Design of a Novel Telerehabilitation System with a Force-Sensing Mechanism
Abstract
:1. Introduction
2. Overview of the Proposed Telerehabilitation System
2.1. Conceptual Design of the Telerehabilitation System
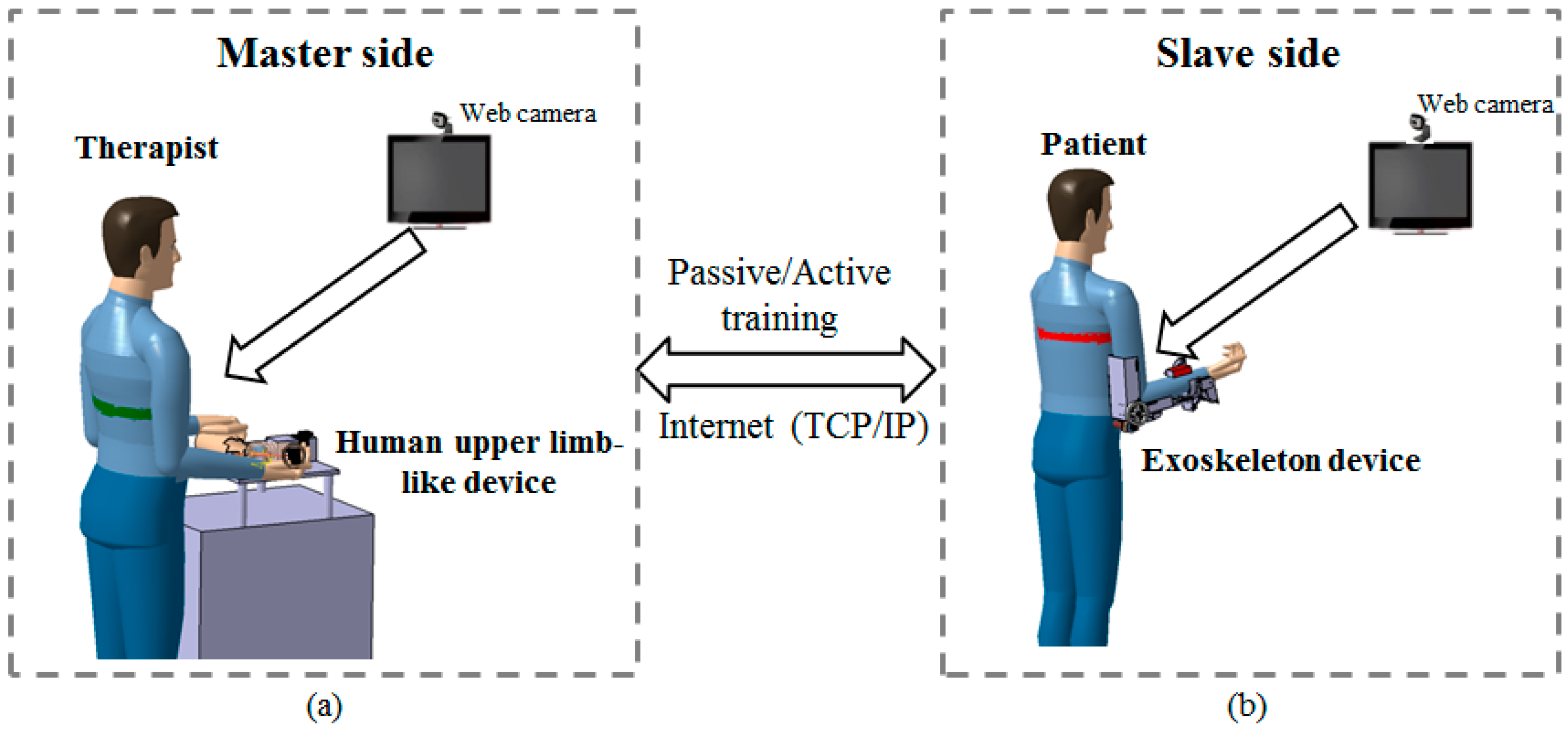
2.2. Passive and Active Training Accomplished by the Proposed Telerehabilitation System
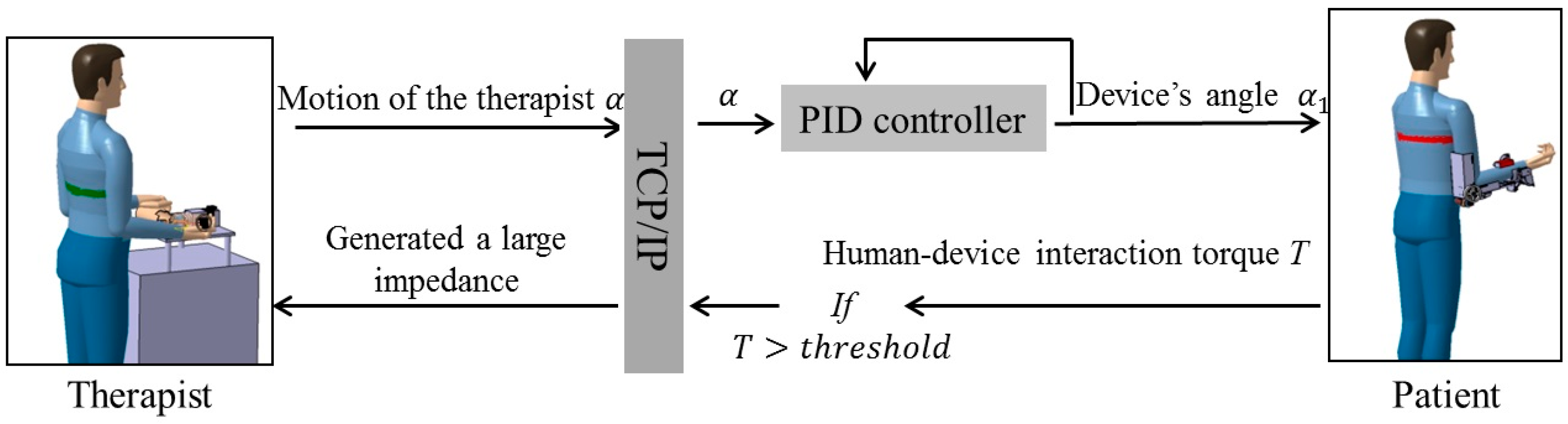
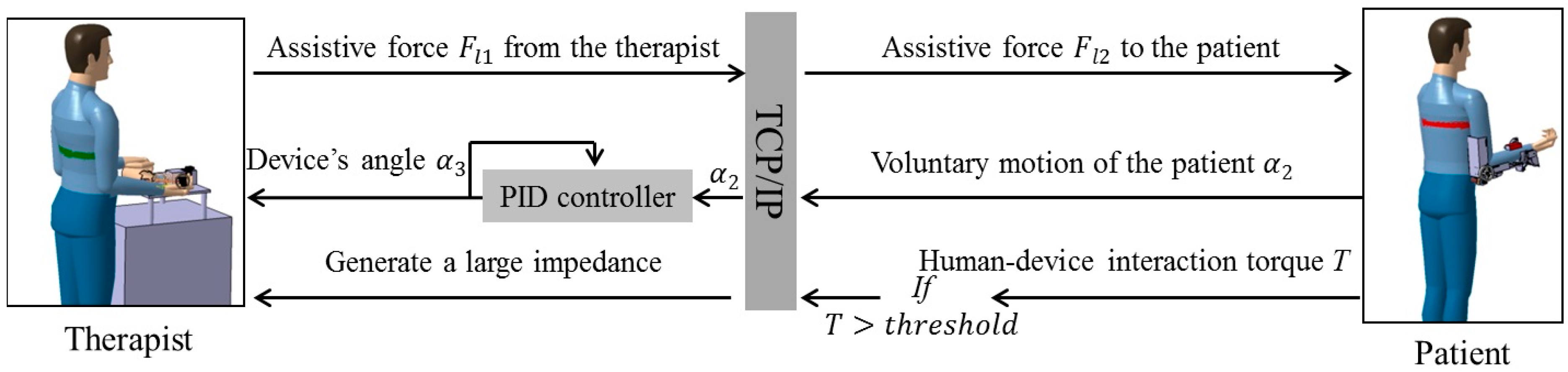
3. Mechanism Design and Control Method
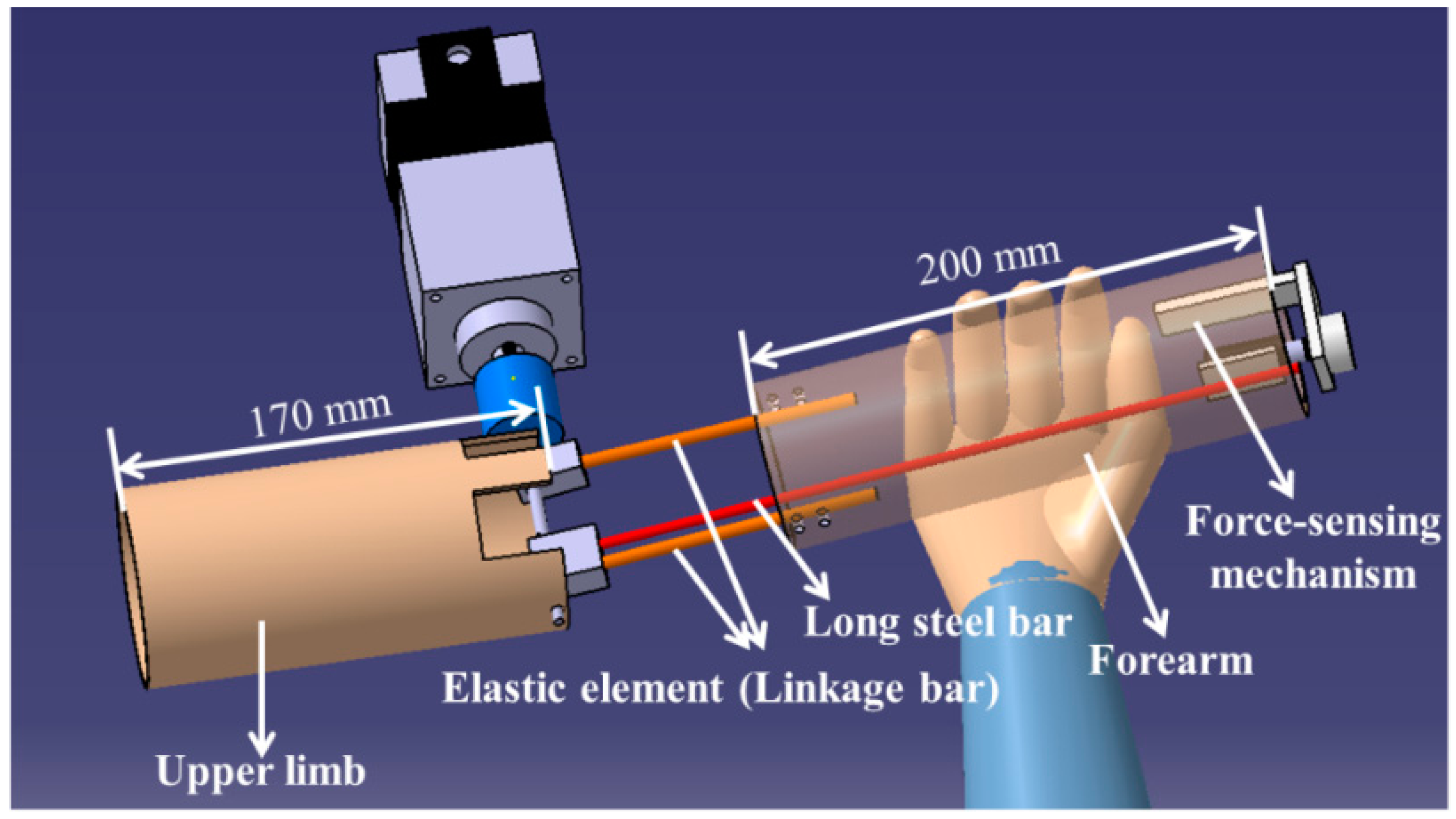
3.1. Mechanism Design and Method of Control for the Master Device

3.2. Torque-Limiter Mechanism Applied in the Exoskeleton Device (Slave Side)
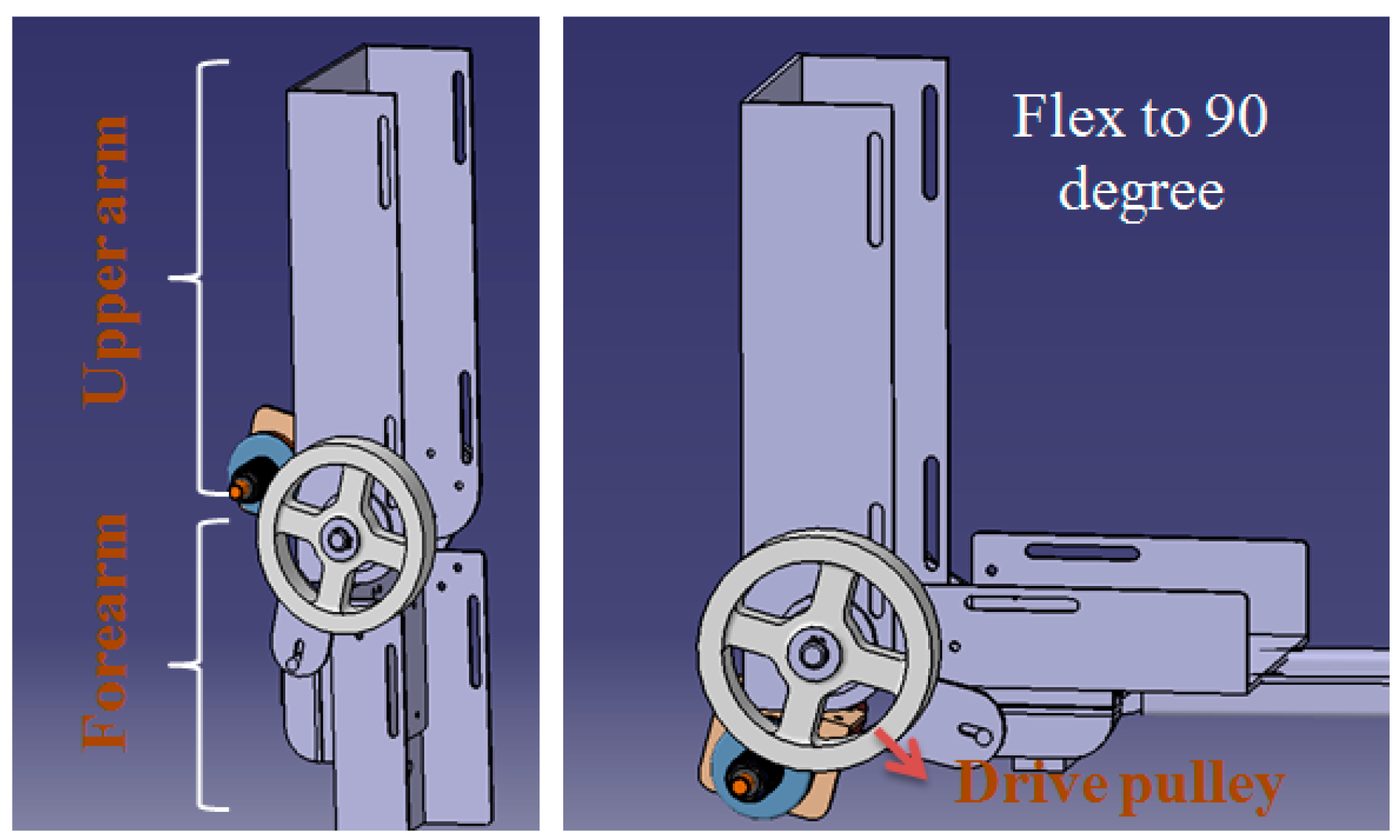
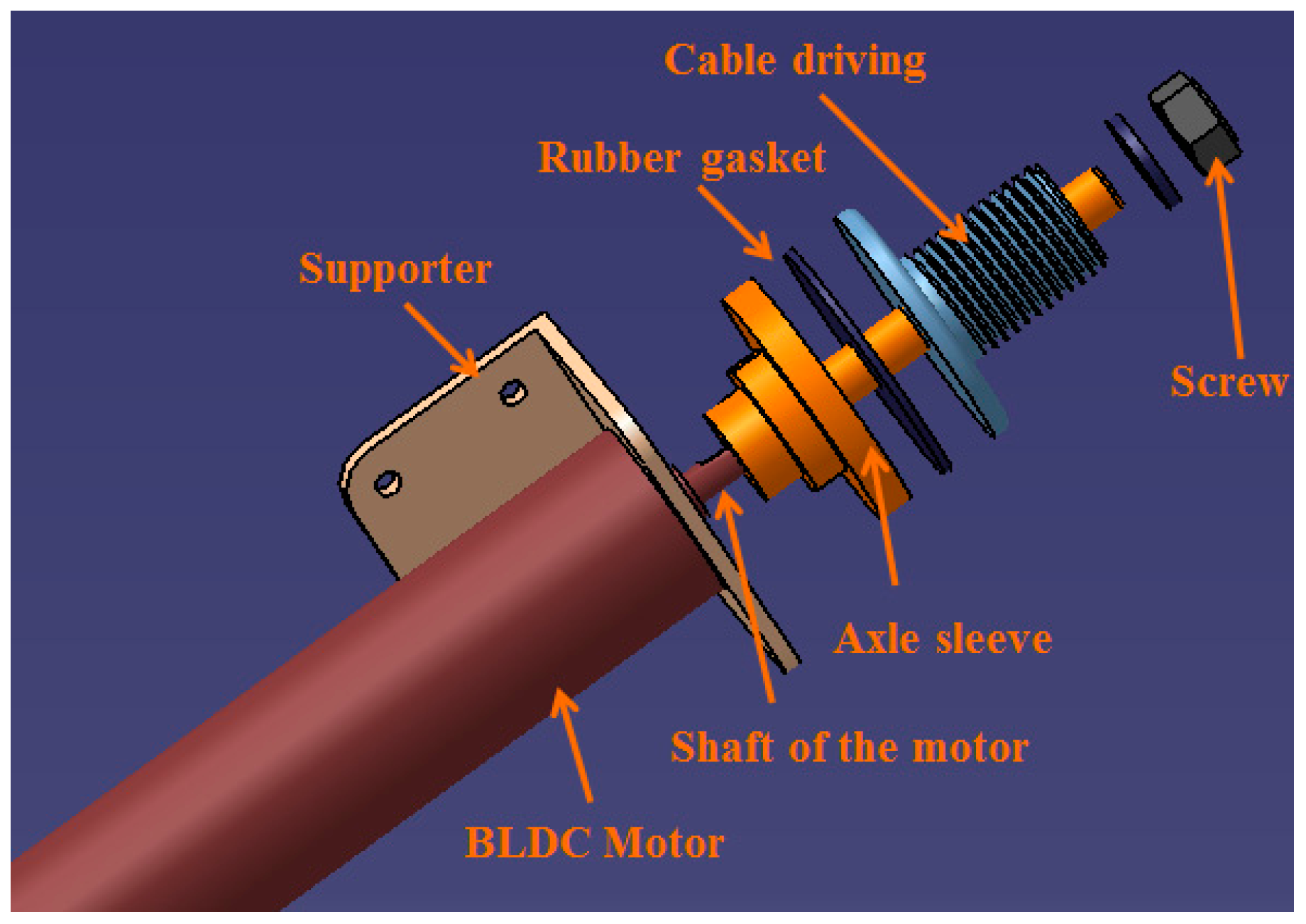
4. Experiments and Results
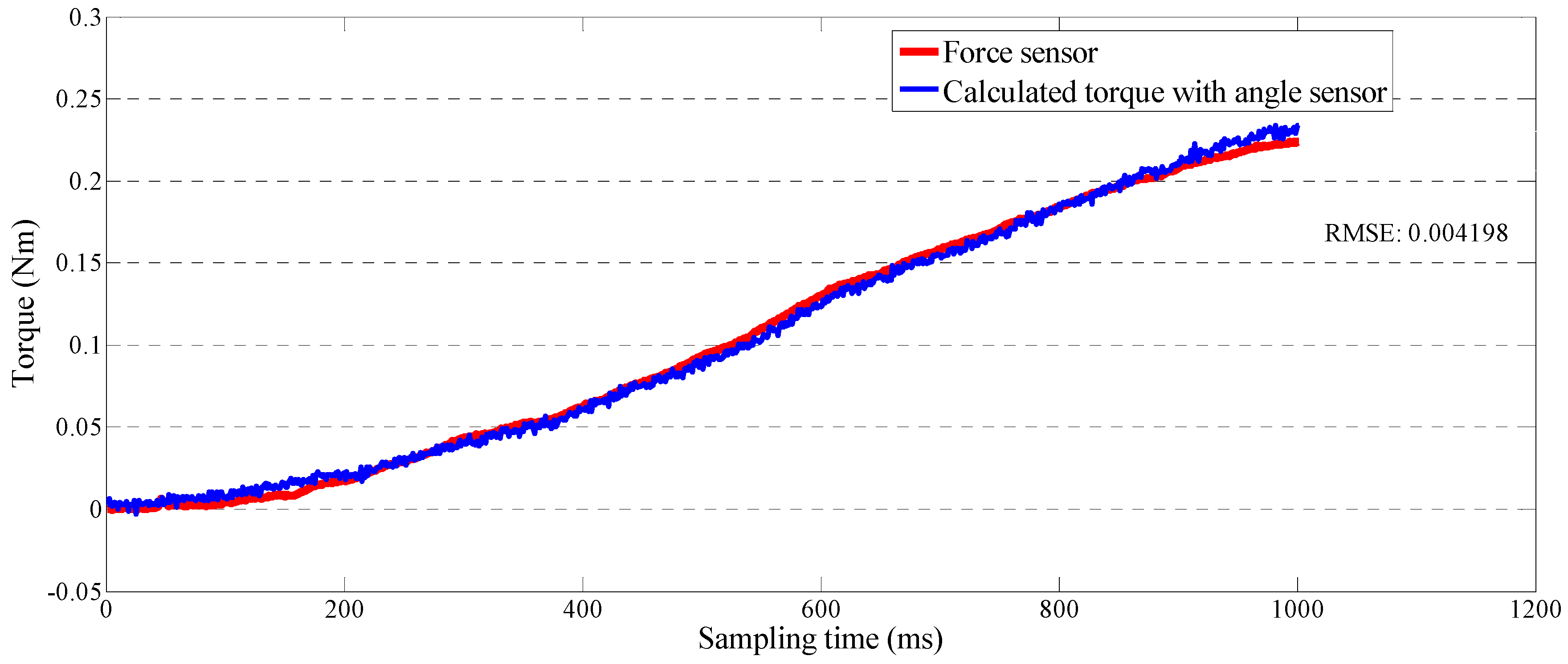
4.1. Calibration of the Force-Sensing Mechanism in the Master Device with the Force Sensor
| Parameters | Value |
|---|---|
| Radius of Part ⑥ (r) | 5 mm |
| Length of elastic elements (L3) | 80 mm |
| Length of long steel bar (L4) | 270 mm |
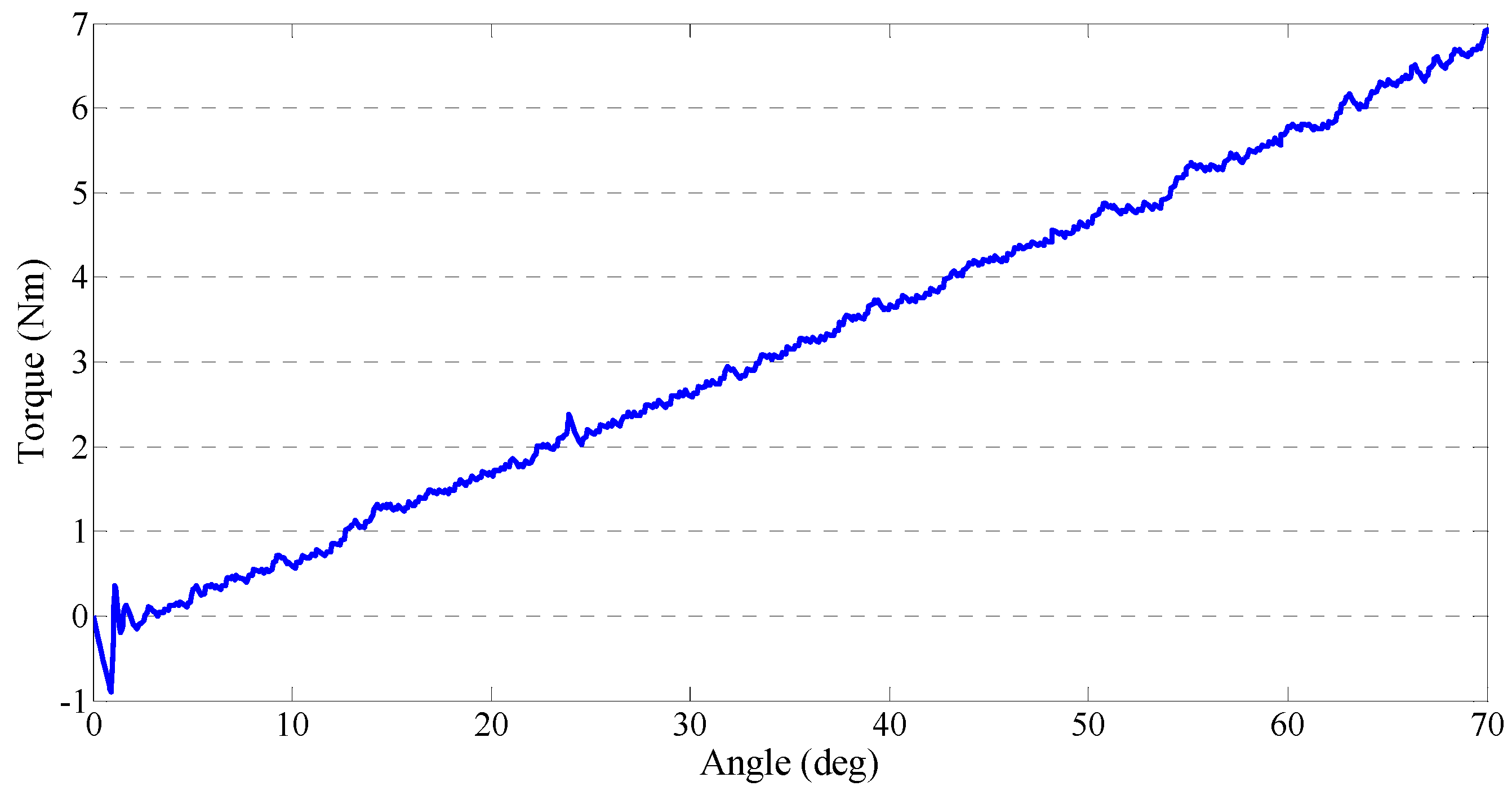
4.2. Performance Test of the Output Impedances of the Master Device

4.3. Teleoperation Performance Test for Passive Training

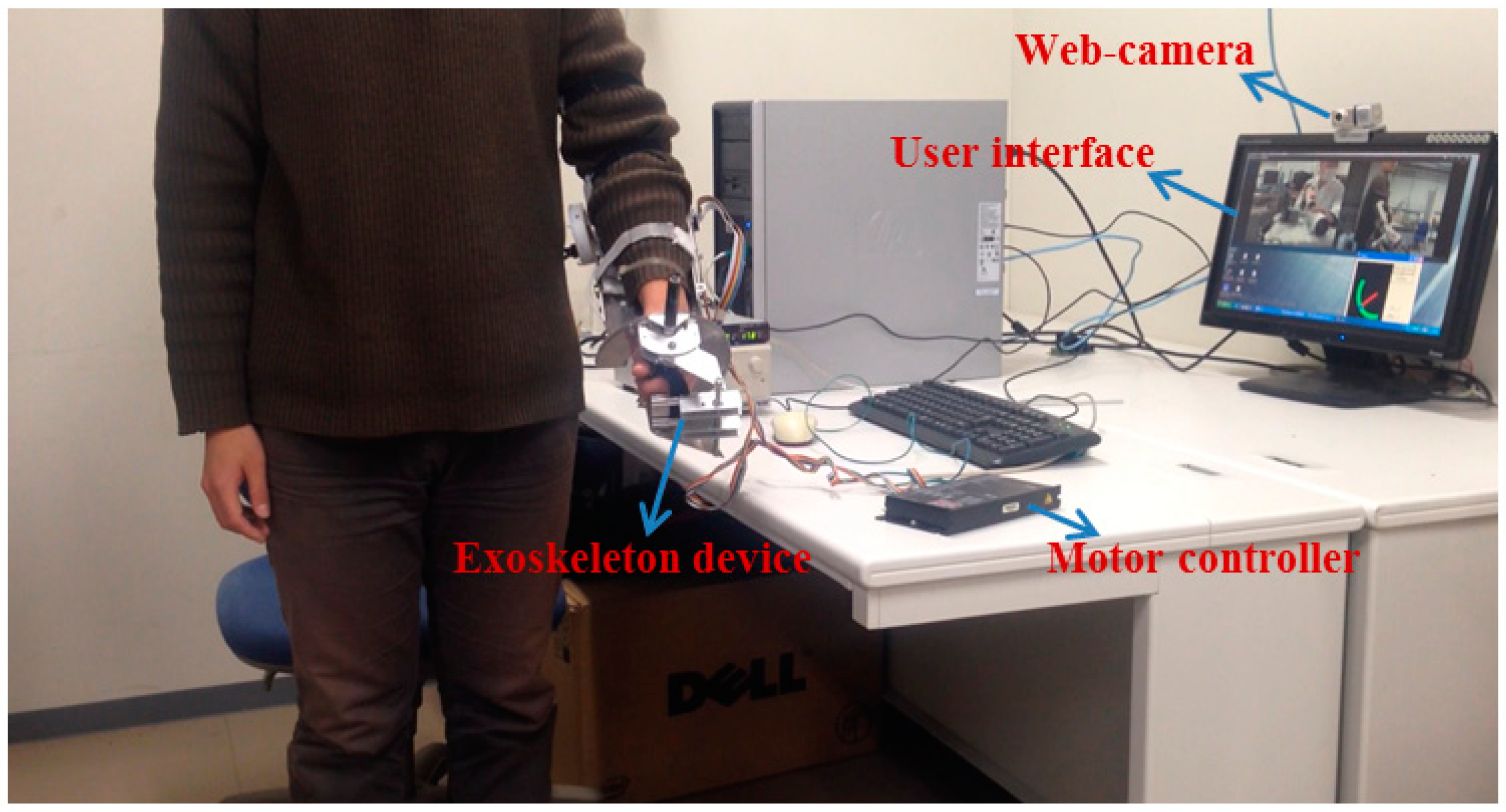
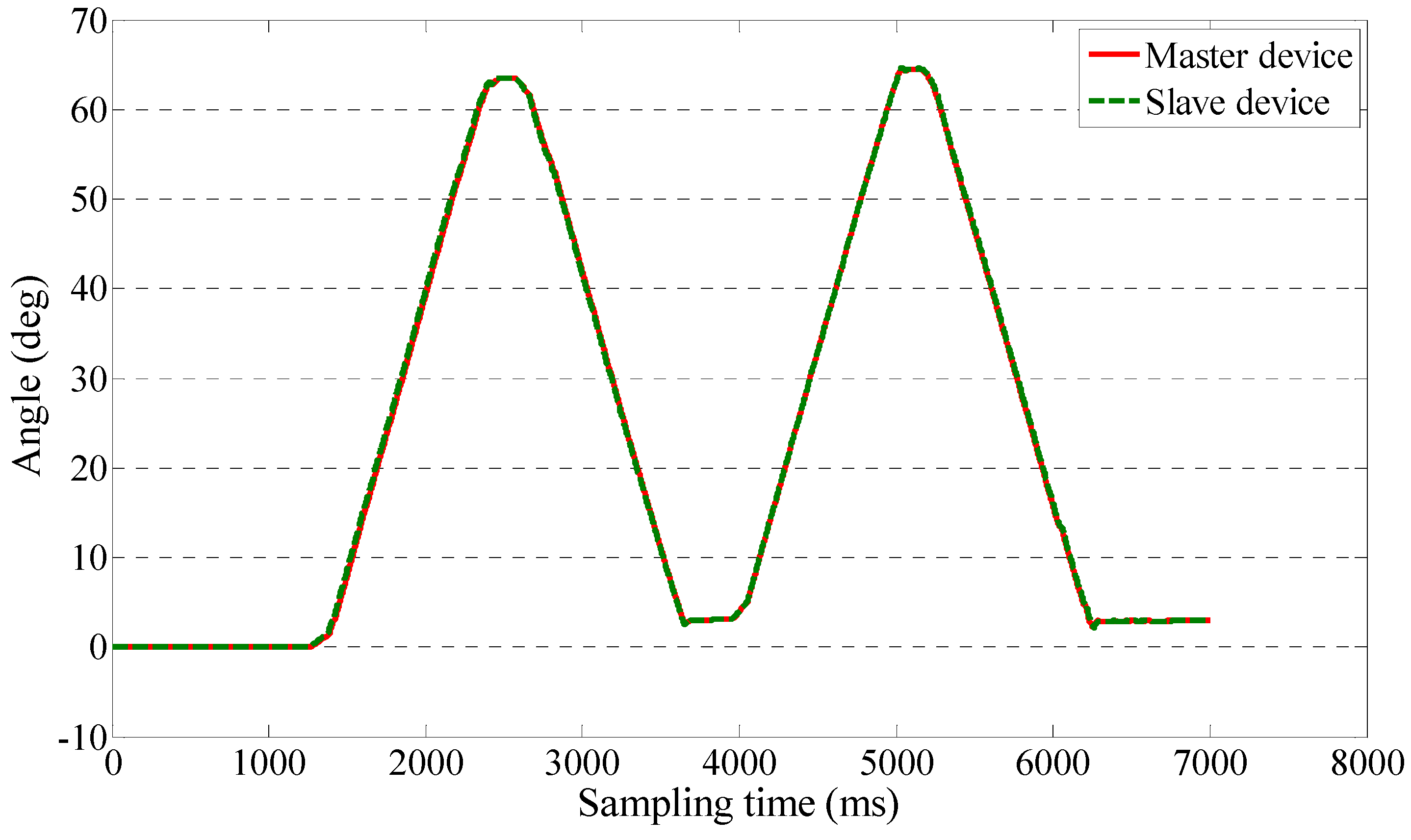

5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lloyd-Jones, D.; Adams, R.J.; Brown, T.M.; Carnethon, M.; Dai, S.; de Simone, G.; Ferguson, T.B.; Ford, E.; Furie, K.; Gillespie, C.; et al. Heart disease and stroke statistics–2010 update: A report from the American Heart Association. Circulation 2010, 121, e46–e215. [Google Scholar] [CrossRef] [PubMed]
- Hesse, S.; Schulte-Tigges, G.; Konrad, M.; Bardeleben, A.; Werner, C. Robot-assisted arm trainer for the passive and active practice of bilateral forearm and wrist movement in hemiparetic subjects. Arch. Phys. Med. Rehabil. 2003, 84, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Novak, D.; Nagle, A.; Keller, U.; Riener, R. Increasing motivation in robot-aided arm rehabilitation with competitive and cooperative gameplay. J. Neuroeng. Rehabil. 2014, 11. [Google Scholar] [CrossRef]
- Hogan, N.; Krebs, H.I.; Sharon, A.; Chamnarong, J. Interactive Robotic Therapist. U.S. Patent 5,466,213, 14 November 1995. [Google Scholar]
- Song, Z.; Guo, S.; Fu, Y. Development of an upper extremity motor function rehabilitation system and assessment system. Int. J. Mechatron. Autom. 2011, 1, 19–28. [Google Scholar] [CrossRef]
- Mayhew, D.; Bachrach, B.; Rymer, W.Z.; Beer, R.F. Development of the MACARM-a novel cable robot for upper limb neurorehabilitation. In Proceedings of the 9th International Conference on Rehabilitation Robotics, Chicago, IL, USA, 28 June–1 July 2005; pp. 299–302.
- Guidali, M.; Duschau-Wicke, A.; Broggi, S.; Klamroth-Marganska, V.; Nef, T.; Riener, R. A robotic system to train activities of daily living in a virtual environment. Med. Biol. Eng. Comput. 2011, 49, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Frisoli, A.; Salsedo, F.; Bergamasco, M.; Rossi, B.; Carboncini, M.C. A force-feedback exoskeleton for upper-limb rehabilitation in virtual reality. Appl. Bionics Biomech. 2009, 6, 115–126. [Google Scholar] [CrossRef]
- Vitiello, N.; Lenzi, T.; Roccella, S.; Rossi, S.M.M.D.; Cattin, E.; Giovacchini, F.; Vecchi, F.; Carrozza, M.C. NEUROExos: A powered elbow exoskeleton for physical rehabilitation. IEEE Trans. Robot. 2013, 29, 220–235. [Google Scholar] [CrossRef]
- Maciejasz, P.; Eschweiler, J.; Gerlach-Hahn, K.; Jansen-Troy, A.; Leonhardt, S. A survey on robotic devices for upper limb rehabilitation. J. Neuroeng. Rehabil. 2014, 11. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Guo, S.; Pang, M.; Zhang, S.; Xiao, N.; Gao, B.; Shi, L. Implementation of resistive training using an upper-limb exoskeleton rehabilitation device in elbow joint. J. Med. Biol. Eng. 2013, 34, 188–196. [Google Scholar] [CrossRef]
- Tang, J.; Carignan, C.; Gattewar, S. Virtual Environment for Robotic Tele-rehabilitation. In Proceedings of the IEEE 9th International Conference on Rehabilitation Robotics, Chicago, IL, USA, 28 June–1 July 2005; pp. 365–370.
- Holden, M.K.; Dyar, T.A.; Schwamm, L.; Bizzi, E. Virtual-environment-based telerehabilitation in patients with stroke. Presence (Camb.) 2005, 14, 214–233. [Google Scholar] [CrossRef]
- Lo, A.C.; Guarino, P.D.; Richards, L.G.; Haselkorn, J.K.; Wittenberg, G.F.; Federman, D.G.; Ringer, R.J.; Wagner, T.H.; Krebs, H.I.; et al. Robot-assisted therapy for long-term upper limb impairment after stroke. Engl. J. Med. 2010, 362, 1772–1783. [Google Scholar] [CrossRef]
- Park, H.; Peng, Q.; Zhang, L. A Portable Telerehabilitation System for Remote Evaluations of Impaired Elbows in Neurological Disorders. IEEE Trans. Neural Syst. Rehabil. Eng. 2008, 16, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Atashzar, S.F.; Polushin, I.G.; Patel, R.V. Networked Teleoperation with Non-Passive Environment: Application to Tele-Rehabilitation. In Proceedings of the 2012 IEEE/RSJ International Conference on Intelligent Robots and Systems, Vilamoura, Algarve, Portugal, 7–12 October 2012; pp. 5125–5130.
- Wang, X.; Li, J. A Tele-rehabilitation System with Bilateral Haptic Feedback to Both the Therapist and the Patient via Time-delay Environment. In Proceedings of the World Haptics Conference, Daejeon, Korea, 14–17 April 2013; pp. 331–334.
- Lenzi, T.; Vitiello, N.; de Rossi, S.M.M.; Roccella, S.; Vecchi, F.; Carrozza, M.C. NEUROExos: A variable impedance powered elbow exoskeleton. In Proceedings of the IEEE International Conference on Robotics and Automation, Shanghai, China, 9–13 May 2011; pp. 1419–1426.
- Rahman, T.; Sample, W.; Jayakumar, S.; King, M.M.; Wee, J.Y.; Seliktar, R.; Alexander, M.; Scavina, M.; Clark, A. Passive exoskeleton for assisting limb movement. J. Rehabil. Res. Dev. 2006, 43, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Guo, S.; Xiao, N.; Gao, B.; Shi, L. Implementation of human-machine synchronization control for active rehabilitation using an inertia sensor. Sensors 2012, 12, 16046–16059. [Google Scholar] [CrossRef] [PubMed]
- Oblak, J.; Cikajlo, I.; Matjačić, Z. Universal Haptic Drive: A Robot for Arm and Wrist Rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 293–302. [Google Scholar] [CrossRef]
- Zinn, M.; Khatib, O.; Roth, B. A new actuation approach for human friendly robot design. Int. J. Robot. Res. 2004, 23, 279–398. [Google Scholar] [CrossRef]
- Ham, R.V.; Sugar, T.G.; Vanderborght, B.; Hollander, K.W.; Lefeber, D. Compliant actuator designs. IEEE Robot. Autom. Mag. 2009, 81–94. [Google Scholar] [CrossRef]
- Kong, K.; Bae, J.; Tomizuka, M.; Control of rotary series elastic actuator for ideal force-mode actuation in human-robot interaction applications. IEEE/ASME Trans. Mech. 2009, 14, 105–118. [Google Scholar] [CrossRef]
- Pratt, G.A.; Williamson, M.M.; Series elastic actuator. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems, Pittsburgh, PA, USA, 5–9 August 1995; pp. 399–406.
- Zhang, S.; Guo, S.; Pang, M. Performance Evaluation of a Novel Master-slave Rehabilitation System. In Proceedings of the 2013 IEEE International Conference on Mechatronics and Automation, Takamatsu, Japan, 4–7 August 2013; pp. 141–145.
- Guo, S.; Zhang, S.; Song, Z.; Pang, M. Development of a Human Upper Limb-like Robot for Master-slave Rehabilitation. In Proceedings of the 2013 ICME International Conference on Complex Medical Engineering, Beijing, China, 25–28 May 2013; pp. 693–696.
- Zhao, G.; Mei, Z.; Liang, D.; Lvanov, K.; Guo, Y.; Wang, Y.; Wang, L. Exploration and implementation of a pre-impact fall recognition method based on an inertial body sensor network. Sensors 2012, 12, 15338–15355. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Lee, S.; Lim, M.; Choi, J.; A Mechanically Adjustable Stiffness Actuator (MASA) of a robot for knee rehabilitation. In Proceedings of the 2014 IEEE International Conference on Robotic & Automation (ICRA), Hong Kong, China, 31 May–7 June 2014; pp. 3384–3389.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Guo, S.; Gao, B.; Hirata, H.; Ishihara, H. Design of a Novel Telerehabilitation System with a Force-Sensing Mechanism. Sensors 2015, 15, 11511-11527. https://doi.org/10.3390/s150511511
Zhang S, Guo S, Gao B, Hirata H, Ishihara H. Design of a Novel Telerehabilitation System with a Force-Sensing Mechanism. Sensors. 2015; 15(5):11511-11527. https://doi.org/10.3390/s150511511
Chicago/Turabian StyleZhang, Songyuan, Shuxiang Guo, Baofeng Gao, Hideyuki Hirata, and Hidenori Ishihara. 2015. "Design of a Novel Telerehabilitation System with a Force-Sensing Mechanism" Sensors 15, no. 5: 11511-11527. https://doi.org/10.3390/s150511511





