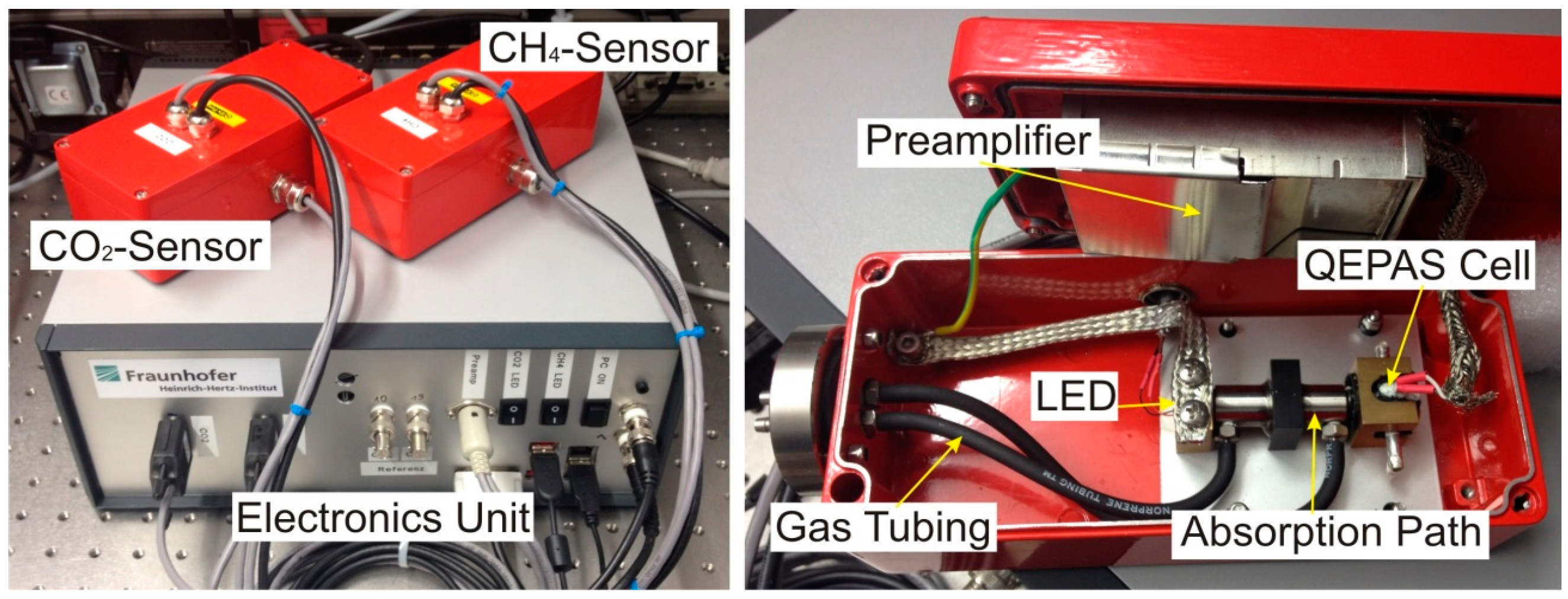
Figure 3 shows two photographs of the absorption-QEPAS sensor unit. The left picture gives an overview of the electronics unit, which contains the current controllers, lock-in amplifier, DAQ device, and PC. The QEPAS sensors are mounted on top of the electronics unit and enclosed in aluminum housings. The right picture in
Figure 3 shows the interior of the methane sensor. The optical components are mounted on a mechanically decoupled aluminum plate. The preamplifier is fixed to the housing cover. The connection to the gas tubing of the biogas plant is realized with stainless steel parts and norprene tubing to minimize corrosion and adsorption effects.
Figure 3.
Picture of the biogas detection system with electronics unit and both absorption-QEPAS sensors (Left); and photograph of the interior of the methane sensor (Right).
Figure 3.
Picture of the biogas detection system with electronics unit and both absorption-QEPAS sensors (Left); and photograph of the interior of the methane sensor (Right).
A major topic in the assembly of the electronics unit and the sensor housings was electrical shielding. Strong electrically induced signals, orders of magnitude higher than the original sensor signal cover the QEPAS signal, if the shielding is insufficient. This occurs because the detected sensor signals are identical to the LED modulation frequency. Furthermore, the LEDs are modulated in the ampere-range, whereas the measured signals are far lower. As can be seen in the asymmetric behavior of the resonance shown in
Figure 2, a complete shielding of electrical interfering signals was not accomplished. However, the major part was suppressed by using coaxial cables, shielding hoses and plates and by carefully avoiding ground loops.