Determining the Leaf Emissivity of Three Crops by Infrared Thermometry
Abstract
:1. Introduction
2. Experimental Section
2.1. Theoretical Considerations
2.2. Plant Materials
2.3. Infrared Thermometer
| Items | Infrared Thermometer | K-Type Thermocouple |
|---|---|---|
| Measuring range | −60–76 °C | −60–14 °C |
| Operating range | 0–50 °C | 0–50 °C |
| Accuracy | 2.0 °C | 1.0 °C |
| Resolution | 0.1 °C | 0.1 °C |
| Response time | 0.5 s | 1.0 s |
| Field of view | 30:1 | |
| Emissivity range | 0.1–1.0 | |
| Adjusted step | 0.01 | |
| Signal indication | LCD screen | LCD screen |
2.4. Standard Temperature
2.5. Calibration of Thermometers
2.6. Leaf Emissivity Measurement
2.7. Statistical Analysis
3. Results and Discussion
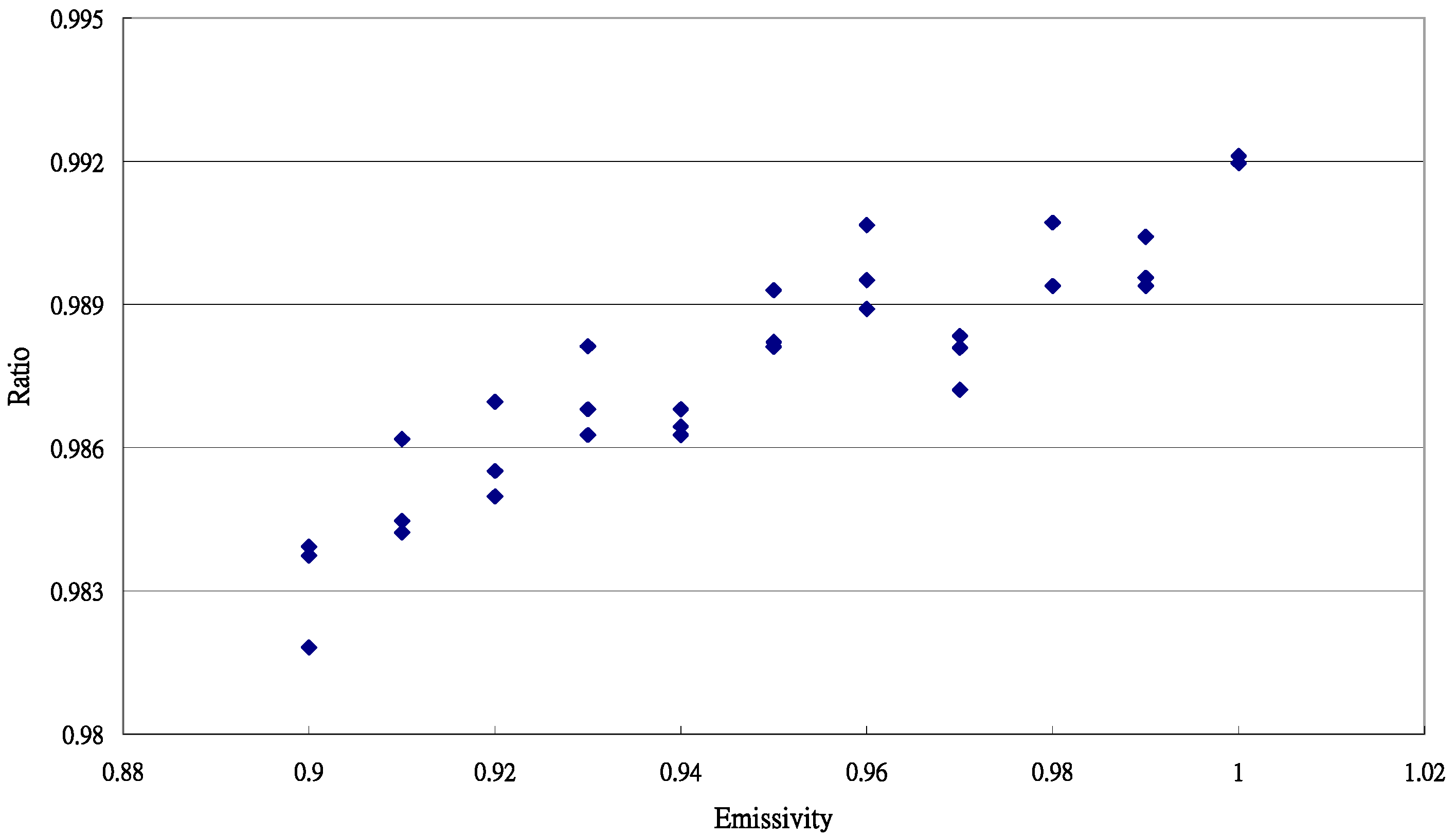
3.1. Performance of the Sentron SI20 LBE Infrared Thermometer
3.2. Emissivity of Phalaenopsis Leaves
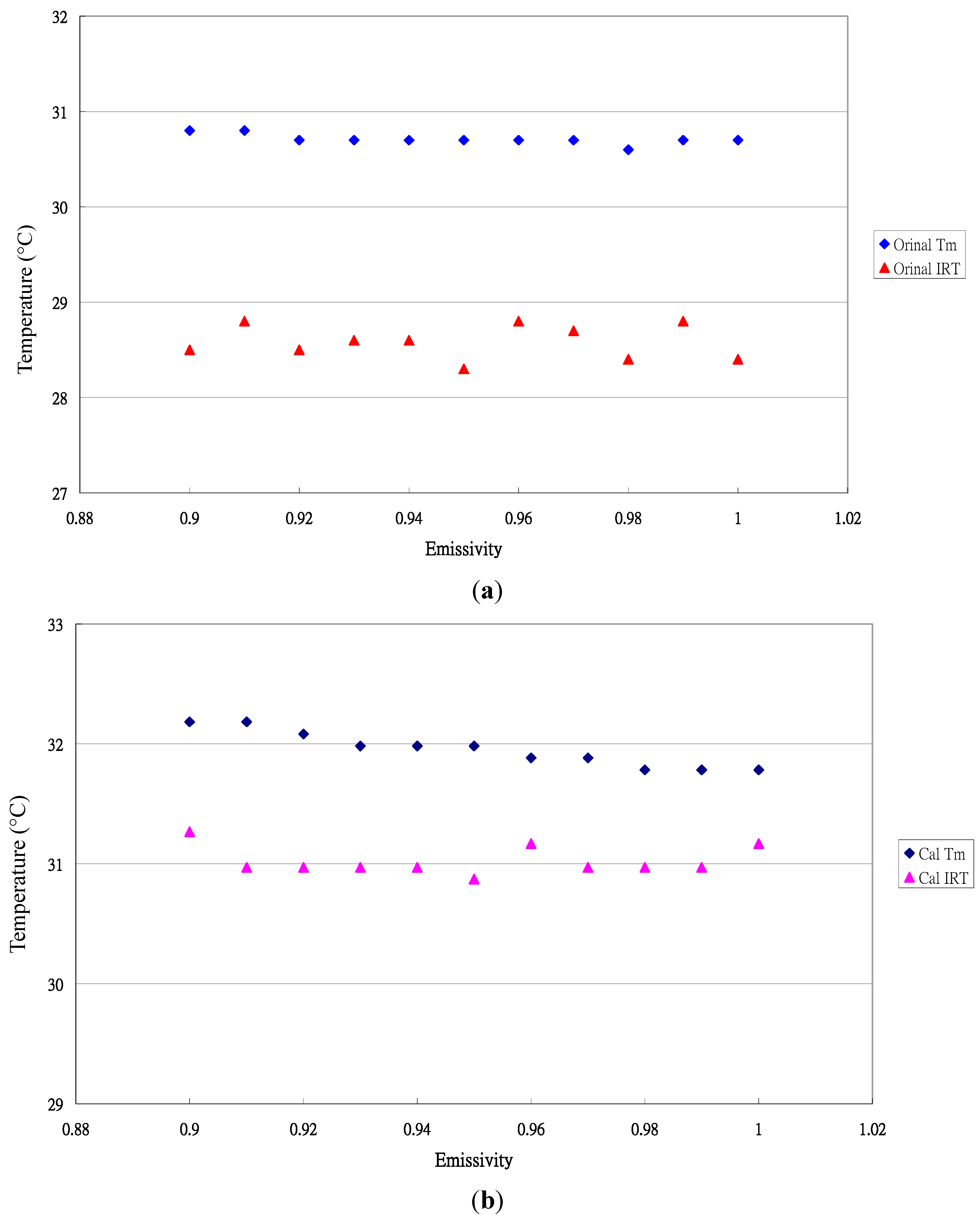
3.3. Emissivity of Paphiopedilum Leaves
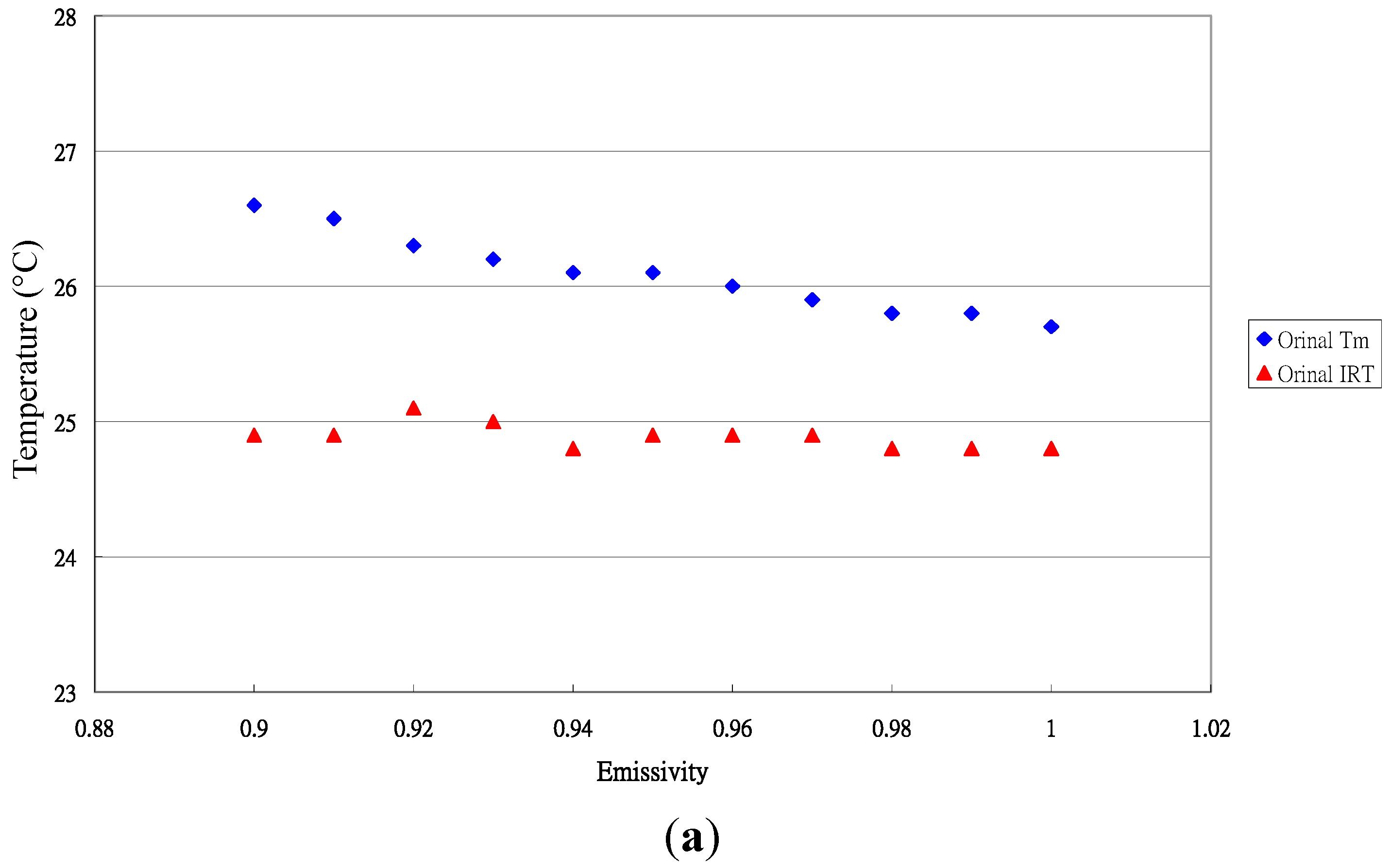
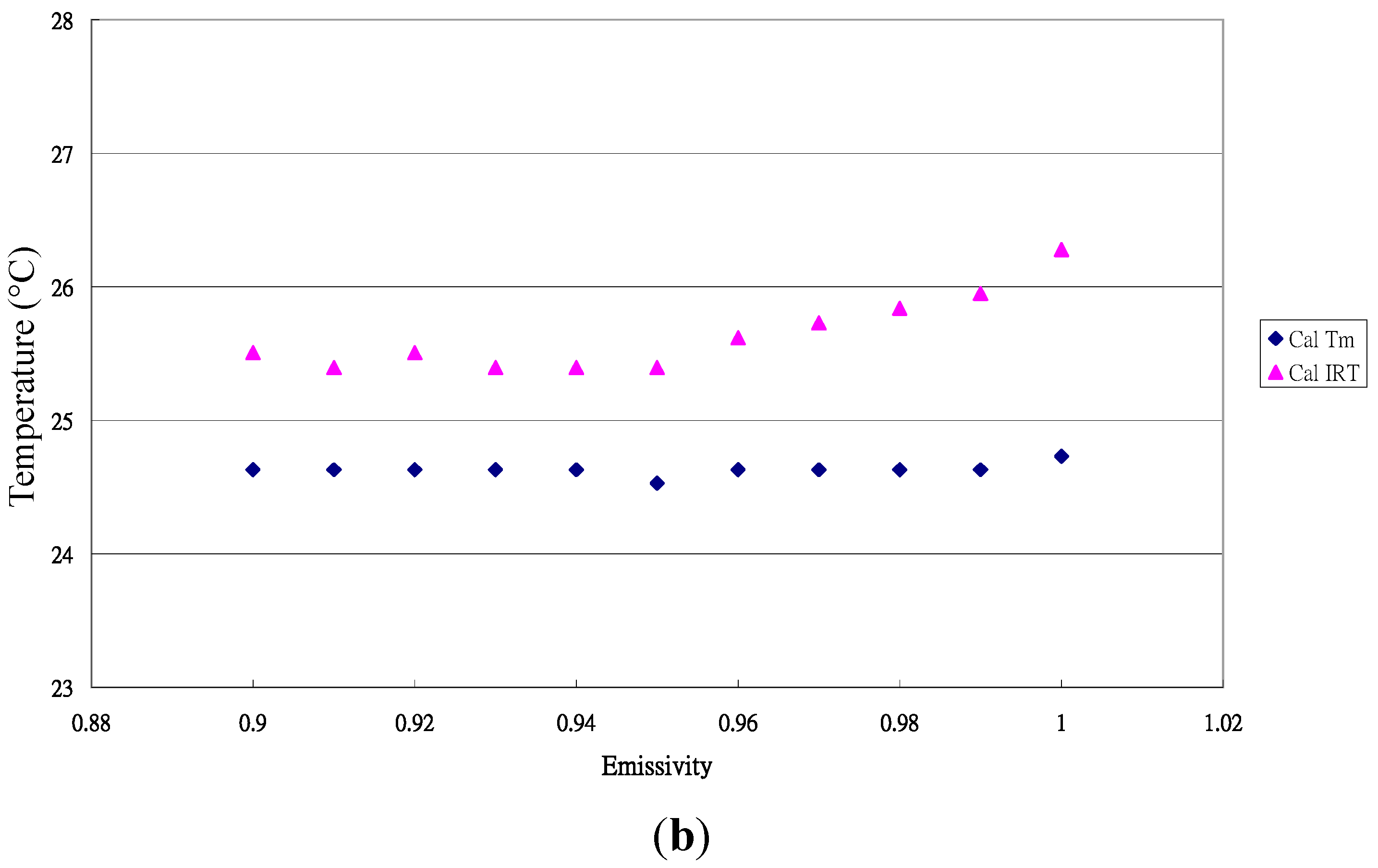

3.4. Emissivity Variation among Crop Leaves in This Study
| Crops | Mean | SD | CV |
|---|---|---|---|
| Phalaenopsis mature leaves | 0.980943 | 0.010391 | 1.06% |
| Phalaenopsis new leaves | 0.978293 | 0.008619 | 0.88% |
| Paphiopedilum leaves | 0.981057 | 0.006641 | 0.68% |
| Achiva macrocarpe Malabar leaves | 0.984777 | 0.005365 | 0.55% |

3.5. Emissivity for Crops in the Literature
| Crops | Mean | sd | CV | Sources |
|---|---|---|---|---|
| Rape | 0.98 | 0.01 | 1.02% | Rahkonen and Jakela [20] |
| Sow-thistle | 0.98 | 0.01 | 1.02% | Rahkonen and Jakela [20] |
| Pine | 0.982 | 0.009 | 0.92% | Arp and Phiuney [29] |
| Olive | 0.976 | 0.006 | 0.62% | Rubio et al. [18] |
| Alfafa | 0.987 | 0.004 | 0.41% | Rubio et al. [18] |
| Pipe | 0.982 | 0.009 | 0.91% | Rubio et al. [18] |
| Holmoak | 0.985 | 0.01 | 1.02% | Rubio et al. [18] |
| Tomato | 0.980 | 0.01 | 1.02% | Lopez et al. [21] |
| Pepper | 0.978 | 0.008 | 0.82% | Lopez et al. [21] |
| Cucumber | 0.983 | 0.008 | 0.81% | Lopez et al. [21] |
| Courgette | 0.985 | 0.007 | 0.71% | Lopez et al. [21] |
| Aubergine | 0.973 | 0.007 | 0.72% | Lopez et al. [21] |
| Melon | 0.978 | 0.006 | 0.61% | Lopez et al. [21] |
| Watermelon | 0.981 | 0.009 | 0.91% | Lopez et al. [21] |
| Green bean | 0.983 | 0.006 | 0.61% | Lopez et al. [21] |
| Red bean | 0.983 | 0.005 | 0.51% | Lopez et al. [21] |
| Bean | 0.957 | 0.005 | 0.52% | Fuchs and Tanner [15] |
| Tobacco | 0.971 | 0.002 | 0.21% | Fuchs and Tanner [15] |
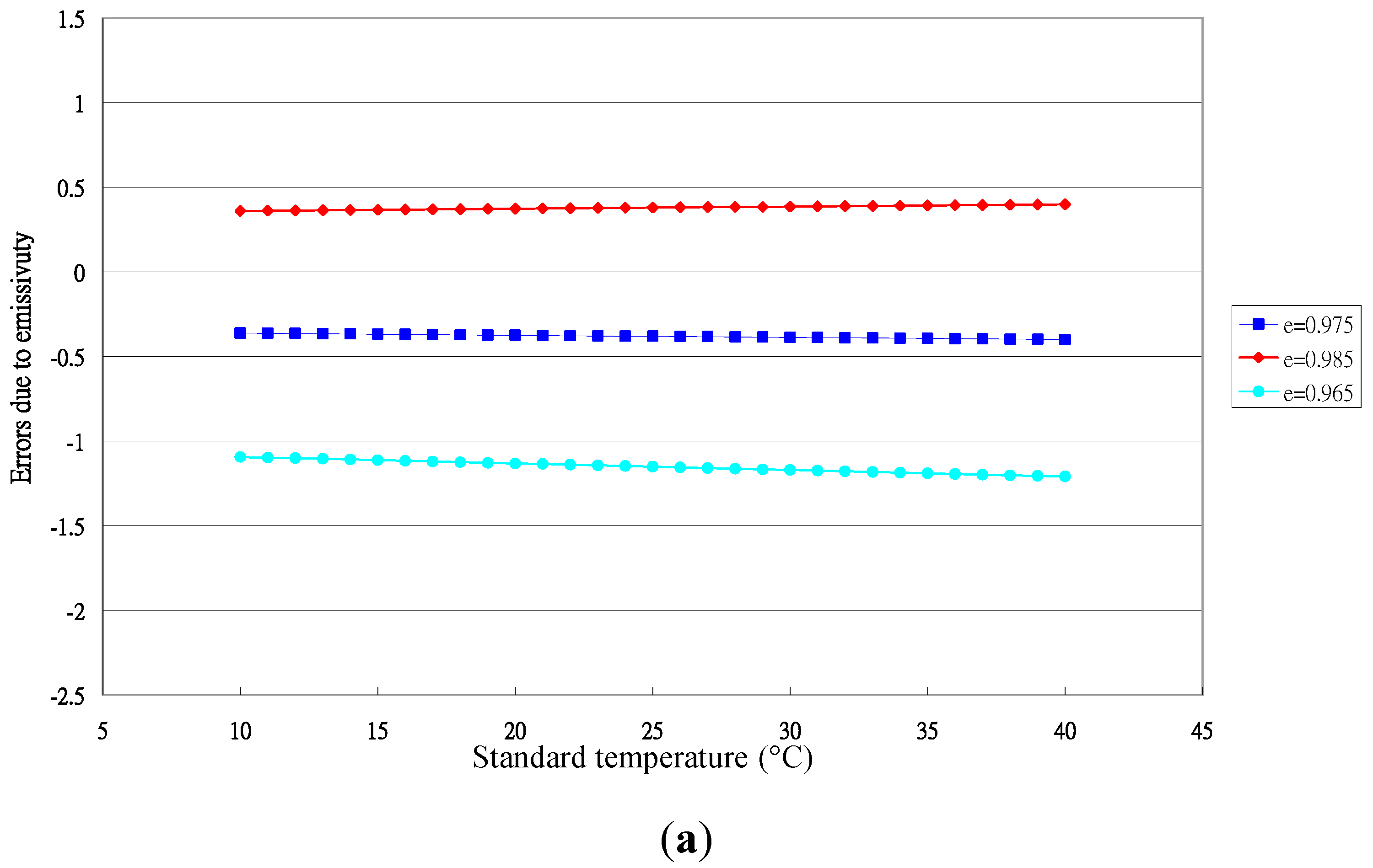
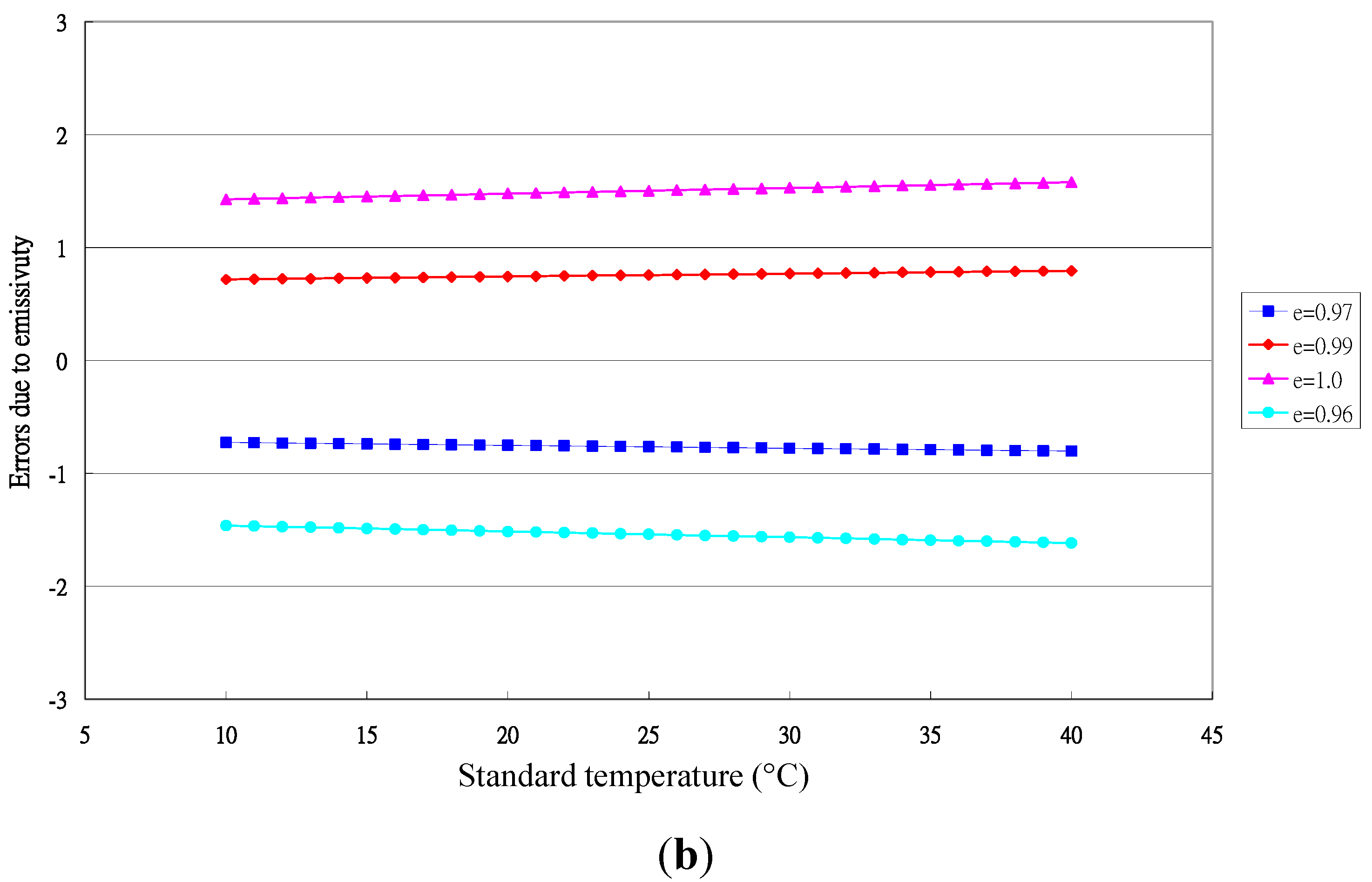
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ehret, D.L.; Lau, A.; Bittman, S.; Lin, W.; Shelford, T. Automated monitoring of greenhouse crops. Agronomie 2001, 21, 403–414. [Google Scholar] [CrossRef]
- Hatfield, J.L. Measuring plant stress with an infrared thermometer. Hortscience 1990, 25, 1535–1538. [Google Scholar]
- Jones, H.G.; Stoll, M.; Santos, T.; de Sousa, C.; Chaves, M.M.; Grant, O.M. Use of infrared thermography for monitoring stomatal closure in the field: application to grapevine. J. Exp. Bot. 2002, 53, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Leinonen, I.; Jones, H.G. Combining thermal and visible imagery for estimating canopy temperature and indentifying plant stress. J. Exp. Bot. 2004, 55, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.T.; Evett, S.R. Modeling diurnal canopy temperature dynamics using one-time-of-day measurements and a reference temperature curve. Agron. J. 2004, 96, 1553–1561. [Google Scholar] [CrossRef]
- Leinonen, I.; Grant, O.M.; Tagliavia, C.P.P.; Chaves, M.M.; Jones, H.G. Estimating stomatal conductance with thermal imagery. Plant Cell Environ. 2006, 29, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Maes, W.H.; Achten, W.M.J.; Reubens, B.; Muys, B. Monitoring stomatal conductance of Jatroptha curcas seedlings under different levels of water shortage with infrared thermography. Agric. For. Meteorol. 2011, 151, 554–564. [Google Scholar] [CrossRef]
- Durigon, A.; de Jong van Lier, Q. Canopy temperature versus soil water head for the prediction of crop water stress. Agric. Water Manag. 2013, 127, 1–6. [Google Scholar] [CrossRef]
- Amiro, B.D.; Thurtell, G.W.; Gillespie, T.J. A small infrared thermometer for measuring leaf temperature in leaf chambers. J. Exp. Bot. 1983, 34, 1569–1576. [Google Scholar] [CrossRef]
- Rapier, C.B.; Michael, K.J. The calibration of a small, low-cost thermal infrared radiometer. Remote Sens. Environ. 1996, 56, 97–103. [Google Scholar] [CrossRef]
- Bugbee, B.; Droter, M.; Monje, O.; Tanner, B. Evaluation and modification of commercial infra-red transducers for leaf temperature measurement. Adv. Space Res. 1998, 10, 1425–1434. [Google Scholar] [CrossRef]
- Savage, M.J.; Heilman, J.L. Infrared calibration of net radiometers and infrared thermometers. Agric. For. Meteorol. 2009, 149, 1279–1293. [Google Scholar] [CrossRef]
- Chen, C.; Weng, Y.; Shen, T. Performance evaluation of the infrared thermocouple. Sensors 2010, 10, 10081–10094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emissivity. Available online: http://www.merriam-webster.com/dictionary/emissivirt (accessed on 2 February 2015).
- Fuchs, M.; Tanner, C.B. Infrared thermometry of vegetation. Agron. J. 1966, 58, 597–601. [Google Scholar] [CrossRef]
- ASTM, Standard E 1933–99a. Standard test methods for measuring and compensating for emissivity using infrared imaging radiometers. ASTM: West Conshohocken, PA, USA, 2006.
- Hipps, L.E. The infrared emissivities of soil and Artemisia tridentate and subsequent temperature corrections in a shrub-steppe ecosystem. Remote Sens. Environ. 1989, 27, 337–342. [Google Scholar] [CrossRef]
- Rubio, E.; Caselles, V.; Badenas, C. Emissivity measurements of several soils and vegetation types in the 8–14 μm wave band: analysis of two field methods. Remote Sens. Environ. 1997, 59, 490–521. [Google Scholar] [CrossRef]
- Sugita, M.; Hiyama, T.; Ikukawa, T. Determination of canopy emissivity: How reliable is it? Agric. For. Meteorol. 1996, 81, 229–239. [Google Scholar] [CrossRef]
- Rahkonen, J.; Jokela, H. Infrared radiometry for measuring plant leaf temperature during thermal weed control treatment. Biosyst. Eng. 2003, 86, 257–266. [Google Scholar] [CrossRef]
- Lopez, A.; Molina-Aiz, F.D.; Valera, D.L.; Peña, A. Determining the emissivity of the leaves of nine horticultural crops by means of infrared thermography. Sci. Hortic-Amsterdam 2012, 137, 49–58. [Google Scholar] [CrossRef]
- Van Alstyne, K.L.; Olson, T.K. Estimating variation in surface emissivities of intertidal macroalgae using an infrared thermometer and the effects on temperature measurements. Mar. Biol. 2014, 161, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Van der Knaap, N.; van Herk, M.; Kuijf, R.; van Rosmalen, N.; de Goeij, L.; Gijzen, W.; van der Leeden, M.; van Spingelen, J.; Lont, A; van Os, A. Cultivation Guide Phalaenopsis: Knowledge for Professionals; Anthura B.V.: Bleiswijk, The Netherlands, 2005. [Google Scholar]
- Floricultura, B.V. Floriclone Phalaenopsis Pot Plant. Available online: http://www.floricultura.nl/pdf/155%2001%20039%20FC%20Phalaenopsis%20pot%20plant%20GB-oct%202012.pdf (accessed on 2 February 2015).
- Chung, W.; Chen, C. Evaluation of performance and uncertainty of infrared tympanic thermometers. Sensors 2010, 10, 3073–3089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, H.; Archer, N.; Rotenberg, E.; Casa, R. Radiation measurement for plant ecophysiology. J. Exp. Bot. 2003, 54, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. Evaluation of measurement uncertainty for thermometers with calibration equations. Accred. Qual. Assur. 2006, 11, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.; Chen, C. Evaluation of piecewise polynomial equations for two types of thermocouples. Sensors 2013, 13, 17084–17097. [Google Scholar] [CrossRef]
- Arp, G.K.; Phinney, D.E. Ecological variations in thermal infrared emissivity of vegetation. Environ. Exp. Bot. 1980, 20, 135–148. [Google Scholar] [CrossRef]
- Brewster, M.Q. Radiative properties and simple transfer. In Thermal Radiative Transfer and Properties; Wiley: New York, NY, USA, 1992; Chapter 2; pp. 26–68. [Google Scholar]
- Meyer, G.E.; Fletcher, M.R.; Fitzgerald, J.B. Calibration and use of a pyroelectric thermal camera and imaging system for greenhouse infrared heating evaluation. Comput. Electron. Agric. 1994, 10, 215–227. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C. Determining the Leaf Emissivity of Three Crops by Infrared Thermometry. Sensors 2015, 15, 11387-11401. https://doi.org/10.3390/s150511387
Chen C. Determining the Leaf Emissivity of Three Crops by Infrared Thermometry. Sensors. 2015; 15(5):11387-11401. https://doi.org/10.3390/s150511387
Chicago/Turabian StyleChen, Chiachung. 2015. "Determining the Leaf Emissivity of Three Crops by Infrared Thermometry" Sensors 15, no. 5: 11387-11401. https://doi.org/10.3390/s150511387






