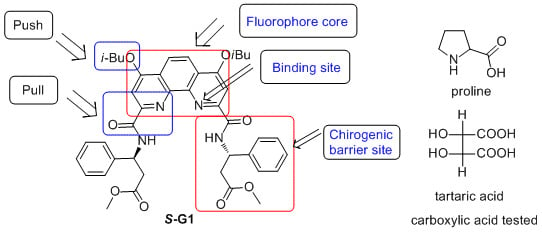
Enantioselective Recognition of Chiral Carboxylic Acids by a β-Amino Acid and 1,10-Phenanthroline Based Chiral Fluorescent Sensor
Abstract
:1. Introduction
2. Experimental Section
2.1. General
2.2. Instrumentation
2.3. Sample Preparations
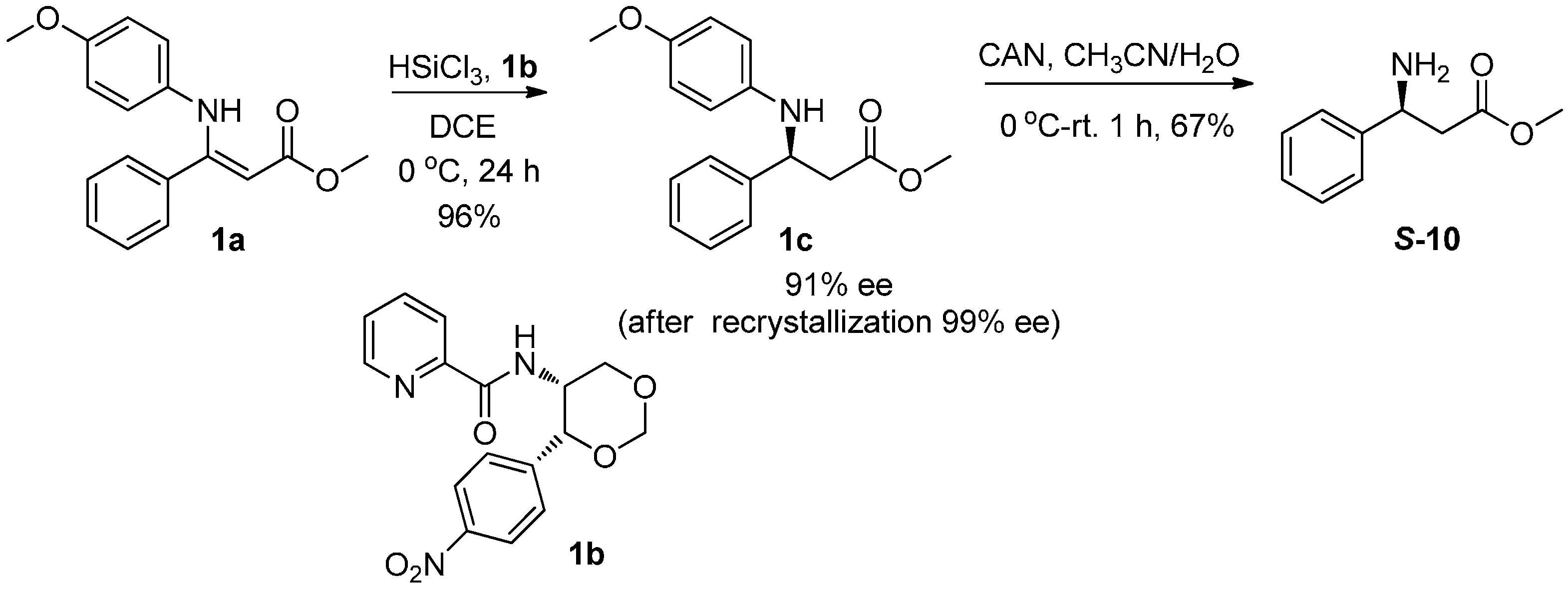
2.3.1. Synthesis of β-Amino Acid (S-10)
2.3.2. Synthesis of Fluorescent Chiral Sensor (S-G1)
Synthesis of S-G1

2.4. Enantioselective Recognition of Chiral Carboxylic Acids with Fluorescent Chiral Sensor S-G1
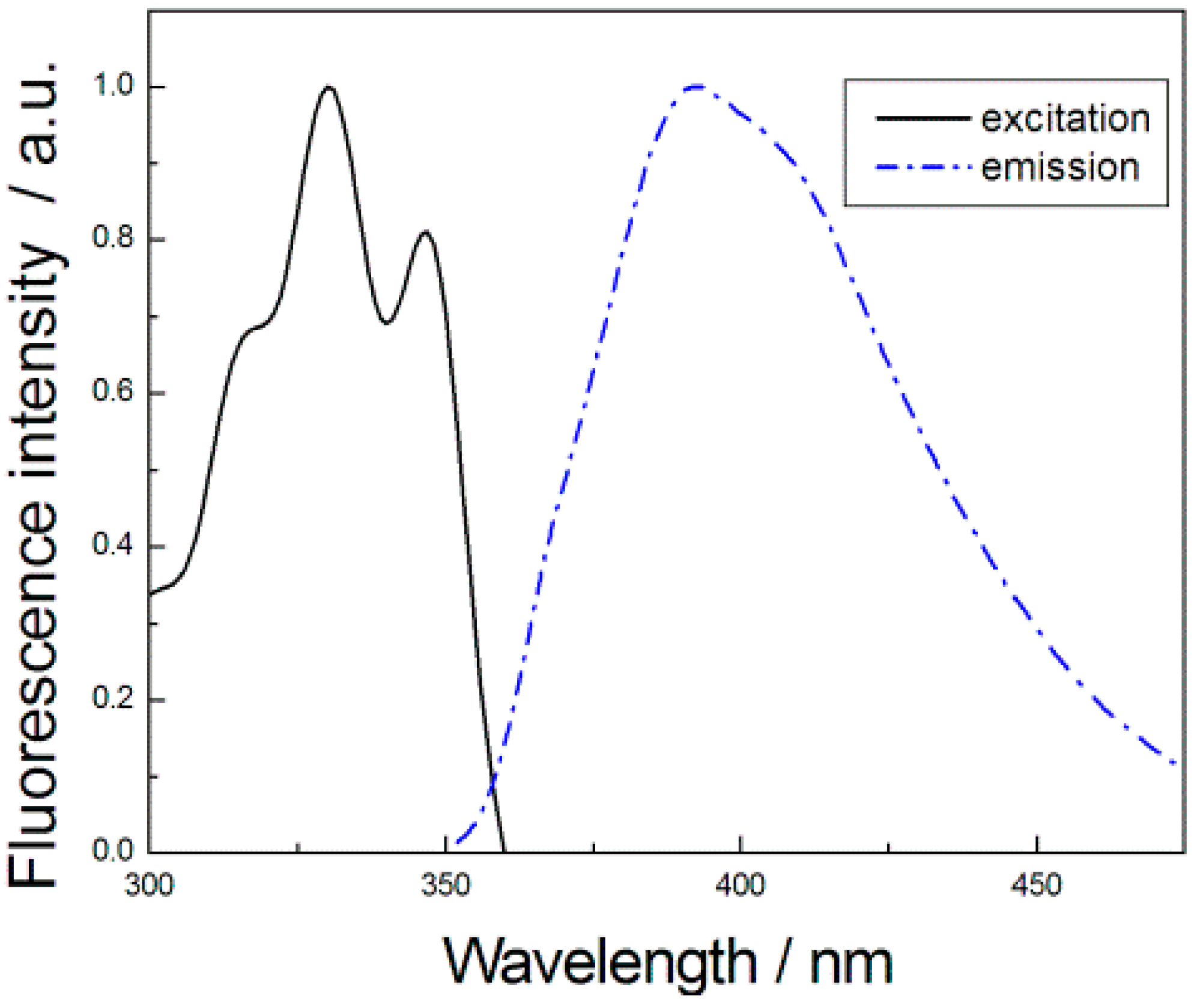
3. Results and Discussion
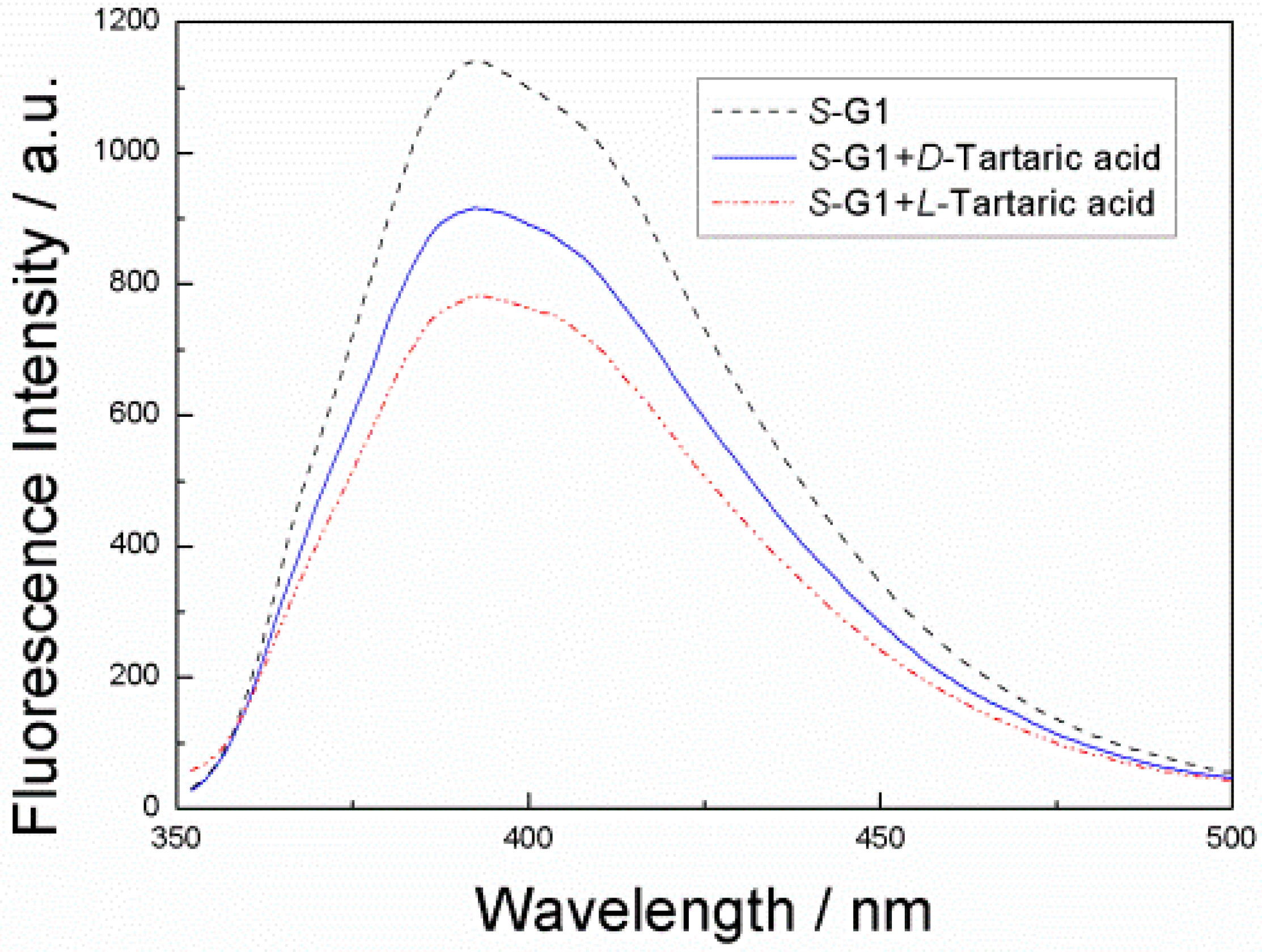
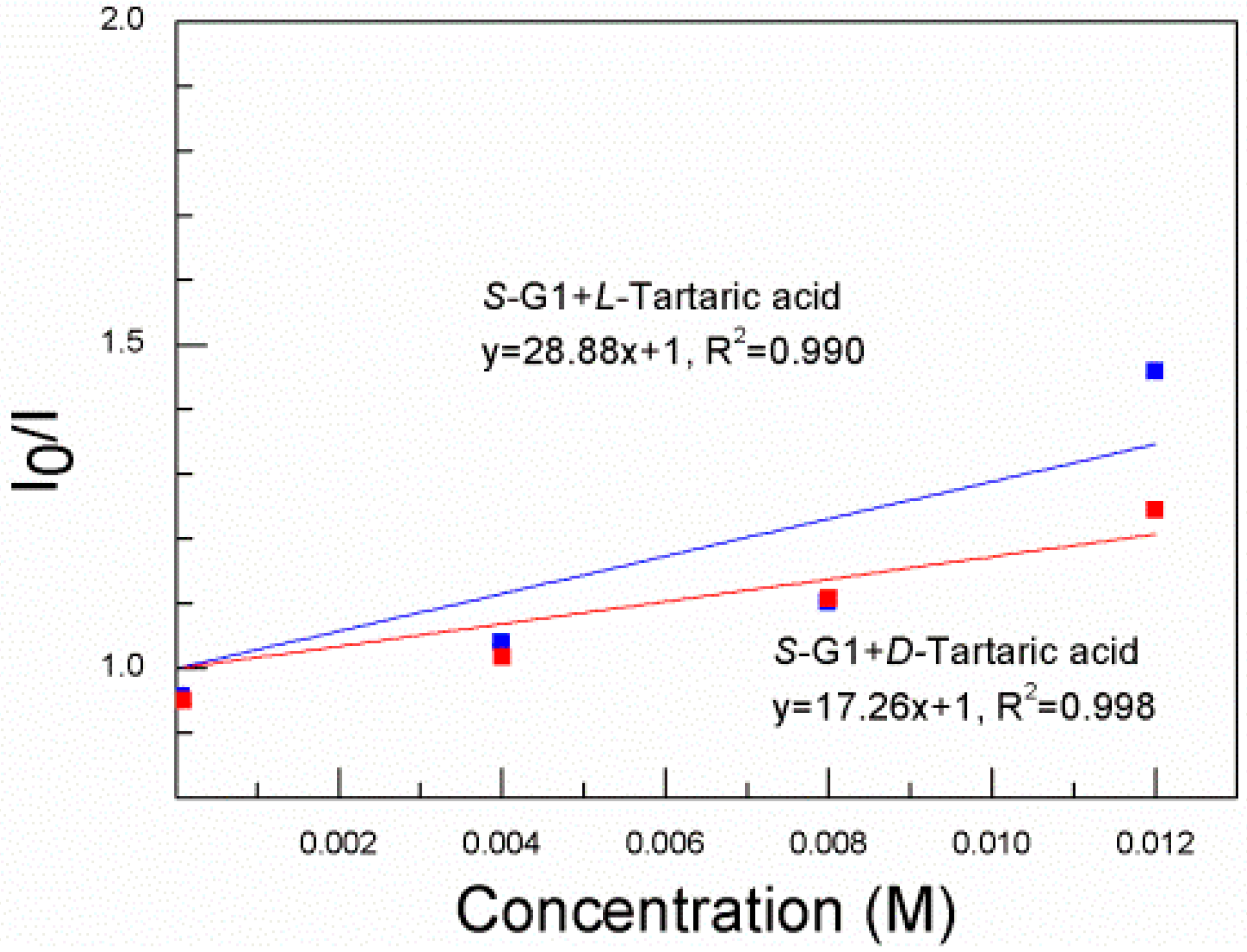

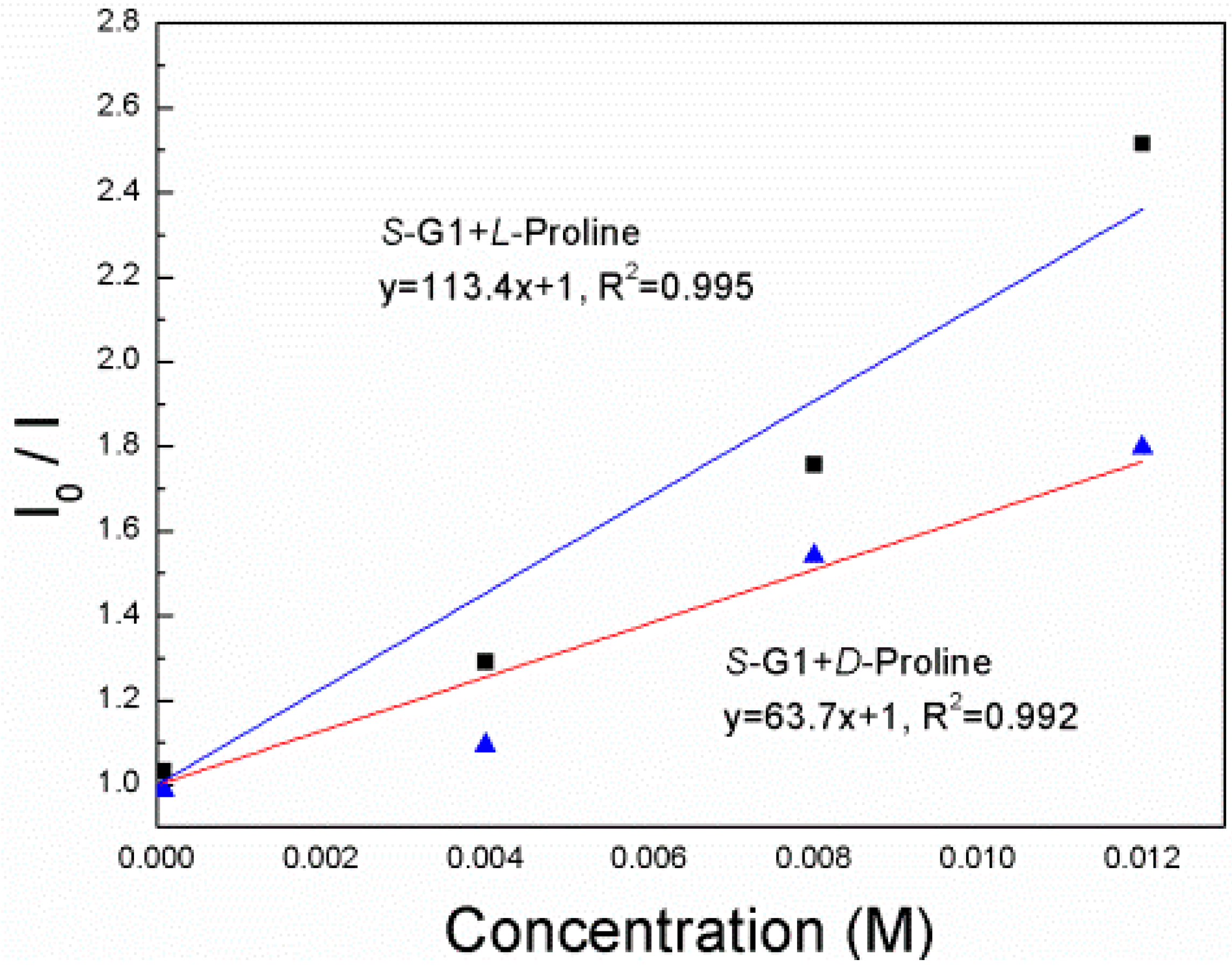
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pu, L. 1,1'-binaphthyl dimers, oligomers, and polymers: Molecular recognition, asymmetric catalysis, and new materials. Chem. Rev. 1998, 98, 2405–2494. [Google Scholar] [CrossRef] [PubMed]
- Kocovský, P.; Vyskocil, Š.; Smrcina, M. Non-symmetrically substituted 1,1'-binaphthyls in enantioselective catalysis. Chem. Rev. 2003, 103, 3213–3246. [Google Scholar] [CrossRef]
- Chen, Y.; Yekta, S.; Yudin, A.K. Modified binol ligands in asymmetric catalysis. Chem. Rev. 2003, 103, 3155–3212. [Google Scholar] [CrossRef] [PubMed]
- Brunel, J.M. Binol: A versatile chiral reagent. Chem. Rev. 2005, 105, 857–898. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-B.; Lin, J.; Qin, Y.-C.; Pu, L. Enantioselective fluorescent recognition of a soluble “supported” chiral acid: Toward a new method for chiral catalyst screening. Org. Lett. 2005, 7, 3441–3444. [Google Scholar] [CrossRef] [PubMed]
- Shahgaldian, P.; Pieles, U. Cyclodextrin derivatives as chiral supramolecular receptors for enantioselective sensing. Sensors 2006, 6, 593–615. [Google Scholar] [CrossRef]
- Webb, T.H.; Wilcox, C.S. Enantioselective and diastereoselective molecular recognition of neutral molecules. Chem. Soc. Rev. 1993, 22, 383–395. [Google Scholar] [CrossRef]
- Pu, L. Fluorescence of organic molecules in chiral recognition. Chem. Rev. 2004, 104, 1687–1716. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Rajaram, A.R.; Pu, L. Enantioselective fluorescent recognition of chiral acids by 3- and 3,3'-aminomethyl substituted binols. Tetrahedron 2004, 60, 11277–11281. [Google Scholar] [CrossRef]
- Lin, J.; Hu, Q.-S.; Xu, M.-H.; Pu, L. A practical enantioselective fluorescent sensor for mandelic acid. J. Am. Chem. Soc. 2002, 124, 2088–2089. [Google Scholar] [CrossRef] [PubMed]
- Tejeda, A.; Oliva, A.I.; Simón, L.; Grande, M.; Cruz Caballero, M.; Morán, J.R. A macrocyclic receptor for the chiral recognition of hydroxycarboxylates. Tetrahedron Lett. 2000, 41, 4563–4566. [Google Scholar] [CrossRef]
- Ishi-i, T.; Crego-Calama, M.; Timmerman, P.; Reinhoudt, D.N.; Shinkai, S. Self-assembled receptors for enantioselective recognition of chiral carboxylic acids in a highly cooperative manner. Angew. Chem. Int. Ed. 2002, 41, 1924–1929. [Google Scholar] [CrossRef]
- Yu, S.; Pu, L. Pseudoenantiomeric fluorescent sensors in a chiral assay. J. Am. Chem. Soc. 2010, 132, 17698–17700. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Z.; Chen, S.-Y.; Li, K.; Yu, X.-Q.; Pu, L. Enantioselective gel collapsing: A new means of visual chiral sensing. J. Am. Chem. Soc. 2010, 132, 7297–7299. [Google Scholar] [CrossRef] [PubMed]
- Ferrand, Y.; Kendhale, A.M.; Kauffmann, B.; Grélard, A.; Marie, C.; Blot, V.; Pipelier, M.; Dubreuil, D.; Huc, I. Diastereoselective encapsulation of tartaric acid by a helical aromatic oligoamide. J. Am. Chem. Soc. 2010, 132, 7858–7859. [Google Scholar] [CrossRef] [PubMed]
- Ema, T.; Tanida, D.; Sakai, T. Versatile and practical macrocyclic reagent with multiple hydrogen-bonding sites for chiral discrimination in NMR. J. Am. Chem. Soc. 2007, 129, 10591–10596. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tamilavan, V.; Hyun, M.H. A fluorescent chiral chemosensor for the recognition of the two enantiomers of chiral carboxylates. Chirality 2012, 24, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Boiocchi, M.; Bonizzoni, M.; Moletti, A.; Pasini, D.; Taglietti, A. Linear recognition of dicarboxylates by ditopic macrocyclic complexes. New J. Chem. 2007, 31, 352–356. [Google Scholar] [CrossRef]
- Bencini, A.; Coluccini, C.; Garau, A.; Giorgi, C.; Lippolis, V.; Messori, L.; Pasini, D.; Puccioni, S. A binol-based chiral polyammonium receptor for highly enantioselective recognition and fluorescence sensing of (S,S)-tartaric acid in aqueous solution. Chem. Commun. 2012, 48, 10428–10430. [Google Scholar] [CrossRef]
- Subramanian, G. Chiral Separation Techniques: A Practical Approach, Third Completely Revised and Updated Edition; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Sun, P.; Krishnan, A.; Yadav, A.; Singh, S.; MacDonnell, F.M.; Armstrong, D.W. Enantiomeric separations of ruthenium(ii) polypyridyl complexes using high-performance liquid chromatography (HPLC) with cyclodextrin chiral stationary phases (CSPS). Inorg. Chem. 2007, 46, 10312–10320. [Google Scholar] [CrossRef] [PubMed]
- Slama, I.; Dufresne, C.; Jourdan, E.; Fahrat, F.; Villet, A.; Ravel, A.; Grosset, C.; Peyrin, E. Vancomycin dimerization and chiral recognition studied by high-performance liquid chromatography. Anal. Chem. 2002, 74, 5205–5211. [Google Scholar] [CrossRef] [PubMed]
- Gokel, G.W.; Leevy, W.M.; Weber, M.E. Crown ethers: Sensors for ions and molecular scaffolds for materials and biological models. Chem. Rev. 2004, 104, 2723–2750. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Wolf, C. A highly congested N,N'-dioxide fluorosensor for enantioselective recognition of chiral hydrogen bond donors. Chem. Commun. 2004, 2078–2079. [Google Scholar] [CrossRef]
- Xu, Y.; McCarroll, M.E. Fluorescence anisotropy as a method to examine the thermodynamics of enantioselectivity. J. Phys. Chem. B 2005, 109, 8144–8152. [Google Scholar] [CrossRef] [PubMed]
- Caricato, M.; Leza, N.J.; Roy, K.; Dondi, D.; Gattuso, G.; Shimizu, L.S.; Vander Griend, D.A.; Pasini, D. A chiroptical probe for sensing metal ions in water. Eur. J. Org. Chem. 2013, 2013, 6078–6083. [Google Scholar] [CrossRef]
- Summers, L.A. The Phenanthrolines. In Adv Heterocycl Chem; Katritzky, A.R., Boulton, A.J., Eds.; Academic Press: Waltham, MA, USA, 1978; Volume 22, pp. 1–69. [Google Scholar]
- Sammes, P.G.; Yahioglu, G. 1,10-phenanthroline: A versatile ligand. Chem. Soc. Rev. 1994, 23, 327–334. [Google Scholar] [CrossRef]
- Chelucci, G.; Addis, D.; Baldino, S. A new approach to the 1,10-phenanthroline core. Tetrahedron Lett. 2007, 48, 3359–3362. [Google Scholar] [CrossRef]
- Luman, C.R.; Castellano, F.N. Comprehensive Coordination Chemistry II; Elsevier: Oxford, UK, 2003; Volume 1, pp. 25–39. [Google Scholar]
- Gladiali, S.; Pinna, L.; Delogu, G.; Graf, E.; Brunner, H. Optically active phenanthrolines in asymmetric catalysis. IV. Enantioselective hydrosilylation of acetophenone by rhodium/chiral alkyl phenanthroline catalysts. Tetrahedron Asymmetry 1990, 1, 937–942. [Google Scholar] [CrossRef]
- Chelucci, G.; Pinna, G.A.; Saba, A.; Sanna, G. 1,10-phenanthrolines derived from natural occurring ketones as ligands for asymmetric catalysis: Enantioselective palladium catalyzed allylic substitution. J. Mol. Catal. A Chem. 2000, 159, 423–427. [Google Scholar] [CrossRef]
- Meynhardt, B.; Lüning, U.; Wolff, C.; Näther, C. Diastereoselective generation of quaternary stereocenters by ligand-controlled palladium-catalyzed allylations. Eur. J. Org. Chem. 1999, 1999, 2327–2335. [Google Scholar] [CrossRef]
- Peña-Cabrera, E.; Norrby, P.-O.; Sjögren, M.; Vitagliano, A.; de Felice, V.; Oslob, J.; Ishii, S.; O’Neill, D.; Åkermark, B.; Helquist, P. Molecular mechanics predictions and experimental testing of asymmetric palladium-catalyzed allylation reactions using new chiral phenanthroline ligands. J. Am. Chem. Soc. 1996, 118, 4299–4313. [Google Scholar] [CrossRef]
- Chelucci, G.; Loriga, G.; Murineddu, G.; Pinna, G.A. Synthesis and application in asymmetric copper(I)-catalyzed allylic oxidation of a new chiral 1,10-phenanthroline derived from pinene. Tetrahedron Lett. 2002, 43, 3601–3604. [Google Scholar] [CrossRef]
- Chelucci, G.; Gladiali, S.; Sanna, M.G.; Brunner, H. Chiral ligands with pyridine donors in transition metal catalyzed enantioselective cyclopropanation and hydrosilylation reactions. Tetrahedron Asymmetry 2000, 11, 3419–3426. [Google Scholar] [CrossRef]
- Schoffers, E. Reinventing phenanthroline ligands—Chiral derivatives for asymmetric catalysis? Eur. J. Org. Chem. 2003, 2003, 1145–1152. [Google Scholar] [CrossRef]
- Chelucci, G.; Thummel, R.P. Chiral 2,2'-bipyridines, 1,10-phenanthrolines, and 2,2':6',2''-terpyridines: Syntheses and applications in asymmetric homogeneous catalysis. Chem. Rev. 2002, 102, 3129–3170. [Google Scholar] [CrossRef] [PubMed]
- Guenard, D.; Gueritte-Voegelein, F.; Potier, P. Taxol and taxotere: Discovery, chemistry, and structure-activity relationships. Acc. Chem. Res. 1993, 26, 160–167. [Google Scholar] [CrossRef]
- Zheng, H.-J.; Chen, W.-B.; Wu, Z.-J.; Deng, J.-G.; Lin, W.-Q.; Yuan, W.-C.; Zhang, X.-M. Highly enantioselective synthesis of β-amino acid derivatives by the lewis base catalyzed hydrosilylation of β-enamino esters. Chem. Eur. J. 2008, 14, 9864–9867. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, X.; Zheng, Y.; Xue, Z.; Shu, C.; Yuan, W.; Zhang, X. Highly diastereoselective and enantioselective synthesis of α-hydroxy β-amino acid derivatives: Lewis base catalyzed hydrosilylation of α-acetoxy β-enamino esters. Angew. Chem. Int. Ed. 2011, 50, 7304–7307. [Google Scholar] [CrossRef]
- Peet, N.P.; Baugh, L.E.; Sunder, S.; Lewis, J.E. Synthesis and antiallergic activity of some quinolinones and imidazoquinolinones. J. Med. Chem. 1985, 28, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Léger, J.-M.; Dolain, C.; Guionneau, P.; Huc, I. Aromatic δ-peptides: Design, synthesis and structural studies of helical, quinoline-derived oligoamide foldamers. Tetrahedron 2003, 59, 8365–8374. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Hu, F.; Wang, B.; Zhang, X.; Liu, C. Enantioselective Recognition of Chiral Carboxylic Acids by a β-Amino Acid and 1,10-Phenanthroline Based Chiral Fluorescent Sensor. Sensors 2015, 15, 10723-10733. https://doi.org/10.3390/s150510723
Zhang Y, Hu F, Wang B, Zhang X, Liu C. Enantioselective Recognition of Chiral Carboxylic Acids by a β-Amino Acid and 1,10-Phenanthroline Based Chiral Fluorescent Sensor. Sensors. 2015; 15(5):10723-10733. https://doi.org/10.3390/s150510723
Chicago/Turabian StyleZhang, Yonghong, Fangzhi Hu, Bin Wang, Xiaomei Zhang, and Chenjiang Liu. 2015. "Enantioselective Recognition of Chiral Carboxylic Acids by a β-Amino Acid and 1,10-Phenanthroline Based Chiral Fluorescent Sensor" Sensors 15, no. 5: 10723-10733. https://doi.org/10.3390/s150510723